Abstract
Anxiety and arousal have been shown to facilitate human vestibulo-ocular reflexes, presumably through direct neural connections between the vestibular nuclei and emotional processing areas of the brain. However, the effects of anxiety, fear and arousal on balance-relevant vestibular reflexes are currently unknown. The purpose of this study was to manipulate standing height to determine whether anxiety and fear can modulate the direct relationship between vestibular signals and balance reflexes during stance. Stochastic vestibular stimulation (SVS; 2–25 Hz) was used to evoke ground reaction forces (GRF) while subjects stood in both LOW and HIGH surface height conditions. Two separate experiments were conducted to investigate the SVS–GRF relationship, in terms of coupling (coherence and cumulant density) and gain, in the medio-lateral (ML) and antero-posterior (AP) directions. The short- and medium-latency cumulant density peaks were both significantly increased in the ML and AP directions when standing in HIGH, compared to LOW, conditions. Likewise, coherence was statistically greater between 4.3 Hz and 6.7 Hz in the ML, and between 5.5 and 17.7 Hz in the AP direction. When standing in the HIGH condition, the gain of the SVS–GRF relationship was increased 81% in the ML direction, and 231% in the AP direction. The significant increases in coupling and gain observed in both experiments demonstrate that vestibular-evoked balance responses are augmented in states of height-induced postural threat. These data support the possibility that fear or anxiety-mediated changes to balance control are affected by altered central processing of vestibular information.
Introduction
Converging evidence suggests that the vestibular system can be influenced by emotional factors such as fear and anxiety, or general autonomic arousal. Animal models have revealed excitatory reciprocal projections between the brainstem vestibular nuclei, vestibular cortex and various neural centres involved with autonomic function and emotion, including the parabrachial nucleus, the dorsal raphe nucleus and locus coeruleus (Balaban & Thayer, 2001; Balaban, 2002; Staab et al. 2013). These excitatory networks have been proposed to be principally activated in states of fear or vigilance in order to alter vestibular-evoked motor responses to self-motion, and to increase sensitivity to imposed motion (Balaban, 2002). This hypothesis is supported by several behavioural studies which demonstrated increased vestibulo-ocular reflex (VOR) gain with arousal in both cats (Crampton & Schwam, 1961; Crampton, 1961) and primates (Furman et al. 1981).
Human studies probing the effects of anxiety or arousal on vestibular reflex function normally focus on the VOR (Collins & Guedry, 1962; Collins & Poe, 1962; Kasper et al. 1992; Yardley et al. 1995). Collins & Guedry (1962) revealed that a minimum level of ‘alertness’ is required to evoke a stable nystagmus to a prolonged rotary acceleration. Similarly, drowsy subjects are less likely to produce VOR responses than awake ones (Kasper et al. 1992). Alerting tasks, such as mental arithmetic, are also more effective at increasing VOR gain than arousal-inducing drugs (amphetamine: Collins & Poe, 1962) or exercise (Yardley et al. 1995). As such, Yardley et al. (1995) have concluded that VOR gain changes are not mediated by generalized autonomic arousal, but rather are specifically related to anxiety. Fear and anxiety are also known to affect balance control in both humans and animals (Kalueff et al. 2008). Mice that are genetically predisposed to anxiety tend to perform poorer on balance and exploration tasks (Lepicard et al. 2000). Balance performance in these mice can be ameliorated with anxiolytic treatments (Venault et al. 2001; Lepicard et al. 2003) or worsened with anxiogenic treatment (Lepicard et al. 2000, 2003). Similarly, human studies have shown that fear or anxiety from a potential fall from an elevated support surface (a height-induced postural threat) leads to significant changes to balance control in static (Carpenter et al. 1999, 2001b), dynamic (Brown & Frank, 1997; Carpenter et al. 2004), and locomotor tasks (Brown et al. 2002; Tersteeg et al. 2012). Since the vestibular system is a significant contributor to standing balance (Fitzpatrick & Day, 2004), altered vestibulospinal function has been proposed as a mediator of postural threat-related changes to balance control (Carpenter et al. 1999, 2004). While the effects of anxiety and arousal on VOR and general balance control are in line with predictions from anatomical models, the effects of fear, anxiety and arousal on vestibular-evoked balance reflexes have yet to be examined directly.
Stochastic vestibular stimulation (SVS) has been proposed as a useful means of probing the human vestibular system during balance (Dakin et al. 2007, 2010, 2011; Mian & Day, 2009; Luu et al. 2012) and locomotor tasks (Blouin et al. 2011; Dakin et al. 2013). SVS evokes ground reaction forces (GRFs, Mian et al. 2010), as well as triceps surae EMG (Dakin et al. 2007), that are comparable to those evoked with discrete galvanic vestibular stimulation (GVS) pulses without inducing prominent whole-body displacements (Dakin et al. 2010) or requiring long stimulation times (Dakin et al. 2007). Certain aspects of the SVS frequency spectrum have been associated with different response features. For example, SVS and body sway are primarily correlated below 2 Hz. As such, the SVS signal can be tailored to produce very little correlated body sway, and yet still produce correlated GRF and EMG responses by omitting frequencies below 2 Hz (Dakin et al. 2010). Limiting whole-body displacements in a height-induced postural threat scenario is important, as balance responses to whole-body perturbations are known to be altered in these situations (Brown & Frank, 1997; Carpenter et al. 2004).
Here, we present two studies that examined the influence of a height-induced postural threat on the magnitude of vestibular-induced balance responses. While previous work has revealed that long-latency stabilizing responses to GVS are affected by a height-induced postural threat (Osler et al. 2013), the purpose of the present studies was to determine whether or not a postural threat modulates the direct relationship between vestibular signals and balance reflexes. Based on the assumption that the vestibular nuclei receive excitatory inputs from emotional processing areas of the brain (Balaban & Thayer, 2001; Balaban, 2002), we hypothesized that threat-induced changes in the relationship between vestibular inputs and balance control would be reflected by an increase in the coupling between SVS and ground reaction forces and an increase in the gain of the evoked responses.
Methods
Study 1
Subjects and ethical approval
Twenty subjects were randomly divided into two experimental groups of 10 subjects each (Head Forward: 3 females; mean (SEM) age 25.2 (1.6) years; Head Turned: 5 females, 22.5 (0.82) years). No subjects reported any known neurological, vestibular, or orthopaedic impairments that may have influenced their balance, or ability to complete the experimental tasks; also, people who self-reported an extreme fear of heights were excluded from the study before participation. All subjects gave written informed consent before their participation in the experiments, and all methods were reviewed and approved by the University of British Columbia Clinical Research Ethics Board. The studies conformed to the standards set by the Declaration of Helsinki.
Stimulation protocol
Bipolar binaural SVS was delivered percutaneously above the mastoid processes to stimulate the nearby vestibular afferents. A unique stimulation profile was generated for each trial using a custom LabVIEW code (National Instruments, Austin, TX, USA). To generate the stimulus, random white noise was digitally band-pass filtered around 2–25 Hz. The resulting 2–25 Hz stimulus had a nearly flat power spectrum. The digital signal was then converted to analog (NI PCI-MIO-16E-1 with NI BNC-2110, National Instruments) and fed to a stimulator (model 2200, A-M Systems, Sequim, WA, USA). The SVS stimulus peak amplitude was 4.5 mA with mean (SEM) root mean square amplitude of 1.1 (0.01) mA. Nominally, the electrodes were positioned such that positive current caused an anode right–cathode left configuration. In both experiments, subjects experienced two 5 min trials of continuous SVS stimulation, one in each threat condition.
