Supplemental Digital Content is available in the text.
Key Words: Wilms tumor gene (WT1), WT1 peptide vaccine, cancer immunotherapy, pancreatic cancer, gemcitabine
Abstract
Wilms tumor gene (WT1) protein is an attractive target for cancer immunotherapy. We aimed to investigate the feasibility of a combination therapy consisting of gemcitabine and WT1 peptide–based vaccine for patients with advanced pancreatic cancer and to make initial assessments of its clinical efficacy and immunologic response. Thirty-two HLA-A*24:02+ patients with advanced pancreatic cancer were enrolled. Patients received HLA-A*24:02-restricted, modified 9-mer WT1 peptide (3 mg/body) emulsified with Montanide ISA51 adjuvant (WT1 vaccine) intradermally biweekly and gemcitabine (1000 mg/m2) on days 1, 8, and 15 of a 28-day cycle. This combination therapy was well tolerated. The frequencies of grade 3–4 adverse events for this combination therapy were similar to those for gemcitabine alone. Objective response rate was 20.0% (6/30 evaluable patients). Median survival time and 1-year survival rate were 8.1 months and 29%, respectively. The association between longer survival and positive delayed-type hypersensitivity to WT1 peptide was statistically significant, and longer survivors featured a higher frequency of memory-phenotype WT1-specific cytotoxic T lymphocytes both before and after treatment. WT1 vaccine in combination with gemcitabine was well tolerated for patients with advanced pancreatic cancer. Delayed-type hypersensitivity-positivity to WT1 peptide and a higher frequency of memory-phenotype WT1-specific cytotoxic T lymphocytes could be useful prognostic markers for survival in the combination therapy with gemcitabine and WT1 vaccine. Further clinical investigation is warranted to determine the effectiveness of this combination therapy.
Pancreatic cancer remains a malignancy with high mortality.1 Gemcitabine has been the standard first-line treatment for patients with advanced pancreatic cancer, but featured a median overall survival time (MST) of about 6 months and a 1-year overall survival (OS) rate of ≤20%.2 Although many trials of gemcitabine-based combination therapies with cytotoxic or biological agents have been attempted, these therapies, with the exception of erlotinib,3 have not achieved any survival results superior to those attained with gemcitabine alone.1 Prognosis of patients with pancreatic cancer thus remains extremely poor, so that novel treatments are urgently needed to improve survival.
Among promising therapeutic strategies, active cancer immunotherapies, such as peptide-based cancer vaccines against tumor-associated antigens (TAAs), which elicit TAA-specific cytotoxic T lymphocytes (CTLs) that eventually eradicate cancer cells, have been and are being developed.4 However, because their clinical efficacy has been limited,5,6 several approaches have been tried to improve their efficacy. One approach is the use of combination therapies with certain chemotherapeutic agents, including gemcitabine, which can stimulate the immune system.7–9 An additional benefit is that chemotherapy makes the tumor cells susceptible to CTL response,10,11 whereas cancer immunotherapy can sensitize the tumor cells to subsequent chemotherapeutic agents. For this reasons, cancer vaccine in combination with certain chemotherapeutic agents can be expected to exert synergistic effects.
The Wilms tumor gene (WT1) is highly expressed in various kinds of malignancies and has been found to perform oncogenic rather than tumor-suppressor functions in tumorigenesis.12,13 Moreover, both cellular and humoral immune responses against the WT1 protein are naturally elicited in cancer patients, indicating that the WT1 gene product is actually immunogenic.14–18 In view of these findings, we and others have been performing clinical studies of the efficacy of WT1 peptide–based immunotherapies for patients, including children, with various kinds of malignancies.13,19–26
This report describes a phase 1 clinical study of a WT1 peptide–based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. The main objective of this study was to investigate the feasibility of this combination therapy and to make initial assessments of its clinical efficacy and the immunologic response to WT1 peptide.
MATERIALS AND METHODS
Patient Characteristics
Patients with pathologically or cytologically confirmed, measurable, locally advanced, or metastatic pancreatic adenocarcinoma or with recurrent disease were recruited for this noncomparative, open-label, phase 1 study at 2 centers: Osaka University Hospital and Jikei University Kashiwa Hospital, in Japan. Another major eligibility criterion was HLA-A*24:02 positivity. We chose this phenotype because about 60% of Japanese population had this phonotype. Other eligibility criteria included age of 20 years and older, 75 years and younger, Karnofsky performance status 60%–100%, no previous history of treatment for locally advanced or metastatic disease, a minimum 6-month interval from completion of any previous treatment for recurrent disease, a life expectancy of ≥3 months, and adequate organ functions. This study was approved by the ethical review boards of the 2 centers and performed in accordance with the Helsinki Declaration. All patients provided written informed consent.
