Abstract
Mammalian carotid bodies are the main peripheral arterial chemoreceptors, strategically located at the bifurcation of the common carotid artery. When stimulated these receptors initiate compensatory respiratory and cardiovascular reflexes to maintain homeostasis. Thus, in response to low oxygen (hypoxia) or increased CO2/H+ (acid hypercapnia), chemoreceptor type I cells depolarize and release excitatory neurotransmitters, such as ATP, which stimulate postsynaptic P2X2/3 receptors on afferent nerve terminals. The afferent discharge is shaped by autocrine and paracrine mechanisms involving both excitatory and inhibitory neuromodulators such as adenosine, serotonin (5-HT), GABA and dopamine. Recent evidence suggests that paracrine activation of P2Y2 receptors on adjacent glia-like type II cells may help boost the ATP signal via the opening of pannexin-1 channels. The presence of an inhibitory efferent innervation, mediated by release of nitric oxide, provides additional control of the afferent discharge. The broad array of neuromodulators and their receptors appears to endow the carotid body with a remarkable plasticity, most apparent during natural and pathophysiological conditions associated with chronic sustained and intermittent hypoxia.
Introduction
The maintenance of homeostasis in the respiratory and cardiovascular systems of mammals depends on signal processing within receptor organs located in the periphery. The carotid bodies, and the less well-studied aortic bodies, represent a class of peripheral chemoreceptor organs involved in the sensing of chemicals in arterial blood such as low O2 (hypoxia), high CO2/H+ (acid hypercapnia) and low glucose (hypoglycaemia). The location of the bilaterally paired carotid bodies at the bifurcation of the common carotid arteries is strategic, as the blood composition is sampled just before it reaches the brain, an organ that is very sensitive to oxygen and glucose deprivation. Thus, in response to hypoxaemia, chemoreceptor cells in the carotid body (CB) depolarize and release neurotransmitters that excite afferent terminals of the carotid sinus nerve. Afferent signals are then relayed to the central pattern generator in the brainstem, leading to reflex hyperventilation and restoration of blood O2 homeostasis (Gonzalez et al. 1994). While central chemoreceptors play a key role in sensing elevated CO2, the relative impermeability of the blood–brain barrier to protons means that pH sensing during metabolic acidosis is dependent mainly on peripheral CB chemoreceptors. The CB is richly vascularized and reputed to have the highest blood flow per unit weight of any tissue in the body (McDonald, 1981).
The main cellular components of the CB are the chemoreceptor cells, also known as glomus or type I cells, and ensheathing glial-like type II cells in a ratio of approximately 4:1 (McDonald, 1981). Type I cells communicate by both chemical and electrical synapses, and are innervated by afferent nerve terminals whose cell bodies reside in the petrosal ganglia. There is morphological evidence for reciprocal chemical synapses (McDonald, 1981), and functional evidence for electrical coupling (Eyzaguirre, 2007), between type I cells and afferent terminals as well as between neighbouring type I cells. In addition, the CB receives an efferent inhibitory innervation via the carotid sinus nerve. A schematic representation of the cellular organization and innervation of the carotid body chemoreceptor complex is shown in Fig.1. The type I cells synthesize a variety of neurotransmitters and neuromodulators, both excitatory and inhibitory, and express a range of corresponding ionotropic and metabotropic receptors. This organization suggests the presence of widespread autocrine and paracrine mechanisms that may help fine tune the afferent discharge during chemoexcitation. There is now compelling evidence that the type I cells are the actual transducers of CB chemostimuli. These cells depolarize in response to hypoxia and acid hypercapnia, primarily as a result of inhibition of K+ channels. The reader is referred to other excellent reviews for a discussion of the transduction mechanisms in type I cells, as this continues to be an active area of investigation (Buckler, 2007; Peers et al. 2010; Kumar & Prabhakar, 2012). In this mini-review I will highlight some of the neurotransmitter and autocrine/paracrine mechanisms involved in signalling processing in the CB, as well as recent data suggesting that type II cells may participate in these events via ‘gliotransmission’ mediated by pannexin 1 channels. Although the focus here is on small molecules, various neuropeptides are known to play important modulatory roles in CB function, as described elsewhere (Prabhakar, 2006; Kumar & Prabhakar, 2012).