Head orientation
Subjects tend to lean away from the edge at height. When subjects stand with their toes at the edge balance changes occur in the antero-posterior (AP) direction, with little to no effect in the medio-lateral (ML) direction (Carpenter et al. 1999, 2001b; Davis et al. 2009). However, a general excitation of the vestibular system, as hypothesized here, should not be limited to one anatomical plane. Therefore, in order to ensure that any observed changes in the relationship between the vestibular and balance control systems were related to general vestibular excitation and not an effect of edge avoidance, we conducted two separate experiments in Study 1, with two different head orientations. SVS responses (like GVS) are oriented in a craniocentric manner, such that the bipolar binaural stimulation evokes a signal of head rotation occurring around a vector directed posteriorly and pointing approximately 18 deg above Reid's plane (Fitzpatrick & Day, 2004). In all conditions subjects stood with their head level and fixated on a visual target for the duration of the trial (∼9 cm2 red square on a white wall, 3.7 m from the subject at eye level). In Study 1, experiment 1, subjects stood with their heads facing forward resulting in a ML perturbation of the body (Fig.1A), and in experiment 2, subjects’ heads were turned 90 deg to the right resulting in an AP perturbation of the body (Fig.1A; Lund & Broberg, 1983; Pavlik et al. 1999; Mian & Day, 2009). In the head turned experiment, the lift was repositioned in the room such that the subjects could use the same visual targets, yet also be positioned in the same spot on the lift. If the changes are to be attributed to a general vestibular excitation, we would expect to see changes in the vestibular–balance control relationship in both the head turned and head forward experiments.
Figure 1. Head orientation and height-induced threat.
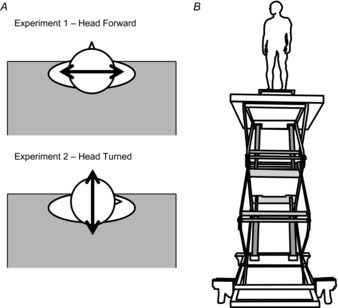
Participant head orientation with respect to the edge of the platform (A) and subject standing position at the edge in the HIGH threat condition in the head turned orientation (B).
Experimental protocol
In both experiments subjects stood with their toes at the edge of a force plate (no. K00407, Bertec, Columbus, OH, USA) positioned at the edge of a hydraulic lift (M419-207B10H01D, Pentalift, Guelph, ON, CAN). Their foot length was measured and their feet were positioned such that the lateral edges of their 5th metatarsals were spaced equal to their foot length. Subjects were exposed to two postural threat conditions: LOW and HIGH. The first SVS trial was always set in the low threat condition (LOW), where the lift rested in its lowest position (80 cm above ground) and a 60 cm wide table was placed in front of the subjects to provide an extended support surface. This is comparable to standing on the ground (Carpenter et al. 2001b). Subjects were then seated such that their feet stayed in the same place on the force plate, and the lift was elevated to 3.2 m (Fig.1B). They then stood up and completed a second trial in this high postural threat condition (HIGH). Participants wore a safety harness and were attached to a rope system at all times. The rope was kept loose enough to allow free movement of the subject on the platform, but would arrest a fall, if needed. There was also an experimenter within reach of the subject at all times; there were no rails bordering the lift platform to prevent a fall (similar to Davis et al. 2009). The LOW condition always preceded the HIGH condition as there is a known order effect of presentation (Adkin et al. 2000), and we desired maximum contrast between conditions.
Measures
Psychosocial measures of fear, anxiety, perceived stability and balance confidence, as well as autonomic arousal were measured. Prior to each trial, subjects evaluated their confidence in maintaining their balance for the stimulation period at that surface height (0: no confidence, to 100: completely confident). After each trial (while seated) subjects rated their experienced fear (0: not at all fearful, to 100: extremely afraid), perceived stability (0: not at all stable, to 100: completely stable), and anxiety (16 questions with a maximum score of 144, higher scores indicate higher anxiety). Each of these questionnaires has been demonstrated to have moderate to high reliability in this threat manipulation protocol (Hauck et al. 2008). Electrodermal activity (EDA) was also measured from the thenar and hypothenar eminences of the non-dominant hand (model 2501, Cambridge Electronic Design (CED), Cambridge, UK). EDA was sampled at 1000 Hz and averaged across the stimulation period to quantify autonomic arousal (Venables, 1991).
Shear GRFs across the force plate as well as the SVS drive signal were measured to represent the balance system output and vestibular input, respectively. GRFs were sampled at 100 Hz (Power 1401, CED) and converted to newtons offline using the plate calibration parameters and a custom MATLAB (MathWorks, Natick, MA, USA) script. GRFs were then low-pass filtered at 50 Hz and up-sampled to 5000 Hz. The SVS signal was concurrently sampled at 5000 Hz.
Calculations
The relationship between vestibular stimulation and the balance control system was examined in terms of coupling and gain. Signal coupling reflects the relationship between two signals and in the context of this study reflects the fidelity of transmission of vestibular signals to the balance control system. Signal coupling was examined using two separate, but congruent measures: cumulant density and coherence. Coherence is a measure of correlation across frequencies bounded between 0 and 1, and indicates where in the frequency spectrum signals are related. Cumulant density, in contrast, is an indicator of degree and direction of correlation between signals, as well as the relative lead or lag times between them. In keeping with previous studies (Mian & Day, 2009; Dakin et al. 2010), the cumulant density function was calculated such that a positive value indicates an association between a positive current (anode right–cathode left) and a rightward GRF (acting on the body) or a negative current (anode left–cathode right) and a leftward GRF in the head forward orientation; a positive value would indicate a positive current-forward or negative current-backward association in the head turned orientation. Gain, in turn, reflects the scale relationship between signals; here it reflects the amplitude of the balance response to a given vestibular input. Coherence, gain and cumulant density estimates between the SVS and GRFs were calculated using the NeuroSpec 2.0 code; a freely available archive of MATLAB code intended for statistical signal processing and based on the methods of Halliday et al. (1995). Specific details pertaining to each factor are outlined below.
Coherence and gain estimates were calculated from concatenated data across all subjects for a single condition; the data were not normalized between subjects prior to concatenation. There were 10 subjects in each experiment, with 300 s of data per condition, of which 299.8 s were used. As such, there was just less than 50 min of data for each condition in each experiment. The concatenated data were split into 1830 disjoint segments (183/subject) that were 1.6384 s long from which SVS–GRFs coherence was calculated, giving a frequency resolution of 0.61 Hz (see Dakin et al. 2010 for details). Within-conditions coherence was determined to be significantly greater than chance when it passed a 95% confidence limit based on the number of disjoint sections used in the analysis (Halliday et al. 1995). Differences in coherence between LOW and HIGH conditions were then assessed with the NeuroSpec 2.0 difference of coherence sub-routine, based on the methods of Rosenberg et al. (1989) and Amjad et al. (1997). This statistic tests the assumption that the coherence estimates are equal with normally distributed variance. The test compares standardized differences (HIGH–LOW) and 95% confidence limits based on the Fisher transform (tanh−1) of the square root of the coherence values, compared to a χ2(1) distribution (Rosenberg et al. 1989; Amjad et al. 1997); any frequencies where the standardized difference of coherence exceeded the 95% confidence limits were taken to be statistically different.