WT1-Peptide–based Cancer Vaccine (WT1 Vaccine)
A HLA-A*24:02-restricted, modified 9-mer WT1 peptide (mp235; CYTWNQMNL; Peptide Institute Inc., Osaka, Japan) was generated according to the Good Manufacturing Practice Guidelines. In our previous report about the first clinical use of WT1 peptide,19 the dose-escalation of WT1 peptide from 0.3 to 3.0 mg was designed to decide the recommended dose in combination with the incomplete Freund’s adjuvant (Montanide ISA51; Seppic, Paris, France), and 3 mg of WT1 peptide in combination with Montanide ISA51 was decided to be well tolerated. In our present study, we chose WT1 vaccine composed of 3 mg of WT1 peptide and Montanide ISA51 adjuvant. WT1 vaccine was prepared, according to our previous report.19 WT1 peptide of 3 mg was dissolved in a small volume of dimethyl sulfoxide (DMSO; Sigma, St Louis, MO). The solution was then diluted to 400 μL with 5% glucose and finally emulsified with an equal weight of Montanide ISA51 adjuvant.
Treatment
Gemcitabine was intravenously administered at a dose of 1000 mg/m2 on days 1, 8, and 15 of a 28-day cycle. WT1 vaccine was intradermally administered at 6 different sites (bilateral upper arms, lower abdomen, and femoral regions) on days 1 and 15 of a 28-day cycle. The initial treatment protocol was planned as 2 courses. Patients without early progressive disease upon the completion of protocol treatment could receive additional treatment until the occurrence of disease progression, unacceptable adverse events, or withdrawal of consent.
Study Assessment
Toxicity was graded using the National Cancer Institute’s Common Toxicity Criteria of Adverse Events (CTCAE version 3.0). Dose-limiting toxicity (DLT) was defined as the following adverse events, during the first 2 courses, which were possibly, probably, or definitely related to treatment: grade 4 hematological toxicity lasting >7 days, grade 3 or worse neutropenia accompanied by high fever (≥38°C) or infection (febril neutropenia), and any nonhematological toxicity of grade 3 or worse in other organ systems, including vaccine-injection sites. Biliary tract infection secondary to biliary obstruction was not considered to be a DLT unless it occurred in conjunction with grade ≥3 neutropenia. Computed tomography was performed every 4 weeks during the protocol treatment and every 6–8 weeks during the additional treatment until disease progression, and tumor response was assessed by the investigators according to the Response Evaluation Criteria in Solid Tumors criteria. Stable disease (SD) was defined as a disease that was stable for ≥8 weeks after the beginning of treatment. The concentration of the tumor marker carbohydrate antigen 19-9 (CA19-9) was measured at baseline and each course.
WT1-specific Immunologic Assessment
As WT1-specific immunologic assessment, delayed-type hypersensitivity (DTH) to WT1 peptide and the WT1 peptide/HLA-A*24:02 tetramer assay was examined. DTH was examined on day 1 of each course during the protocol treatment and optionally at suitable time during the additional treatment. All DTH tests were performed and measured by the investigators. Briefly, 30 μg of WT1 peptide in saline and saline alone were intradermally injected in the forearm, and the maximum diameter of erythema and other skin reaction, including induration, were measured after 48 hours. DTH-positivity was defined as erythema ≥2 mm in diameter, which size was the minimum size measurable with a ruler at the clinical practice.
Peripheral blood (PB) mononuclear cells for WT1 peptide/HLA-A*24:02 tetramer assay were collected on day 1 of each course during the protocol treatment and appropriately during the additional treatment, and cryopreserved until use. The following tetramer and monoclonal antibodies were used: PE-conjugated WT1235 tetramer [HLA-A*24:02-restricted natural 9-mer WT1 peptide (CMTWNQMNL)] (MBL, Nagoya, Japan), anti-CD4-FITC, anti-CD16-FITC, anti-CD45RA-APC (BioLegend, San Diego, CA), anti-CD19-FITC, anti-CCR7-PE-Cy7 (BD Pharmingen, San Diego, CA), anti-CD3-PerCP, anti-CD8-APC-Cy7, anti-CD14-FITC (BD Biosciences, San Jose, CA), and anti-CD56-FITC (eBioscience, San Diego, CA). Lineage antigen (CD4, CD14, CD16, CD19, and CD56)-negative, CD3−, CD8−, and WT1235 tetramer+ lymphocytes were defined as WT1 tetramer+CD3+CD8+ T lymphocytes (WT1-CTLs). Data acquisition were performed on a FACS Aria instrument (BD Biosciences), and data analysis were performed with FACS Diva software (BD Biosciences).