Figure 1. Cellular organization and innervation of the rat carotid body.
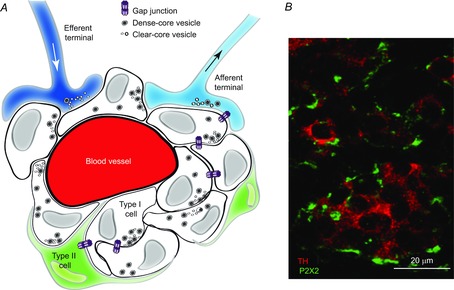
A, schematic illustration of type I cell clusters in intimate association with glial-like type II cells. Type I cells contain dense-core granules and receive afferent innervation from the petrosal ganglia; inhibitory efferent terminals from autonomic neurones terminate near type I cells. Adapted from Piskuric and Nurse (2013). B, section of rat carotid body showing clusters of type I cells immunostained for tyrosine hydroxylase (red); type I cells are innervated by afferent terminals, immunostained for purinergic P2X2 subunit (green).
A brief historical perspective on signal processing in the CB
The first description of the CB appeared around the mid 18th century and is attributable to the studies of von Haller (reviewed by Kumar & Prabhakar, 2012). However, it was the seminal histological studies of De Castro in the 1920s that led to the idea that the CB was a sensory organ, designed to detect chemicals in arterial blood. Subsequently, the physiological role of the CB in controlling ventilation in response to changes in blood levels of O2, CO2 and H+ ions was demonstrated in the laboratory of Corneille Heymans, who later received the Nobel Prize in Physiology and Medicine (Heymans et al. 1930). Early studies on signalling mechanisms focused on acetylcholine (ACh) as a major excitatory neurotransmitter in the CB (Hollinshead & Sawyer, 1945). However, this view was later challenged in the mid 1950s (Douglas, 1954), only to be resurrected in more recent times (Eyzaguirre & Zapata, 1984; Gonzalez et al. 1994; Nurse & Zhang, 1999; Fitzgerald, 2000; Nurse, 2010). Other transmitter candidates including dopamine and substance P were also considered as excitatory mediators in the CB (Gonzalez et al. 1994; Iturriaga & Alcayaga, 2004; Prabhakar, 2006). Many studies, based on extracellular nerve recording during exogenous application of transmitter candidates to the whole CB, identified several agents, including ACh, that were capable of exciting the afferent carotid sinus nerve (Gonzalez et al. 1994; Iturriaga & Alcayaga, 2004). Indeed ATP, now considered a key CB excitatory neurotransmitter (Nurse, 2010; Piskuric & Nurse, 2013), has been known for some time to cause an increase sinus nerve discharge during intra-carotid injections (Jarish et al. 1952). Additionally, exogenous administration of the ATP breakdown product, adenosine, increased ventilation in vivo, as well the afferent sensory discharge in vivo and in vitro (reviewed by Conde et al. 2009). Other small molecules, including dopamine, serotonin (5-HT) and histamine, may act as direct chemoexcitants, or modulators of chemosensory activity (Gonzalez et al. 1994; Iturriaga & Alcayaga, 2004; Jacono et al. 2005; Kumar & Prabhakar, 2012; Nurse & Piskuric, 2013), and in many cases, receptors for these ligands have been localized on chemoreceptor cells and/or afferent nerve terminals. However, dopamine still remains the best studied CB neurotransmitter and there is abundant evidence that it is released from type I cells during chemoexcitation (Gonzalez et al. 1994; Lopez-Barneo, 2003). However, with the exception of in the rabbit, its role appears to be that of an inhibitory neuromodulator in most species, as described in detail elsewhere (Gonzalez et al. 1994; Iturriaga & Alcayaga, 2004; Iturriaga et al. 2009; Nurse, 2010; Kumar & Prabhakar 2012).