SVS–GRFs gain magnitude estimates and point-wise 95% confidence limits were also calculated from the concatenated data using the method described by Halliday et al. (1995). Point-wise 95% confidence limits were calculated about the gains, and any frequencies where the confidence limits did not overlap were assumed to be statistically different. Ratios between signal gains (HIGH:LOW) were calculated to determine the magnitude of the difference in gain estimates at each frequency where both LOW and HIGH trials had significant within-conditions coherence. Means and standard errors of these ratios were then calculated within these ranges from the grouped data.
Cumulant densities, in contrast to coherence and gain, were calculated on a subject-by-subject basis in both AP and ML directions using a modified version of the NeuroSpec 2.0 software that served to gain-normalize the cumulant density function to provide values of correlation bounded between −1 and +1 (see Dakin et al. 2010). As such, the cumulant density estimates, like coherence, reflected signal coupling, but the subject-by-subject analysis allowed us to ensure that our results were not skewed by outliers. Both coherence and cumulant density provide converging evidence to address our coupling hypothesis in these experiments. Short- (SLP) and medium-latency peaks (MLP) in the vestibular-induced GRFs were identified in plots of the cumulant density using a custom MATLAB script and were visually confirmed by an experimenter. Peak (or trough) amplitudes were recorded for each subject in the relevant direction of stimulation.
Statistical analyses
Normality was assessed with Kolmogorov–Smirnov tests for all variables; P < 0.05 was interpreted as a failure to meet the assumption of normality. SLP, MLP, EDA and ratings of anxiety all met assumptions of normality, and, therefore, paired-samples t tests were used to identify changes across conditions for these variables. Differences in ratings of fear of falling, balance confidence and stability were assessed with non-parametric Wilcoxon signed rank tests. The criterion for statistical significance was set to α = 0.05 for all tests and effect sizes are reported as eta squared (η2 = t2/(t2 + (n – 1)); parametric tests) or r (r = z/√N; non-parametric). As reported earlier, differences of coherence tests were used to identify changes in coherence estimates across threat conditions and confidence limits were used to examine differences in gain across threat conditions. In both cases 95% confidence limits were used as thresholds for statistical significance. As expected, there was no significant coherence between SVS and the GRF in the AP direction for the head forward experiment (orthogonal to stimulation) and only minimal coherence in the ML direction for the head turned experiment at either height; as such, effects across height conditions were not evaluated for these planes.
Study 2
One potential confound for height effects on vestibular function in Study 1 is the change in peripheral visual cues caused by changes to the distance to the floor and ceiling in raising the platform to the HIGH height. This may affect vestibular-evoked balance reflexes by altering visual–vestibular interactions (Britton et al. 1993; Day & Guerraz, 2007), which are known to be important for balance control (Vidal et al. 1982; Guerraz & Bronstein, 2008). While distance to foveal target was kept constant between height conditions, we recognize the potential change in peripheral cues in Study 1 as a potential confound. Therefore, a follow-up study was conducted to address this issue.
Five subjects (4 males; 27.2 (3.3) years) participated in this control study. The heights, stimulation parameters, measures and foveal targets were all the same as the head turned group in Study 1. In this study, however, subjects wore a set of goggles fitted with an upper visor and lower cardboard surface extending 19 cm forward (29.5 cm wide, and 5.5 cm separating upper and lower surfaces) that blocked the superior and inferior peripheral visual fields, to ensure the subjects could not see the floor or ceiling in either height condition. Lateral peripheral vision was also kept constant by hanging sheets from ceiling to floor on both sides of the subject (minimum 2.3 m from subject). All measures and calculations described in Study 1 were also used here. Due to the smaller sample, all within-subjects differences are described with descriptive statistics; statistical tests were used for concatenated data.
Results
Study 1
Psychosocial state and arousal
Standing at height was effective at inducing significant physiological and psycho-social changes in the participants of these experiments. Participants were more fearful and more anxious in the HIGH versus the LOW condition (Fear: Head Forward, z = −2.62, P = 0.009, r = 0.585; Head Turned, z = −2.81, P = 0.005, r = 0.628; Anxiety: Head Forward, t9 = −3.49, P = 0.007, η2 = 0.575; Head Turned, t9 = −4.54, P = 0.001, η2 = 0.696). Participants also felt less confident in their ability to maintain balance at height (Head Forward, z = −2.72, P = 0.007, r = 0.607; Head Turned, z = −2.41, P = 0.016, r = 0.538) and reported feeling less stable (Head Forward, z = −2.82, P = 0.005, r = 0.631; Head Turned, z = −2.83, P = 0.005, r = 0.633). EDA was significantly increased in both HIGH conditions, indicating that participants were also more aroused in the HIGH, compared to LOW, conditions (Head Forward, t8 = −4.81, P = 0.001, η2 = 0.743; Head Turned, t8 = −3.15, P = 0.014, η2 = 0.553).
SVS–GRF relationships
Standing at height modulated the relationship between vestibular inputs and balance control in both experiments. There were significant increases in the peak amplitudes of SVS–GRF cumulant density functions in the HIGH, compared to LOW, condition in both head forward and head turned experiments (Fig.2). There were significant peaks in the cumulant density at lag times of 136.2 ± 5.4 ms (SLP) and 292.9 ± 16.5 ms (MLP) in the LOW condition, and 129.5 ± 6.0 ms (SLP) and 300.3 ± 18.0 ms (MLP) in the HIGH condition with the head facing forward (Fig.2A). Short- and medium-latency peaks were 39.8 ± 9.5% and 53.7 ± 19.1% larger with height (SLP: t9 = 3.34, P = 0.009, η2 = 0.553; MLP: t9 = −2.59, P = 0.029, η2 = 0.427; Fig.2B). Similarly, when subjects stood with their heads turned peaks occurred at 150.9 ± 8.9 ms (SLP) and 296.4 ± 22.9 ms (MLP) in the LOW condition and at 122.5 ± 3.6 ms and 260.0 ± 20.9 ms in the HIGH condition (Fig.2C). In this experiment, the SLP amplitudes were 83.4 ± 23.6% larger and the MLP amplitudes were 96.6 ± 34.7% larger in the HIGH, compared to LOW, conditions (Fig.2D); these increases were also statistically significant (SLP: t9 = −3.38, P = 0.008, η2 = 0.559; MLP: t9 = 2.91, P = 0.017, η2 = 0.485).
Figure 2. Study 1 – Effects of height on SVS-GRF cumulant density.
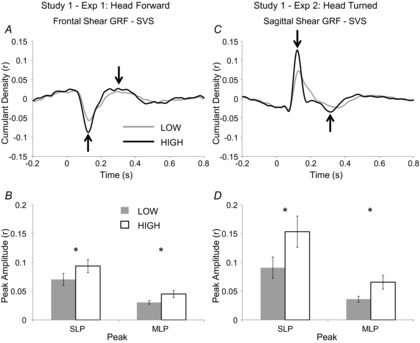
Mean LOW and HIGH cumulant density plots with peaks marked (arrows) for head forward (A) and head turned (C) experiments of Study 1. A positive deflection in the head forward cumulant density plot (A) means a positive current (anode right) is associated with a rightward GRF acting on the body or a negative current (anode left) causing a leftward GRF; a positive deflection in the head turned (C) trace indicates a positive current is associated with a forward GRF applied to the body. Mean SLP and MLP amplitudes in the head forward (B; n = 10) and head turned (D; n = 10) experiments; bars indicate SEM.