Statistical Analysis
The safety profile constituted the primary end point. A treatment schedule was considered to be acceptable if the probability of developing DLT was estimated to be <20%. If the estimated probability of DLT occurrence was 10%, the upper limit of the 90% (one-sided) confidence interval (CI) of DLT probability was <20%, based on the projected sample size of 20 patients. For a more accurate determination of the associations with clinical efficacy and immunologic parameters, in total 32 patients were enrolled (8 patients were further enrolled after the completion of safety assessment with the initial 24 patients as shown in Fig. 1). The secondary end points included objective response, CA19-9 response, defined as a decrease in CA19-9 concentration of at least 50% in the patients with ≥100 U/mL of CA19-9 at baseline, progression-free survival defined as time from date of beginning of the treatment to date of disease progression as confirmed by the investigators or death without progression, OS, immunologic responses to WT1 peptide, and correlations between clinical benefit response (CBR)2 and quality of life (QOL) assessed using by the Functional Assessment of Cancer Therapy-General (FACT-G) measurement system.27 The nonparametric, Wilcoxon signed-rank test or Mann-Whitney U test was used to calculate P values for change in immune cells because the data were skewed. We judged P values of <0.01 to be significant. χ2 test was used to calculate P values for associations between DTH and clinical efficacy. The statistical analyses were performed with SAS for Windows version 9.2 (SAS Institute Inc., Cary, NC). Correlations between CBR and the physical and functional scores based on replies to the FACT-G QOL questionnaire were analyzed with a linear mixed-effects model, for which SAS for Windows release 9.1 (SAS Institute Inc.) was used.
FIGURE 1.
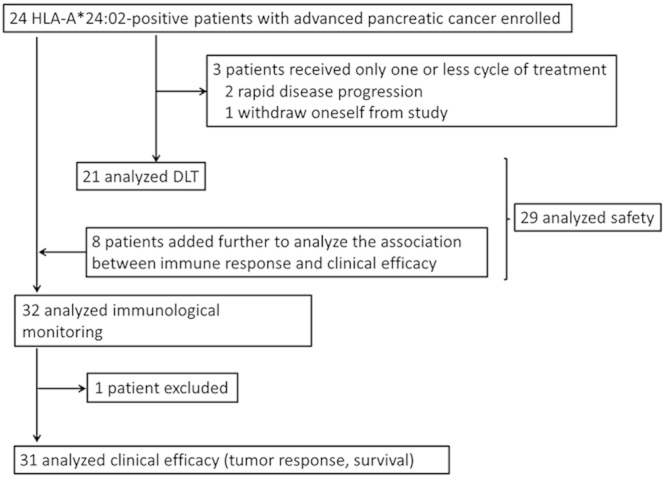
Study profile.