Synaptic transmission between chemoreceptors and afferent nerve terminals
To identity the main excitatory neurotransmitters that cause the increase in afferent discharge during chemoexcitation, several laboratories have relied on the isolated CB–sinus nerve preparation in vitro (Gonzalez et al. 1994; Iturriaga & Alcayaga; 2004; Kumar & Bin-Jaliah, 2007; Donnelly, 2009; Kumar and Prabhakar, 2012). CB output as reflected by sinus nerve discharge frequency is directly recorded in these preparations, which have the advantage that secondary cardiovascular variables are eliminated. The development of a functional co-culture model consisting of isolated rat CB chemoreceptor cell clusters and juxtaposed petrosal neurones has contributed significantly to our understanding of synaptic mechanisms (Nurse & Zhang, 1999; Nurse, 2010). More recently, this model has been used to record simultaneously responses in both chemoreceptor type I cell(s) and the adjacent postsynaptic neurone using double patch-clamp recording or ratiometric Ca2+ imaging (Zhang et al. 2009; Nurse & Piskuric, 2013). In this co-culture model, ATP, acting via postsynaptic P2X2/3 receptors, was identified as a key excitatory neurotransmitter during hypoxia and hypercapnia (Zhang et al. 2000; Zhang & Nurse, 2004). In concert with these findings, P2X2 receptor subunit expression has been detected by immunochistochemistry on afferent nerve terminals apposed to tyrosine hydroxylase-positive type I cells in the rat carotid body in situ (Fig.1B). A critical role of P2X2 receptor subunits in the sinus nerve chemosensory discharge, and in the whole animal hypoxic ventilatory response, was validated in a transgenic mouse model deficient in the P2X2 subunit (Rong et al. 2003). An excitatory role of co-released ACh was also identified in the co-culture model, although controversy still surrounds its importance in sensory transmission (Fitzgerald, 2000; Donnelly, 2009; Nurse, 2010; Kumar & Prabhakar, 2012; Piskuric & Nurse, 2013). The co-culture model also provided evidence that GABA, released from chemoreceptor cells, may act postsynaptically on ligand-gated GABAA receptors to inhibit the sensory discharge by a shunting mechanism (Zhang et al. 2009).
Paracrine and autocrine mechanisms in the CB: interactions among type I and type II cells
Several endogenous neuromodulators, acting via G-protein coupled receptors, appear to play important roles in regulating CB output via paracrine and autocrine mechanisms. Some of them play predominantly inhibitory roles such as dopamine acting via D2 receptors (Iturriaga et al. 2009), and GABA acting via GABAB receptors (Fearon et al. 2003), on type I cells. Here, I briefly consider the roles of predominantly excitatory neuromodulators, i.e. adenosine, 5-HT and ATP, acting at type I or type II cells.
Adenosine
Adenosine (Ado), generated via the breakdown of extracellular ATP by ecto-5′-nucleotidase, or released from type I cells via an equilibrative nucleoside transporter, appears to play important autocrine/paracrine roles during acute hypoxia (Conde et al. 2012). There is evidence that Ado may act on high affinity A2a receptors present on type I cells to enhance type I cell depolarization via protein kinase A-dependent inhibition of TASK-like background K+ channels (Tse et al. 2012). Also, selective blockers of A2a receptors appear to inhibit the hypoxia-induced receptor potential in type I cells at nanomolar concentrations (Nurse & Piskuric, 2013). Low affinity A2b receptors are also present on type I cells, allowing for further modulation of their secretory functions (Conde et al. 2009, 2012; Livermore & Nurse, 2013). Indeed, exposure to chronic hypoxia appears to alter Ado signalling in the CB via mechanisms that probably involve regulation of presynaptic A2 receptors in type I cells. For example, it was recently shown that exposure of rat CB cultures to chronic hypoxia (2% O2; 24 h) led to a marked increase in Ado-evoked intracellular Ca2+ transients and catecholamine secretion in type I cells, mediated via A2b receptors (Livermore & Nurse, 2013). This pathway could potentially play a role during ventilatory acclimatization to hypoxia, as occurs during sojourns to high altitude, when CB sensitivity to acute hypoxia is augmented (Conde et al. 2009; Teppema & Dahan, 2010). Ado may also act postsynaptically via A2a receptors to enhance the firing pattern in the afferent nerve (Conde et al. 2009, 2012; Piskuric & Nurse, 2013).