The coupling observed on a subject-by-subject basis was also demonstrated in the pooled coherence estimates. There was significant ML direction SVS–GRF within-conditions coherence when subjects stood with their heads forward in the LOW condition from 1.8 Hz to 11.0 Hz and in the HIGH condition from 1.8 Hz to 15.3 Hz (Fig.3A). SVS–GRF coherence was significantly greater at height than when participants were near the ground. This significant increase in SVS–GRF coherence at height was localized to between 4.3 Hz and 6.7 Hz, approximately (Fig.3B). Likewise, when subjects’ heads were turned, significant SVS–GRF coherence was observed in the AP direction between 1.8 Hz and 16.5 Hz in the LOW condition, and 1.8 Hz and 22.5 Hz in the HIGH condition (Fig.3D). There was also greater SVS–GRF coherence in the HIGH, compared to the LOW, condition between 5.5 Hz and 17.7 Hz (Fig.3E).
Figure 3. Study 1 – Effects of height on SVS-GRF coherence and gain.
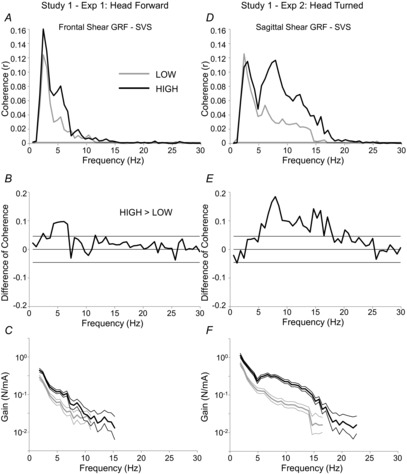
Pooled coherence plots for the LOW and HIGH conditions from the head forward (A) and head turned (D) experiments. Note: thin horizontal line above abscissa represents the threshold for significant within-conditions coherence for both LOW and HIGH conditions. Difference of coherence plotted (thick continuous line) for head forward (B) and head turned (E) experiments of Study 1. Thin horizontal lines represent the upper and lower 95% confidence limits for the test. Any points where the difference of coherence exceeds the 95% confidence limit are taken to be statistically different. Finally, LOW and HIGH gains (thick lines) of the pooled SVS–GRF data are plotted (on a log scale) for head forward (C) and head turned (F) experiments. Thin lines surrounding the gain traces represent point-wise 95% confidence limits; regions where the confidence limits do not overlap are taken to be statistically different.
Qualitatively, the signal gain estimates were similar in both experiments. The gains always peaked at the lowest frequency represented (1.8 Hz) and gradually decayed as frequency increased (Fig.3C and F). There were significant increases in signal gain estimates across threat conditions in both head forward and head turned experiments. In the head forward experiment signal gains significantly differed across threat conditions between 1.8 Hz and 8.5 Hz (Fig.3C; except 7.3 Hz). On average, the gain was 81 ± 12% larger in the HIGH, compared to the LOW, condition. This effect was more prominent in the head turned experiment, where signal gains were larger for all frequencies where the SVS and GRF signals cohered in both LOW and HIGH conditions (Fig.3F). Here, the HIGH signal gain was 231 ± 23% larger than the LOW gain.
Study 2
Subjects in Study 2, with peripheral vision kept constant, had similar arousal and psychosocial responses to height compared to those in the experiments of Study 1. EDA (45.7 ± 7.3%), fear of falling (30 ± 10.5%) and anxiety (80 ± 27%) were all larger in the HIGH condition compared to LOW. Likewise, balance confidence (24 ± 11.2%) and perceived stability (22.4 ± 9.5%) were both lower in the HIGH condition, compared to LOW.
SVS–GRF resultant data are plotted in Fig.4A. In line with Study 1, SLP amplitudes were 109 ± 59% larger in the HIGH, compared to LOW, condition. Likewise, MLP amplitudes increased 47 ± 29% in the HIGH condition, compared to LOW. The concatenated SVS and GRF data from the five subjects revealed significant within-conditions coherence between 1.8 Hz and 15.9 Hz in the LOW condition and between 1.8 Hz and 20.8 Hz in the HIGH condition. As shown in Fig.4A, the magnitude of coherence was generally greater in the HIGH, compared to the LOW, condition between 4.8 Hz and 20.8 Hz; the difference of coherence test revealed statistically greater coherence in the HIGH condition at most points in this range. Finally, the gain was significantly larger in the HIGH condition at all frequencies where there was significant within-conditions coherence in both conditions (1.8–15.9 Hz); on average, the gain was 597 ± 136% larger in the HIGH condition.
Figure 4. Results of follow-up control experiments.
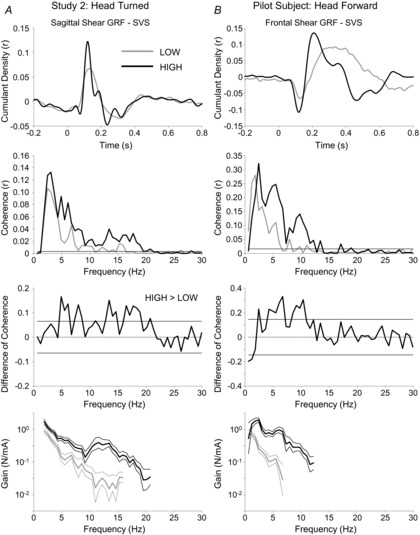
Cumulant density, coherence, difference of coherence, and gain are plotted for LOW and HIGH conditions concatenated from a sample of 5 subjects with head right and visual fields controlled in Study 2 (A), and a single subject standing with head forward and receiving 0–25 Hz SVS stimulation (B). Conventions are the same as in Figs 2 and 3.
Discussion
The purpose of these experiments was to determine the effects of a postural threat on the relationship between vestibular signals and balance control. Our results confirmed the first hypothesis that coupling, as indicated by coherence and cumulant density, would increase with increased postural threat. The pattern and timing of cumulant density peaks observed in the current studies are consistent with previous studies that have used SVS under similar quiet standing conditions (Mian & Day, 2009; Dakin et al. 2010; Mian et al. 2010). As hypothesized, both short- and medium-latency peaks were increased in the HIGH, compared to LOW, conditions in all experiments. Significant coherence between SVS and GRFs was observed between 1.8 and 11.0 Hz, and between 1.8 and 16.5 Hz in the LOW conditions of the head forward and head turned experiments of Study 1, respectively. There was significantly greater SVS–GRF coherence in the HIGH, compared to LOW, conditions between 4.3 Hz and 6.7 Hz in the head forward and between 5.5 Hz and 17.7 Hz in the head turned experiment. Changes in the amount of vestibular–balance coupling reflect changes to the balance-correcting response to a known vestibular error. Such changes in coupling have been shown to be influenced by postural state (Mian & Day, 2009; Mian et al. 2010; Reynolds, 2010) and task (Fitzpatrick et al. 1996; Luu et al. 2012). For example, coupling can be unconsciously disengaged when people are discreetly stabilized by a robotic support surface (Luu et al. 2012), a state where the subject is not self-balancing. It is also reduced as gait speed and cadence are increased (Dakin et al. 2013), states where a vestibular error evokes smaller step-by-step balance corrections (either by disengagement or mechanical stability; Brandt et al. 1999). Likewise, muscle-specific coupling changes throughout the gait cycle. Coupling to individual anti-gravity muscles is modulated by the phase of gait such that coupling rises and falls as the muscle becomes more or less suited to stabilize the body (e.g. early stance versus mid swing for triceps surae; Blouin et al. 2011). Vestibular coupling, in fact, cycles through different muscle groups and across limbs, such that a vestibular error signal evokes balance corrective responses throughout the gait cycle but only phasically in any given muscle (Dakin et al. 2013). Therefore, we interpret the increase in coupling observed in our experiments as state-specific changes in vestibular-motor coupling, whereby the response to a vestibular perturbation is increased, yet the task (standing upright) is essentially unchanged. While these changes in coupling reflect transfer of vestibular error signals to balance responses, they do not reveal changes in the gain of the vestibular–motor relationship; this question is more appropriately addressed using signal gain estimates.