RESULTS
Patient Characteristics
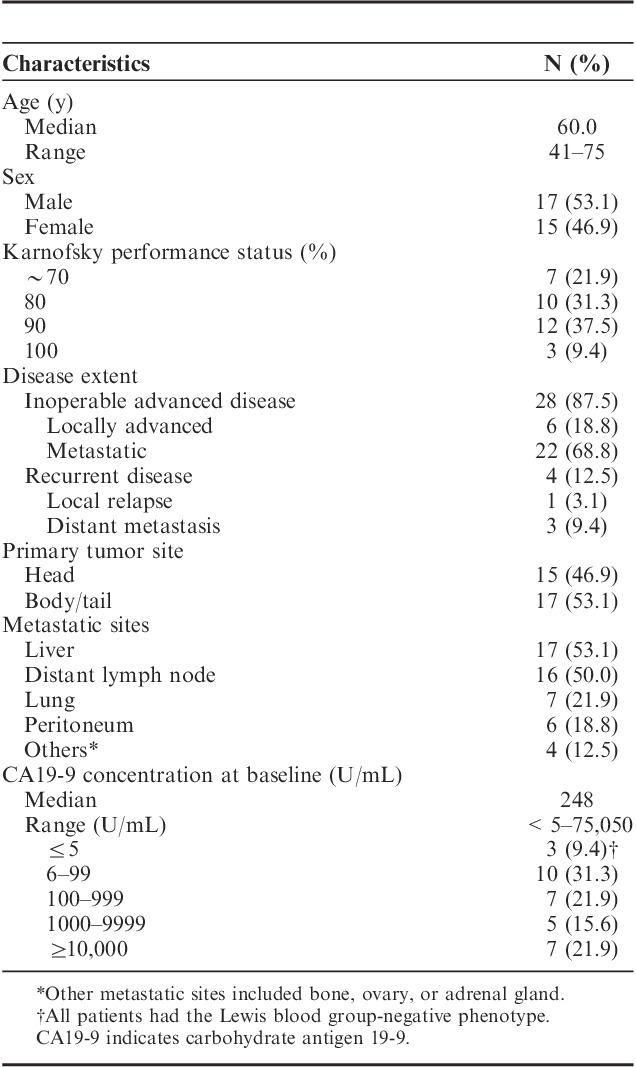
A total of 63 patients with advanced pancreatic cancer, whose median age was 63.0 years old, were screened and checked a phenotype in HLA-A locus. Twenty-two patients failed to enroll in this trial because of lack of HLA-A*24:02 phenotype. A total of 32 HLA-A*24:02+ patients with advanced pancreatic cancer were finally enrolled in this trial between 2008 and 2010. Of 32 patients, 28 had inoperable advanced pancreatic cancer (6 locally advanced and 22 metastatic diseases), and the remaining 4 had recurrent disease. Table 1 summarizes the patient baseline characteristics. Three patients did not complete the first 2 courses of treatment: 2 patients showed rapid disease progression, and 1 refused to continue the treatment. It was determined by the supervising Data Safety and Monitoring Board that the elimination of these cases was unlikely to be or was not related to the protocol treatment. Of the initial 24 patients, 21 could thus to be used for assessment of DLT, 29 of all 32 patients for assessment of adverse events (Fig. 1).
TABLE 1.
Patients Characteristics at Baseline
Safety
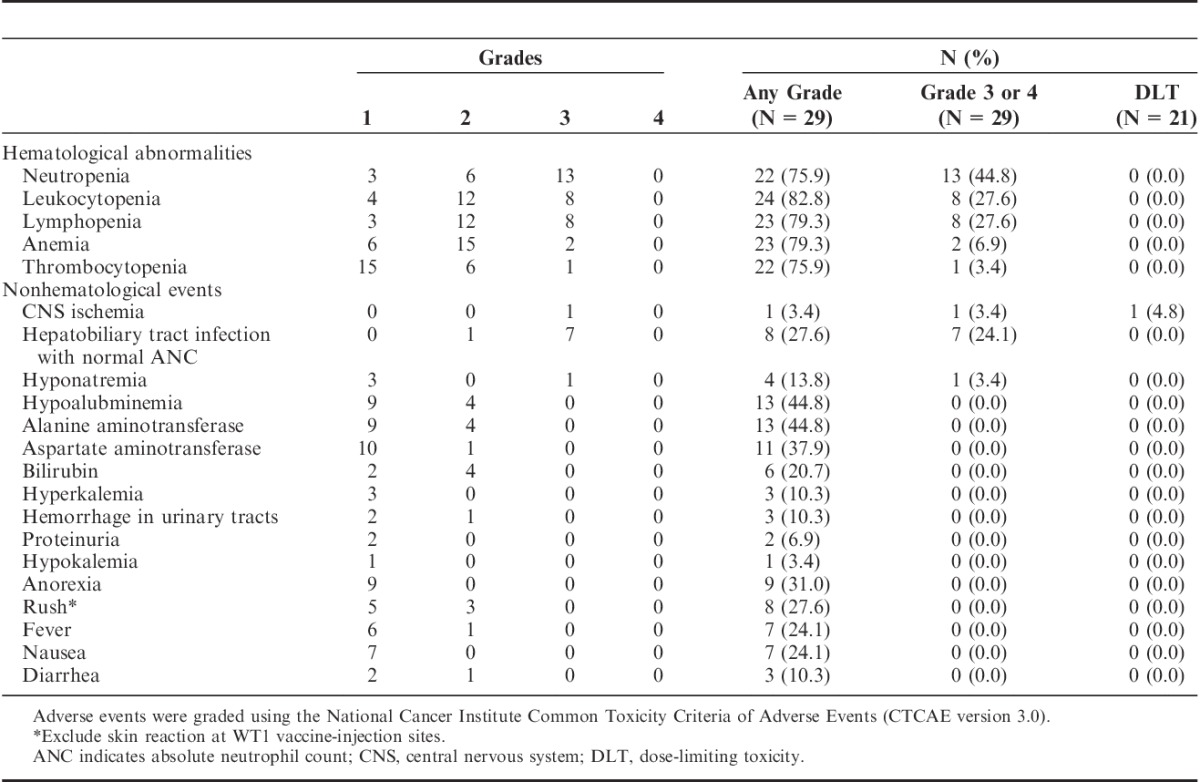
Administration of WT1 vaccine in combination with gemcitabine was well tolerated. All adverse events are listed in Table 2. The initial assessment of safety for 21 patients found that a grade 4 central nervous system cerebrovascular ischemia considered to be a DLT had occurred in 1 patient. The most commonly reported adverse event was skin toxicity related to WT1 vaccine. All patients developed grade 1 or 2 skin reactions with swelling, redness, erythema, and induration with or without involvement of small vesicles at the local vaccine-injection sites. Hematological abnormalities were similar to those observed with the administration of gemcitabine alone, and none of the patients developed DLTs associated with hematological abnormalities or febril neutropenia. Eight grade 3 nonhematological