Serotonin (5-HT)
Using immunocytochemistry, 5-HT and 5-HT2a receptors have been localized to type I cells in tissue sections of the rodent CB (Zhang et al. 2003). In recent studies, the 5-HT biosynthetic enzyme tryptophan hydroxylase and the serotonin transporter have also been localized to type I cells in situ (Yokoyama et al. 2013). Moreover, detection of 5-HT release from whole rodent CB by high-performance liquid chromatography has been observed under certain conditions (Jacono et al. 2005; Peng et al. 2009). In the co-culture model of the CB, the hypoxia-induced receptor potential in type I cells was reversibly inhibited by 5-HT2a receptor blockers, consistent with a paracrine positive feedback role for endogenously released 5-HT (Zhang et al. 2003). In some type I cells, exogenous 5-HT induced membrane depolarization via a protein kinase C (PKC)-dependent inhibition of a resting K+ conductance. 5-HT also appears to play an important modulatory role in CB plasticity. For example, exposure of rats to chronic intermittent hypoxia leads to sensory long-term facilitation where the CB sinus nerve discharge in response to acute hypoxia is markedly potentiated (Peng et al. 2009). These authors showed the mechanism involved 5-HT release and activation of 5HT2a receptors coupled to PKC. Taken together, these studies support a neuromodulatory role for 5-HT via paracrine interactions that facilitate the chemoexcitatory response of the type I cell.
ATP
In addition to its major role as a fast-acting excitatory neurotransmitter in the CB, there is increasing evidence that ATP may have paracrine functions involving glial-like type II cells (Tse et al. 2012; Piskuric & Nurse, 2013; Piskuric & Nurse, 2012). Earlier studies showed that ATP, acting via G-protein coupled P2Y2 receptors, caused a rise in intracellular Ca2+ in isolated rat type II cells (Xu et al. 2003; Tse et al. 2012). More recently, activation of these P2Y2 receptors was shown to lead not only to a rise in intracellular Ca2+ but also to the opening of large-pore, pannexin-1 channels in type II cells (Piskuric & Nurse, 2012; Zhang et al. 2012), as illustrated in Fig.2. In fact, these open pannexin-1 channels acted as conduits for the further release of ATP, and could be reversibly blocked by the selective blocker carbenoxolone (5 μm; Fig.2). This ‘ATP-induced ATP release’ appears to be a potential paracrine mechanism for boosting the excitatory ATP signal at the chemosensory synapse. Preliminary evidence suggests that other CB neuromodulators can also regulate intracellular Ca2+ levels in type II cells (Tse et al. 2012), as well as the opening of pannexin-1 channels (S. Murali, M. Zhang and C. A. Nurse, unpublished observations). ATP itself may also help control its own extracellular levels within the sensory synapse. For example, at high concentrations ATP can act in a negative feedback manner to inhibit pannexin-1 channels in type II cells (Nurse & Piskuric, 2013), and to inhibit the hypoxia-induced receptor potential in type I cells via P2Y1 receptors (Tse et al. 2012). In another chemosensory organ, i.e. the taste bud, high extracellular levels of the excitatory neurotransmitter ATP can lead to severely diminished taste responses, attributable to desensitization of the rapidly adapting P2X3-containing receptors on the afferent nerve (Kinnamon & Finger, 2013). Similarly, in the CB, the necessity to prevent the accumulation of high levels of ATP via these (and other, see below) negative feedback inhibitory pathways may have evolved in part to limit desensitization of P2X2–P2X3 heteromeric receptors on petrosal chemoafferent terminals (Prasad et al. 2001).