The change in signal gains across threat conditions in all experiments confirms our second hypothesis, that gain would be increased with increased postural threat. The amplitude of evoked GRF responses steadily decreased as SVS frequency was increased, as indicated by the shape of the signal gain estimate, in both experiments in Study 1 and also in Study 2. This relationship is consistent with previous reports using a 0–25 Hz SVS bandwidth (Dakin et al. 2010), although absolute gain values were on average lower in the study presented here. Compared to the LOW conditions, the signal gain estimates in the HIGH conditions were on average 81% larger in the head forward experiment and 231% larger in the head turned experiment of Study 1. This altered relationship corresponds with previously reported observations of changes in VOR reflex gain in response to anxiety (Yardley et al. 1995) and/or alertness (Collins & Guedry, 1962; Collins & Poe, 1962; Kasper et al. 1992). As a whole, these findings support an increased gain at the level of the vestibular nuclei.
The changes to SVS–GRF gain and coupling observed in our studies may be attributed to greater excitation of the vestibular nuclei. The amygdala, activated by fear-inducing stimuli, can influence the vestibular nuclei through two parallel networks (Lang et al. 2000; Balaban & Thayer, 2001; Balaban, 2002; Liddell et al. 2005; Öhman, 2005). In the first network, the amygdala excites the parabrachial nucleus either directly, or indirectly through the locus coeruleus and dorsal raphe nucleus (Lang et al. 2000; Balaban & Thayer, 2001; Balaban, 2002; Staab et al. 2013). Since the parabrachial nucleus has excitatory (and reciprocal) connections with each of the vestibular nuclei (Balaban, 2004), as well as the vestibulo-cerebellum (Staab et al. 2013), it could potentially facilitate vestibular-evoked reflexes through these pathways. The second network involves amygdalofugal projections to multiple cortical regions (Balaban & Thayer, 2001; Balaban, 2002; Liddell et al. 2005; Staab et al. 2013), including the parieto-insular vestibular cortex and anterior cingulate cortex, which are part of the multi-site vestibular cortex (Dieterich & Brandt, 2008). There is indirect evidence to suggest that the vestibular cortex can modulate the function of vestibular reflexes, presumably via cortico-bulbar projections (Doricchi et al. 2002; Ventre-Dominey et al. 2003; Marsden et al. 2005; Arshad et al. 2013, 2014). For example, middle cerebral artery stroke causes asymmetries in GVS-evoked balance responses, but unilateral pyramidal compression (affecting corticospinal tract in medulla, but not affecting pons) does not (Marsden et al. 2005). Likewise, VOR gain and bias have been shown to be affected by neglect caused by cortical damage (Doricchi et al. 2002; Ventre-Dominey et al. 2003), hemispheric dominance (Arshad et al. 2013) and experimental cortical inhibition with trans-cranial direct current stimulation (Arshad et al. 2014). Therefore, cortical-induced excitation of the brainstem vestibular nuclei could also potentially contribute to the observed facilitation of vestibular reflexes in our studies.
The above explanations for the change in the SVS–GRF relationship all assume that a change in gain occurs at the vestibular nuclei; however, alternative mechanisms must be acknowledged. First, the vestibular cortex can evoke a motor response via projections through sensory association cortices to the motor cortex and corticospinal tract (Staab et al. 2013). Therefore, an excitation at the cortical level, independent of the vestibular nuclei, could potentially induce the changes in gain and coherence. However, the evidence from GVS experiments in people with middle cerebral artery stroke suggests that cortical contributions to GVS-evoked balance reflexes do not bypass the brainstem (Marsden et al. 2005), making a corticospinal explanation of our results less likely. Similarly, the amygdala has direct excitatory projections to the caudal pontine reticular formation (Lang et al. 2000). Based on extensive reciprocal connections between the reticular formation, vestibular nuclei and vestibulo-cerebellum (Wilson & Peterson, 1981), the reticular formation has been implicated in GVS-evoked reflexes (Britton et al. 1993) and therefore may contribute to the larger SVS-evoked reflexes observed in this study. Finally, the changes in SVS–GRF gain could potentially be attributed to tonic increases in lower motor neuron pool excitability in the spinal cord. Fear- and anxiety-based amygdalic activation of autonomic centres, including the locus coeruleus and dorsal raphe nucleus (Balaban & Thayer, 2001; Balaban, 2002) can cause diffuse monoaminergic activation of spinal motor neuron and interneuron pathways (Baldissera et al. 1981; Jankowska et al. 2000; Johnson & Heckman, 2010), which can cause persistent excitation of the motor neuron pool and change the input–output gain of the pool (Johnson & Heckman, 2010). However, it is unlikely that the motor neuron pool is in a general state of excitation in this scenario, as Hoffmann reflexes, which can be used to probe motor neuron pool excitability (Zehr, 2002; Misiaszek, 2003), are either not changed (Horslen et al. 2013) or suppressed (Sibley et al. 2007) while standing at height.
Gaze behaviour, particularly when subjects are not given specific instructions on where to look, can be different when standing at height, compared to standing on the ground. For example, when non-fearful people stand at height they tend to explore their visual scene, whereas fearful people restrict their gaze behaviour to a stable target (reviewed in Brandt & Huppert, 2014), possibly to help stabilize balance. While we attempted to control this effect by providing, and instructing subjects to look at, comparable visual targets in both conditions of the experiments in Study 1, the peripheral visual scene was different between height conditions. Controlling peripheral visual scene in Study 2 by use of blinders that occluded the view of the floor and ceiling provides evidence against a confounding visual effect related to standing at height. Changes in SVS–GRF coupling and gain in Study 2 were similar to those observed in Study 1 (Fig.4A). This is in line with other studies that have shown height-related behavioural changes to quiet standing persist when subjects stand at 3.2 m with eyes closed, or while wearing peripheral vision blinders (Davis et al. 2009). Likewise, height-related changes to gait are still observed when the drop at the edge is obscured by a sheet that would not support weight (Tersteeg et al. 2012), a situation where the threat of injury persists but the visual scene is similar across threat levels. Therefore, it is unlikely the changes in vestibular-evoked balance reflexes observed here, or the changes in general balance behaviours seen in other studies, can be attributed solely to changes in the visual scene related to standing at height.