adverse events (1 instance of hyponatremia and 7 hepatobiliary/pancreas infections) were detected and attributed to complications associated with disease progression or biliary obstruction. Other major nonhematological adverse events included grade 1 or 2 skin rash, anorexia, nausea, and fever, all of which were previously reported as major adverse events associated with gemcitabine. Hepatic transaminase elevation was principally related to disease progression and/or hepatobiliary infection. Except for local skin reactions, none of the patients experienced adverse events considered to be related to WT1 vaccination.
TABLE 2.
Adverse Events Reported in 29 Patients who Completed the First 2 Courses of Treatment
Clinical Response and Survival Analysis
The clinical efficacy results for all 32 patients are summarized in Table 3. Two patients were excluded from some of these analyses. One patient, who had followed a satisfactory and interesting treatment course and finally undergone a surgical resection (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/JIT/A317 and Table 3), was excluded from the evaluations of response and survival because the diagnosis of pancreatic cancer could not be pathologically confirmed due to the lack of viable tumor cells in the resected specimens. The other patient was excluded from the evaluation of response because of withdrawal of consent before the first evaluation. Thus, of the total of 32 patients, 30 could be used to evaluate response to treatment and 31 to assess survival. Six of 30 patients (20.0%) reached partial response (PR), and 16 of them (53.3%) showed SD at least for ≥8 weeks (Table 3). Median progression-free survival was 4.2 months (95% CI, 3.6–4.6) (Fig. 2A) and MST was 8.1 months (95% CI, 6.3–10.0) (Fig. 2B). Six-month and 1-year OS rates were 71.0% (95% CI, 54.9–87.1) and 29.0% (95% CI, 12.9–45.1), respectively (Fig. 2B).
TABLE 3.
Summary of Clinical Efficacy Results
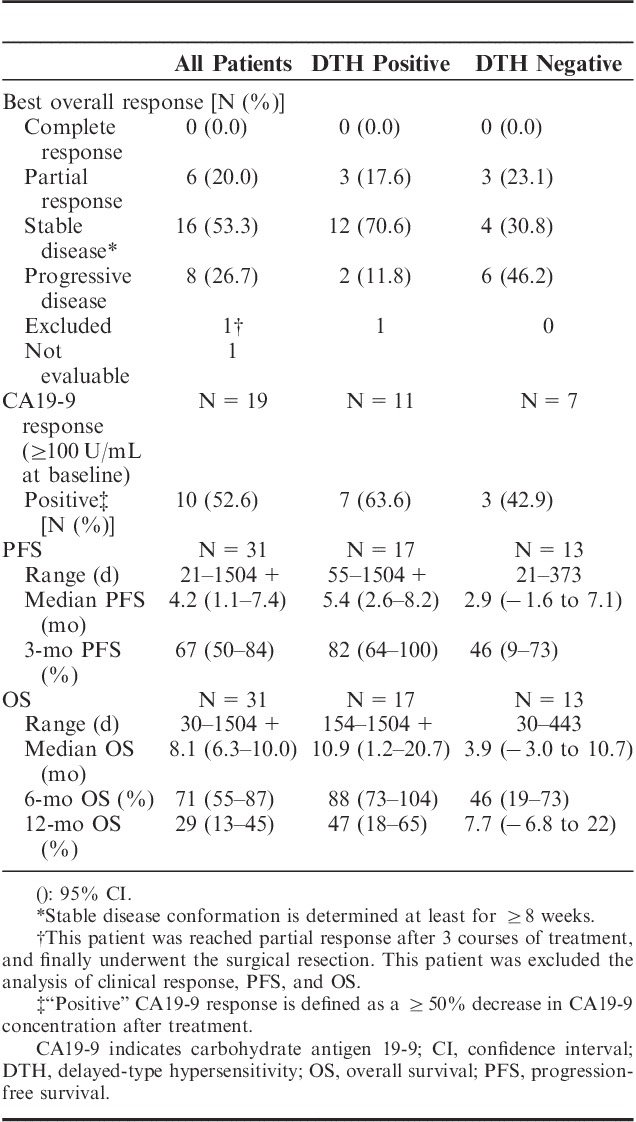
FIGURE 2.