Figure 2. Effects of P2 receptor agonists on type I and type II cells.
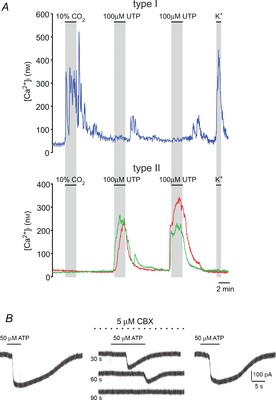
A, intracellular calcium transients elicited by different stimuli in type I versus type II cells. In the upper trace, a type I cell responds with an increase in [Ca2+]i when exposed to hypercapnia (10% CO2) and high K+, but not to UTP or ATP; delayed off responses of unknown origin were sometimes seen on removal of UTP and ATP. In lower traces, two different type II cells failed to respond to hypercapnia and high K+, but elicited robust increases in [Ca2+]i when exposed to UTP and ATP, consistent with activation of P2Y2 receptors. B, in a type II cell, ATP activated an inward current that was reversibly inhibited by 5 μm carbenoxolone (CBX; middle traces at time intervals indicated), a selective blocker of pannexin-1 channels; holding potential was –60 mV. Data for both A and B were reproduced from Zhang et al. (2012).
Role of nitric oxide in efferent inhibition
Evidence for an inhibitory efferent innervation of the CB via autonomic (parasympathetic) neurones has been reviewed elsewhere (Prabhakar, 1999; Campanucci & Nurse, 2007; Moya et al. 2012). These efferent neurones are immunopositive for vesicular acetylcholine transporter (VAChT) and neuronal nitric oxide synthase (nNOS) and are distributed in small groups along the glossopharyngeal and carotid sinus nerves. Inhibition of CB function during hypoxia is mediated by nitric oxide (NO) released from these efferent fibres situated near chemoreceptor type I cells (Fung et al. 2001). The nNOS-positive efferent neurones express a variety of P2X purinergic and nicotinic ACh receptors (Campanucci et al. 2006, 2012; Campanucci & Nurse, 2007). Stimulation of these receptors with ATP and ACh leads to an increase in intracellular Ca2+, activation of nNOS and synthesis of NO that can be detected by diaminofluorescein (DAF) fluorescence (Campanucci et al. 2012; Lowe et al. 2013). Moreover, in co-cultures containing CB type 1 cell clusters and juxtaposed efferent neurones, application of ATP led to type I cell hyperpolarization that could be prevented by the NO scavenger carboxy PTIO (Campanucci et al. 2006). These data suggest that release of the excitatory neurotransmitter ATP from chemoreceptor type I cells during hypoxia may have an additional, paracrine, negative feedback role, i.e. the trigger for NO synthesis and release from efferent fibres, leading to inhibition of the receptor potential in type I cells. Given that nicotinic ACh receptor stimulation can also lead to NO synthesis in efferent neurones (Campanucci et al. 2012), it is plausible that local release of ACh from type I cells, or from VAChT-positive efferent neurones themselves, could lead to a similar NO-mediated chemoreceptor inhibition.