By limiting the frequency range of stimulation to avoid the potential confounding effects of correlated body sway, we arguably have focused our stimulus to optimize the SLP component of the response at the expense of the MLP (Dakin et al. 2010). Some researchers argue that the MLP component of the balance response to vestibular stimulation is more likely to be of vestibular origin than the SLP (Britton et al. 1993; Marsden et al. 2002; Fitzpatrick & Day, 2004; Mian & Day, 2009). There is, in fact, some debate as to what the short- and medium-latency peaks actually represent. For example, Britton et al. (1993) have suggested that the short- and medium-latency peaks might be of reticulospinal and vestibulospinal origin, respectively. In contrast, Cathers et al. (2005) have suggested that the different peaks reflect otolith and canal inputs; however, Mian et al. (2010) tested and failed to support the otolith hypothesis. We cannot contribute to this debate with these data. However, the direction of both peaks is dependent on head orientation (Mian et al. 2010), suggesting vestibular modulation of each. In addition, recent work from our group has demonstrated that all frequencies in our stimulation band contribute to both peaks (Dakin et al. 2011). As such, it is unlikely that the contributor of the MLP was not stimulated in this study. Finally, both peaks were amplified in HIGH conditions of this study, which implies that they were both modulated by a similar, if not the same, mechanism. However, in anticipation of this potential criticism related to the stimulus profile used in this study, we tested a single case study subject in the head forward protocol of Study 1, experiment 1, with an open 0 Hz (>DC) to 25 Hz stimulation bandwidth to encourage prominent MLP responses. The results from this subject are shown in Fig.4B. In line with the results from the full experiments, this subject had greater coherence, signal gain, as well as larger SLP and MLP amplitudes in the HIGH, compared to the LOW, condition. This further demonstrates that our results are generalizable beyond the SLP, and whatever subsystem(s) it might represent.
Another potential limitation to this study is the directional aspect of the postural threat created by having the edge of the platform always located in front of the subject. Several studies have demonstrated that when subjects stand quietly at the edge of an elevated platform, the changes to balance control only occur in the AP direction (Carpenter et al. 1999, 2001b; Adkin et al. 2000; Davis et al. 2009). People tend to increase body sway stiffness in the AP direction (Carpenter et al. 2001b), and to lean backward away from the edge (Carpenter et al. 1999, 2001b; Davis et al. 2009). The purpose of using two different head orientations in this study was to control for potential confounding effects of the edge location. Since the direction of the balance responses to GVS (Lund & Broberg, 1983) or SVS (Pavlik et al. 1999; Mian & Day, 2009) are dictated by head orientation, then we would expect that threat effects should be aligned with the direction of stimulation, not the edge, if the effects relate to an altered vestibular–balance relationship. Since increases in coupling and gain occurred in both the AP and ML directions, the changes observed in these experiments cannot be attributed entirely to an attempt to avoid the edge or to changes in balance control related to the presence of the edge.
The current results are in conflict with a previous report which suggested early postural responses to square-wave GVS are not altered when people stand on a narrow elevated beam (Osler et al. 2013). Osler et al. (2013) found that late (∼800 ms) trunk sway responses in the ML direction were attenuated at height (i.e. subjects swayed less towards the edge), but early responses were not affected. In contrast, our study was only concerned with early latency responses (136 ms for SLP and 296 ms for MLP). While early responses to GVS (or in our case SVS) reflect predominantly vestibular effects, responses occurring 400 ms or more after stimulus onset are thought to be dependent on multi-sensory feedback (Day & Guerraz, 2007), which would be subject to proprioceptive changes thought to occur with threat (Davis et al. 2011, Horslen et al. 2013), and which can be voluntarily attenuated (Reynolds, 2010). Likewise, mechanical filtering is known to reduce high frequency content from SVS-evoked responses in GRFs compared to EMG, and from GRFs to trunk sway (Dakin et al. 2010). In fact, there is negligible correlation between trunk sway and SVS at and above 2 Hz (Dakin et al. 2010), which is where all changes were observed across threat conditions in the current study. Thus, the measures used by Osler et al. (2013) are probably insensitive to the early, high frequency, changes in the vestibular–postural relationship observed here.
These results add to a growing body of evidence suggesting that fear, anxiety and arousal can influence vestibular function, and that these effects translate to changes in balance control in humans. Temporary changes in state-anxiety and arousal are linked to increased VOR gain (Collins & Guedry, 1962; Collins & Poe, 1962; Kasper et al. 1992; Yardley et al. 1995), suggesting that ascending vestibular reflexes are subject to autonomic or emotional modulation. Similarly, changes in mood and anxiety have been linked to changes in vestibular control of balance (Bolmont et al. 2002) and changes to the centre of pressure in frequency bands thought to be under vestibular control (Wada et al. 2001). Furthermore, pathological fear and anxiety disorders have been linked to balance dysfunction (Balaban & Thayer, 2001; Yardley & Redfern, 2001) and vertigo (Balaban & Jacob, 2001; Furman & Jacob, 2001).
Our confirmation that vestibular control of posture is altered with threat sheds new light onto previously unresolved issues related to the effects of fear and anxiety on balance control. Automatic balance responses to perturbations are known to be altered when people stand at the edge of an elevated platform (Brown & Frank, 1997; Carpenter et al. 2004). Specifically, the amplitude of muscle activity in the ‘balance-correcting’ phase (120–220 ms post-perturbation) is increased with threat (Carpenter et al. 2004). The amplitude of this ‘balance-correcting’ phase is known to be modulated by vestibular inputs (Allum & Pfaltz, 1985; Keshner et al. 1987; Horak et al. 1994; Carpenter et al. 2001a). As such, a functional implication of the increased gain observed here is that the same head acceleration evoked by a perturbation may generate a larger vestibular-evoked balance response when the consequences of falling are elevated. Similarly, it has been postulated that continuous postural sway in undisturbed standing might serve to generate a certain quantity or quality of balance-related afferent information that the body can then use to monitor the postural state (Carpenter et al. 2010; Murnaghan et al. 2011, 2013). Undisturbed postural sway is reduced when people stand at the edge of an elevated platform (Carpenter et al. 2001b). We have previously proposed that changes in muscle spindle sensitivity with a postural threat might serve to facilitate this afferent acquisition process, thereby reducing the amount of sway required to meet the afferent demands in a threatening scenario (Horslen et al. 2013). As such, a second implication for these results is that the increased gain, and implied increased excitability, of the vestibular system in a threatening scenario might also facilitate sway reduction without compromising the amount of afferent information available to the central nervous system.
In conclusion, we have demonstrated that the gain and coupling of balance responses to SVS are increased in a high postural threat scenario. These responses are in agreement with the proposal made by Balaban (2002) that vestibular pathways are excited in states of fear or vigilance to augment vestibular-evoked responses to imposed or self-motion. Furthermore, these data also support the emergent theme of a general sensitization to balance-relevant sensory information in high postural threat scenarios.
Glossary
- AP
antero-posterior
- EDA
electrodermal activity
- GRF
ground reaction force
- GVS
galvanic vestibular stimulation
- HIGH
high threat condition
- LOW
low threat condition
- ML
medio-lateral
- MLP
medium-latency peak
- SLP
short-latency peak
- SVS
stochastic vestibular stimulation
- VOR
vestibulo-ocular reflex
Key points
The vestibular system is an important sensory contributor to the control of standing balance.
Fear, anxiety and arousal are thought to influence the excitability of the vestibular system, but it is not clear if these changes lead to altered vestibular-evoked balance reflexes.
Low and high standing surface heights were used to manipulate fear and anxiety in this study, while stochastic vestibular stimulation was used to evoke balance reflexes.
High surface heights lead to greater coupling between vestibular inputs and balance reflexes, as well as larger responses.