Summary and concluding remarks
This short review has highlighted several features of information processing in the chemosensory CB with a focus on only few selected ligands. The presence within this tiny organ of a plethora of neurotransmitters and neuromodulators, as well as an even broader spectrum of their receptors, emphasizes the complexity of information processing. Nevertheless, compared to the challenges posed in understanding comparable mechanisms in the brain, the CB offers an attractive alternative. Indeed, it provides a unique opportunity for studying fast chemical synaptic transmission, autocrine–paracrine signalling, electrical coupling, efferent inhibition and even ‘gliotransmission’ within a fairly localized region (Figs.1, 3). Moreover, the sensitivity of the CB to chemostimuli can be modified by different patterns of environmental challenges (e.g. hypoxia), providing an opportunity for studies on synaptic plasticity (Kumar & Prabhakar, 2012; Teppema & Dahan, 2010). Understanding these mechanisms can have benefits to human health. For example, enhanced CB activity occurs during exposure to chronic intermittent hypoxia (e.g. sleep apnoea), thereby increasing the risk for hypertension (Peng et al. 2009; Kumar & Prabhakar 2012). Furthermore, increased CB activity and disordered breathing patterns occur in congestive heart failure, serving only to exacerbate the condition and increase the risk of mortality (Schultz et al. 2013).
Figure 3. Proposed model for purinergic paracrine signalling in the rat carotid body.
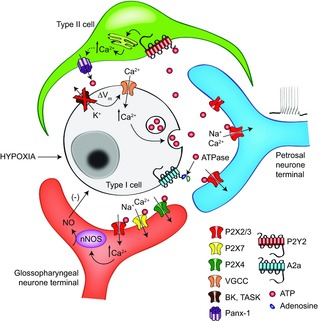
Type I cells release the excitatory neurotransmitter ATP following hypoxia-induced membrane depolarization. ATP excites afferent terminals via postsynaptic P2X2/3 receptors. ATP may also activate P2Y2 receptors on neighbouring type II cells, leading to a rise in intracellular Ca2+ and opening of pannexin-1 channels. When open, pannexin-1 channels cause further release of ATP, thereby boosting the excitatory signal. The inhibitory efferent pathway may also be activated by ATP acting on a variety of P2X receptors on nNOS-positive, autonomic terminals; stimulation of these P2X receptors leads to synthesis and release of NO, which in turn hyperpolarizes type I cells. Breakdown of extracellular ATP by ecto-5′-nucleotidase generates adenosine, which may also be transported directly from type I cells. Adenosine can further enhance excitatory pathways in the CB via presynaptic A2a and A2b receptors on type I cells, and postsynaptic A2a receptors on afferent terminals. Some of these receptors, as well as other paracrine pathways, have been omitted for clarity. Adapted from Piskuric & Nurse (2013).
Acknowledgments
I am indebted to several colleagues and collaborators for their contributions to some of the studies mentioned in this review, especially Min Zhang, Cathy Vollmer, Nikol Piskuric and Veronica Campanucci. I thank Sindy Murali, Nikol Piskuric and Cathy Vollmer for help with preparing the figures.
Glossary
- ACh
acetylcholine
- Ado
adenosine
- NO
nitric oxide
- nNOS
nitric oxide synthase
- PKC
protein kinase C
- VAChT
vesicular acetylcholine transporter
Biography
Colin A. Nurse obtained his PhD degree in Neurobiology from Harvard University in 1977. He has since held academic positions at McMaster University, where he is currently Professor of Biology since 1994. His research interests are in the mechanisms of sensory processing at peripheral arterial chemoreceptors, and on the developmental regulation of O2 and CO2 chemosensitivity in adrenal chromaffin cells.
Additional information
Competing interests
The author declares no conflict of interest.
Funding
Work from my laboratory was supported by operating grants from the Canadian Institutes of Health Research (MOP 12037 & MOP 57909).
References
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Dookhoo L, Vollmer C. Nurse CA. Modulation of the carotid body sensory discharge by NO: an up-dated hypothesis. Respir Physiol Neurobiol. 2012;184:149–157. doi: 10.1016/j.resp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Campanucci VA. Nurse CA. Autonomic innervation of the carotid body: role in efferent inhibition. Respir Physiol Neurobiol. 2007;157:83–92. doi: 10.1016/j.resp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Zhang M, Vollmer C. Nurse CA. Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition. J Neurosci. 2006;26:9482–9493. doi: 10.1523/JNEUROSCI.1672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC, Obeso A. Gonzalez C. Adenosine in peripheral chemoreception: new insights into a historically overlooked molecule – invited article. Adv Exp Med Biol. 2009;648:145–159. doi: 10.1007/978-90-481-2259-2_17. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC, Rigual R, Obeso A. Gonzalez C. Hypoxic intensity: a determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J Appl Physiol. 2012;112:2002–2010. doi: 10.1152/japplphysiol.01617.2011. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. Nicotinic acetylcholine receptors do not mediate excitatory transmission in young rat carotid body. J Appl Physiol. 2009;107:1806–1816. doi: 10.1152/japplphysiol.00135.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW. Is there chemical transmission at chemoreceptors? Pharmacol Rev. 1954;6:81–83. [PubMed] [Google Scholar]
- Eyzaguirre C. Electrical synapses in the carotid body-nerve complex. Respir Physiol Neurobiol. 2007;157:116–122. doi: 10.1016/j.resp.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C. Zapata P. Perspectives in carotid body research. J Appl Physiol. 1984;57:931–957. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- Fearon IM, Zhang M, Vollmer C. Nurse CA. GABA mediates autoreeptor feedback inhibition in the rat carotid body via presynaptic GABAB receptors and TASK-1. J Physiol. 2003;553:83–94. doi: 10.1113/jphysiol.2003.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RS. Oxygen and carotid body chemotransduction: the cholinergic hypothesis – a brief history and new evaluation. Respir Physiol. 2000;120:89–104. doi: 10.1016/s0034-5687(00)00091-8. [DOI] [PubMed] [Google Scholar]
- Fung ML, Ye JS. Fung PC. Acute hypoxia elevates nitric oxide generation in rat carotid body in vitro. Pflugers Arch. 2001;442:903–909. doi: 10.1007/s004240100610. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A. Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Heymans C, Bouckhaert JJ. Dautrebande L. Sinus carotidien et reflexes respiratoires II. Influences respiratoires reflexes de l'acidose, de l'alcalose, de l'anhydride carbonique, de l'ion hydrogene et de l'anoxeme. Sinus carotidiens et echanges respiratores dans les poumons et au dela des poumons. Arch Intern Pharmacodyn. 1930;39:400–448. [Google Scholar]
- Hollinshead WH. Sawyer CH. Mechanisms of carotid body stimulation. Am J Physiol. 1945;122:79–86. [Google Scholar]
- Iturriaga R. Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Brain Res Rev. 2004;47:46–53. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J. Gonzalez C. Neurotransmitters in carotid body function: the case of dopamine – invited article. Adv Exp Med Biol. 2009;648:137–143. doi: 10.1007/978-90-481-2259-2_16. [DOI] [PubMed] [Google Scholar]
- Jacono FJ, Peng YJ, Kumar GK. Prabhakar NR. Modulation of the hypoxic sensory response of the carotid body by 5-hydroxytryptamine: role of the 5-HT2 receptor. Respir Physiol Neurobiol. 2005;145:135–142. doi: 10.1016/j.resp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Jarisch A, Landgren S, Neil E. Zotterman Y. Impulse activity in the carotid sinus nerve following intra-carotid injection of potassium chloride, veratrine, sodium citrate, adenosine-triphosphate and α-dinitrophenol. Acta Physiol Scand. 1952;25:195–211. doi: 10.1111/j.1748-1716.1952.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC. Finger T. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 2013;7:264. doi: 10.3389/fncel.2013.00264. (1–7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. Bin-Jaliah I. Adequate stimuli of the carotid body: more than an oxygen sensor? Respir Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Kumar P. Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore S. Nurse CA. Enhanced adenosine A2b receptor signalling facilitates stimulus-induced catecholamine secretion in chronically hypoxic carotid body type I cells. Am J Physiol Cell Physiol. 2013;305:C739–750. doi: 10.1152/ajpcell.00137.2013. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J. Oxygen and glucose sensing by carotid body glomus cells. Curr Opin Neurobiol. 2003;13:493–499. doi: 10.1016/s0959-4388(03)00093-x. [DOI] [PubMed] [Google Scholar]