These results support the idea that the manner in which vestibular information is processed is altered when people are exposed to a threat to their balance, and this altered processing may explain why normal balance behaviour is different in threatening scenarios.
Additional information
Competing interests
The authors do not have any competing interests to report.
Author contributions
All data were collected in the Neural Control of Posture and Movement Lab at the University of British Columbia. All authors contributed to the conception and design of the study. B.C.H. and C.J.D. collected and analysed the data. B.C.H. drafted the manuscript under the supervision of M.G.C. All authors critically reviewed the manuscript and approved it for submission for publication.
Funding
The authors wish to thank the Natural Sciences and Engineering Research Council of Canada, and Canada Foundation for Innovation for project funding, and for salary support provided by: Natural Sciences and Engineering Research Council of Canada to B.C.H. and C.J.D.; Michael Smith Foundation for Health Research and the Canadian Chiropractic Research Foundation to J.-S.B.; Canada Research Chair to M.G.C.
References
- Adkin AL, Frank JS, Carpenter MG, Peysar GW. Postural control is scaled to level of postural threat. Gait Posture. 2000;12:87–93. doi: 10.1016/s0966-6362(00)00057-6. [DOI] [PubMed] [Google Scholar]
- Allum JH, Pfaltz CR. Visual and vestibular contributions to pitch sway stabilization in the ankle muscles of normals and patients with bilateral peripheral vestibular deficits. Exp Brain Res. 1985;58:82–94. doi: 10.1007/BF00238956. [DOI] [PubMed] [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Bronstein AM. Handedness-related cortical modulation of the vestibular-ocular reflex. J Neurosci. 2013;33:3221–3227. doi: 10.1523/JNEUROSCI.2054-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad Q, Nigmatullina Y, Roberts RE, Bhrugubanda V, Asavarut P, Bronstein AM. Left cathodal trans-cranial direct current stimulation of the parietal cortex leads to an asymmetrical modulation of the vestibular-ocular reflex. Brain Stimul. 2014;7:85–91. doi: 10.1016/j.brs.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77:469–475. doi: 10.1016/s0031-9384(02)00935-6. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses. Brain Res. 2004;996:126–137. doi: 10.1016/j.brainres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Jacob RG. Background and history of the interface between anxiety and vertigo. J Anxiety Disord. 2001;15:27–51. doi: 10.1016/s0887-6185(00)00041-4. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Thayer JF. Neurological bases for balance–anxiety links. J Anxiety Disord. 2001;15:53–79. doi: 10.1016/s0887-6185(00)00042-6. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H. Integration in spinal neuronal systems. In: Brooks VB, Illert M, editors. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control, part 1. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Blouin JS, Dakin CJ, van den Doel K, Chua R, McFadyen BJ, Inglis JT. Extracting phase-dependent human vestibular reflexes during locomotion using both time and frequency correlation approaches. J App Physiol. 2011;111:1484–1490. doi: 10.1152/japplphysiol.00621.2011. [DOI] [PubMed] [Google Scholar]
- Bolmont B, Gangloff P, Vouriot A, Perrin PP. Mood states and anxiety influence abilities to maintain balance control in healthy human subjects. Neurosci Lett. 2002;329:96–100. doi: 10.1016/s0304-3940(02)00578-5. [DOI] [PubMed] [Google Scholar]
- Brandt T, Huppert D. Fear of heights and visual height intolerance. Curr Opin Neurol. 2014;27:111–117. doi: 10.1097/WCO.0000000000000057. [DOI] [PubMed] [Google Scholar]
- Brandt T, Strupp M, Benson J. You are better off running than walking with acute vestibulopathy. Lancet. 1999;354:746. doi: 10.1016/S0140-6736(99)03179-7. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Brown LA, Gage WH, Polych MA, Sleik RJ, Winder TR. Central set influences on gait. Age-dependent effects of postural threat. Exp Brain Res. 2002;145:286–296. doi: 10.1007/s00221-002-1082-0. [DOI] [PubMed] [Google Scholar]
- Brown LA, Frank JS. Postural compensations to the potential consequences of instability: kinematics. Gait Posture. 1997;6:89–97. [Google Scholar]
- Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res. 2001a;140:95–111. doi: 10.1007/s002210100802. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JHJ. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol. 2004;92:3255–3265. doi: 10.1152/jn.01139.2003. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP. Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res. 1999;9:277–286. [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res. 2001b;138:210–218. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Murnaghan CD, Inglis JT. Shifting the balance: evidence of an exploratory role for postural sway. Neuroscience. 2010;171:196–204. doi: 10.1016/j.neuroscience.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005;563:229–234. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WE, Guedry FE., Jr Arousal effects and nystagmus during prolonged constant angular acceleration. Acta Otolaryngol. 1962;54:349–362. doi: 10.3109/00016486209126954. [DOI] [PubMed] [Google Scholar]
- Collins WE, Poe RH. Amphetamine, arousal, and human vestibular nystagmus. J Pharmacol Exp Ther. 1962;138:120–125. [PubMed] [Google Scholar]
- Crampton GH. Habituation of vestibular nystagmus in the cat during sustained arousal produced by d-amphetamine. Rep US Army Med Res Lab. 1961;488:1–15. doi: 10.21236/ad0263258. [DOI] [PubMed] [Google Scholar]
- Crampton GH, Schwam WJ. Effects of arousal reaction on nystagmus habituation in the cat. Am J Physiol. 1961;200:29–33. doi: 10.1152/ajplegacy.1961.200.1.29. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Inglis JT, Blouin JS. Short and medium latency muscle responses evoked by electrical vestibular stimulation are a composite of all stimulus frequencies. Exp Brain Res. 2011;209:345–354. doi: 10.1007/s00221-011-2549-7. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Inglis JT, Chua R, Blouin JS. Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J Neurophysiol. 2013;110:86–94. doi: 10.1152/jn.00843.2012. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Lee Son GM, Inglis JT, Blouin JS. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol. 2007;583:1117–1127. doi: 10.1113/jphysiol.2007.133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin JS. Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol. 2010;103:1048–1056. doi: 10.1152/jn.00881.2009. [DOI] [PubMed] [Google Scholar]
- Davis JR, Campbell AD, Adkin AL, Carpenter MG. The relationship between fear of falling and human postural control. Gait Posture. 2009;29:275–279. doi: 10.1016/j.gaitpost.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Davis JR, Horslen BC, Nishikawa K, Fukushima K, Chua R, Inglis JT, Carpenter MG. Human proprioceptive adaptations during states of height-induced fear and anxiety. J Neurophysiol. 2011;106:3082–3090. doi: 10.1152/jn.01030.2010. [DOI] [PubMed] [Google Scholar]
- Day BL, Guerraz M. Feedforward versus feedback modulation of human vestibular-evoked balance responses by visual self-motion information. J Physiol. 2007;582:153–161. doi: 10.1113/jphysiol.2007.132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131:2538–2552. doi: 10.1093/brain/awn042. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Siegler I, Iaria G, Berthoz A. Vestibulo-ocular and optokinetic impairments in left unilateral neglect. Neuropsychologia. 2002;40:2084–2099. doi: 10.1016/s0028-3932(02)00049-0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J App Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9–26. doi: 10.1016/s0887-6185(00)00040-2. [DOI] [PubMed] [Google Scholar]