- Lowe M, Park SJ, Nurse CA. Campanucci VA. Purinergic stimulation of carotid body efferent glossopharyngeal neurones increases intracellular Ca2+ and nitric oxide production. Exp Physiol. 2013;98:1199–1212. doi: 10.1113/expphysiol.2013.072058. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Peripheral chemoreceptors: structure–function relationships of the carotid body. In: Hornbein TF, editor. Regulation of Breathing. Lung Biol. Health Dis. New York: Marcel Dekker; 1981. pp. 105–320. Vol. 17. [Google Scholar]
- Moya EA, Alcayaga J. Ituriaga R. NO modulation of carotid body chemoreception in health and disease. Respir Physiol Neurobiol. 2012;184:158–164. doi: 10.1016/j.resp.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Nurse CA. Zhang M. Acetylcholine contributes to hypoxic chemotransmission in co-cultures of rat type I cells and petrosal neurons. Respir Physiol. 1999;115:189–199. doi: 10.1016/s0034-5687(99)00017-1. [DOI] [PubMed] [Google Scholar]
- Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol. 2010;95:657–667. doi: 10.1113/expphysiol.2009.049312. [DOI] [PubMed] [Google Scholar]
- Nurse CA. Piskuric NA. Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol. 2013;24:22–30. doi: 10.1016/j.semcdb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN. Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK. Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskuric NA. Nurse CA. Effects of chemostimuli on [Ca2+]i responses of rat aortic body type I cells and endogenous local neurons: comparison with carotid body cells. J Physiol. 2012;590:2121–2135. doi: 10.1113/jphysiol.2012.229468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskuric NA. Nurse CA. Expanding role of ATP as a versatile messenger at carotid and aortic body chemoreceptors. J Physiol. 2013;591:415–422. doi: 10.1113/jphysiol.2012.234377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respir Physiol. 1999;115:161–168. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C. Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol. 2001;537:667–677. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM. Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ. Del Rio R. Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep. 2013;15:356–362. doi: 10.1007/s11906-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ. Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;60:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Tse A, Yan L, Lee AK. Tse FW. Autocrine and paracrine actions of ATP in rat carotid body. Can J Physiol Pharmacol. 2012;90:705–711. doi: 10.1139/y2012-054. [DOI] [PubMed] [Google Scholar]
- Xu J, Tse FW. Tse A. ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J Physiol. 2003;549:739–747. doi: 10.1113/jphysiol.2003.039735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Misuzu YY. Yamamoto Y. Immunohistochemical localization of tryptophan hydroxylase and serotonin transporter in the carotid body of the rat. Histochem Cell Biol. 2013;140:147–155. doi: 10.1007/s00418-012-1066-5. [DOI] [PubMed] [Google Scholar]
- Zhang M, Clarke K, Zhong H, Vollmer C. Nurse CA. Postsynaptic action of GABA in modulating sensory transmission in co-cultures of rat carotid body via GABAA receptors. J Physiol. 2009;587:329–344. doi: 10.1113/jphysiol.2008.165035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fearon IM, Zhong H. Nurse CA. Presynaptic modulation of rat arterial chemoreceptor function by 5-HT: role of K+ channel inhibition via protein kinase C. J Physiol. 2003;551:825–842. doi: 10.1113/jphysiol.2002.038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. Nurse CA. CO2/pH chemosensory signalling in co-cultures of rat carotid body receptors and petrosal neurons: role of ATP and ACh. J Neurophysiol. 2004;92:3433–3445. doi: 10.1152/jn.01099.2003. [DOI] [PubMed] [Google Scholar]
- Zhang M, Piskuric NA, Vollmer C. Nurse CA. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. J Physiol. 2012;590:4335–4350. doi: 10.1113/jphysiol.2012.236265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C. Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