- Furman JM, O'Leary DP, Wolfe JW. Changes in the horizontal vestibulo-ocular reflex of the rhesus monkey with behavioral and pharmacological alerting. Brain Res. 1981;206:490–494. doi: 10.1016/0006-8993(81)90553-9. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Bronstein AM. Mechanisms underlying visually induced body sway. Neurosci Lett. 2008;443:12–16. doi: 10.1016/j.neulet.2008.07.053. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data—Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hauck LJ, Carpenter MG, Frank JS. Task-specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture. 2008;27:676–682. doi: 10.1016/j.gaitpost.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Horak FB, Shupert CL, Dietz V, Horstmann G. Vestibular and somatosensory contributions to responses to head and body displacements in stance. Exp Brain Res. 1994;100:93–106. doi: 10.1007/BF00227282. [DOI] [PubMed] [Google Scholar]
- Horslen BC, Murnaghan CD, Inglis JT, Chua R, Carpenter MG. Effects of postural threat on spinal stretch reflexes: evidence for increased muscle spindle sensitivity? J Neurophysiol. 2013;110:899–906. doi: 10.1152/jn.00065.2013. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Hedén CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ. Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann N Y Acad Sci. 2010;1198:35–41. doi: 10.1111/j.1749-6632.2010.05430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Ishikawa K, Griffith AJ. Anxiety and otovestibular disorders: linking behavioral phenotypes in men and mice. Behav Brain Res. 2008;186:1–11. doi: 10.1016/j.bbr.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Kasper J, Diefenhardt A, Mackert A, Thoden U. The vestibulo-ocular response during transient arousal shifts in man. Acta Otolaryngol. 1992;112:1–6. doi: 10.3109/00016489209100775. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Allum JH, Pfaltz CR. Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp Brain Res. 1987;69:77–92. doi: 10.1007/BF00247031. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Öhman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Venault P, Negroni J, Perez-Diaz F, Joubert C, Nosten-Bertrand M, Berthoz A, Chapouthier G. Posture and balance responses to a sensory challenge are related to anxiety in mice. Psychiatry Res. 2003;118:273–284. doi: 10.1016/s0165-1781(03)00069-6. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Venault P, Perez-Diaz F, Joubert C, Berthoz A, Chapouthier G. Balance control and posture differences in the anxious BALB/cByJ mice compared to the non anxious C57BL/6J mice. Behav Brain Res. 2000;117:185–195. doi: 10.1016/s0166-4328(00)00304-1. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Luu BL, Inglis JT, Huryn TP, Van der Loos HF, Croft EA, Blouin JS. Human standing is modified by an unconscious integration of congruent sensory and motor signals. J Physiol. 2012;590:5783–5794. doi: 10.1113/jphysiol.2012.230334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden JF, Castellote J, Day BL. Bipedal distribution of human vestibular-evoked postural responses during asymmetrical standing. J Physiol. 2002;542:323–331. doi: 10.1113/jphysiol.2002.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden JF, Playford ED, Day BL. The vestibular control of balance after stroke. J Neurol Neurosurg Psychiatry. 2005;76:670–678. doi: 10.1136/jnnp.2004.046565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Dakin CJ, Blouin JS, Fitzpatrick RC, Day BL. Lack of otolith involvement in balance responses evoked by mastoid electrical stimulation. J Physiol. 2010;588:4441–4451. doi: 10.1113/jphysiol.2010.195222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Day BL. Determining the direction of vestibular-evoked balance responses using stochastic vestibular stimulation. J Physiol. 2009;587:2869–2873. doi: 10.1113/jphysiol.2009.171256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve. 2003;28:144–160. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- Murnaghan CD, Horslen BC, Inglis JT, Carpenter MG. Exploratory behavior during stance persists with visual feedback. Neuroscience. 2011;195:54–59. doi: 10.1016/j.neuroscience.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Murnaghan CD, Squair JW, Chua R, Inglis JT, Carpenter MG. Are increases in COP variability observed when participants are provided explicit verbal cues prior to COM stabilization? Gait Posture. 2013;38:734–738. doi: 10.1016/j.gaitpost.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Osler CJ, Tersteeg MC, Reynolds RF, Loram ID. Postural threat differentially affects the feedforward and feedback components of the vestibular-evoked balance response. Eur J Neurosci. 2013;38:3239–3247. doi: 10.1111/ejn.12336. [DOI] [PubMed] [Google Scholar]
- Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins JJ. The effects of stochastic galvanic vestibular stimulation on human postural sway. Exp Brain Res. 1999;124:273–280. doi: 10.1007/s002210050623. [DOI] [PubMed] [Google Scholar]
- Reynolds RF. The effect of voluntary sway control on the early and late components of the vestibular-evoked postural response. Exp Brain Res. 2010;201:133–139. doi: 10.1007/s00221-009-2017-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Sibley KM, Carpenter MG, Perry JC, Frank JS. Effects of postural anxiety on the soleus H-reflex. Hum Mov Sci. 2007;26:103–112. doi: 10.1016/j.humov.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Staab JP, Balaban CD, Furman JM. Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol. 2013;33:297–306. doi: 10.1055/s-0033-1356462. [DOI] [PubMed] [Google Scholar]
- Tersteeg MCA, Marple-Horvat DE, Loram ID. Cautious gait in relation to knowledge and vision of height: is altered visual information the dominant influence? J Neurophysiol. 2012;107:2686–2691. doi: 10.1152/jn.00875.2011. [DOI] [PubMed] [Google Scholar]
- Venables PH. Autonomic activity. Ann N Y Acad Sci. 1991;620:191–207. doi: 10.1111/j.1749-6632.1991.tb51584.x. [DOI] [PubMed] [Google Scholar]
- Venault P, Rudrauf D, Lepicard EM, Berthoz A, Jouvent R, Chapouthier G. Balance control and posture in anxious mice improved by SSRI treatment. Neuroreport. 2001;12:3091–3094. doi: 10.1097/00001756-200110080-00022. [DOI] [PubMed] [Google Scholar]
- Ventre-Dominey J, Nighoghossian N, Denise P. Evidence for interacting cortical control of vestibular function and spatial representation in man. Neuropsychologia. 2003;41:1884–1898. doi: 10.1016/s0028-3932(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Berthoz A, Millanvoye M. Difference between eye closure and visual stabilization in the control of posture in man. Aviat Space Environ Med. 1982;53:166–170. [PubMed] [Google Scholar]
- Wada M, Sunaga N, Nagai M. Anxiety affects the postural sway of the antero-posterior axis in college students. Neurosci Lett. 2001;302:157–159. doi: 10.1016/s0304-3940(01)01662-7. [DOI] [PubMed] [Google Scholar]
- Wilson VJ. Vestibulospinal and reticulospinal systems. In: Brooks VB, Peterson BW, editors. Handbook of Physiology. Bethesda, MD, USA: American Physiological Society; 1981. pp. 667–702. part 1 &, section 1,, vol. IIThe Nervous SystemMotor Control. [Google Scholar]
- Yardley L, Redfern MS. Psychological factors influencing recovery from balance disorders. J Anxiety Disord. 2001;15:107–119. doi: 10.1016/s0887-6185(00)00045-1. [DOI] [PubMed] [Google Scholar]
- Yardley L, Watson S, Britton J, Lear S, Bird J. Effects of anxiety arousal and mental stress on the vestibulo-ocular reflex. Acta Otolaryngol. 1995;115:597–602. doi: 10.3109/00016489509139373. [DOI] [PubMed] [Google Scholar]
- Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]