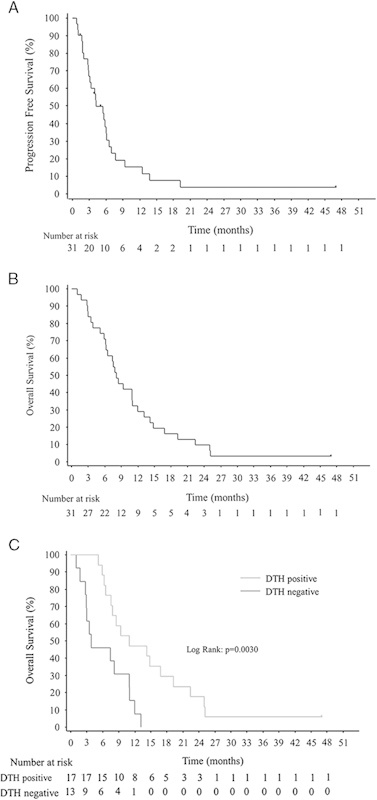
Kaplan-Meier survival curves. A, Progression-free survival (N=31). B, Overall survival (N=31). C, Overall survival in DTH-positive (gray line) or DTH-negative patients (black line). DTH indicates delayed-type hypersensitivity.
Ten of 19 patients with ≥100 U/mL of CA19-9 at baseline (52.6%) showed a decrease in CA19-9 serum concentration of at least 50% (Table 3).
Correlations between CBR and either physical or functional scores assessed with the FACT-G QOL questionnaire were analyzed. For assessment of CBR, 16 of the initial 24 patients (66.7%) could be used. Nine (56.3%) of these patients (3 with PR, 5 with SD, and 1 with progressive disease) were classified as CBR responders (data not shown). CBR responders showed improvement in physical and functional scores during the first 2 courses, whereas both scores for CBR nonresponders tended to become worse (Supplementary Figure 2, Supplmental Digital Content 2, http://links.lww.com/JIT/A318).
WT1-specific Immune Response
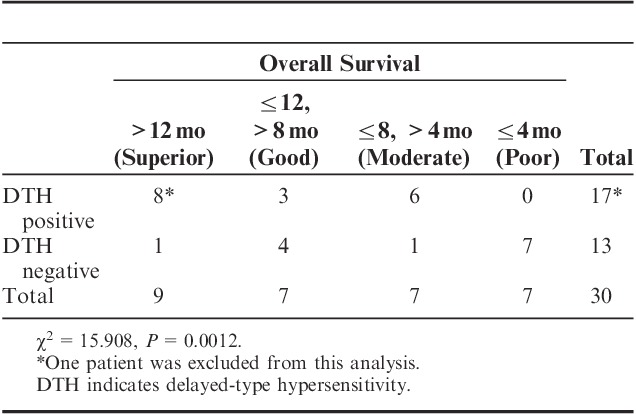
Exploratory analyses of the immune response consisted of assessment of DTH to WT1 peptide and WT1 tetramer+CD3+CD8+ T lymphocytes (WT1-CTLs) in PB of all 32 patients. All patients were DTH-negative at baseline, but 31 were at least once assessed as DTH after WT1 vaccination and 18 patients (58.1%) showed DTH-positivity, all of which conversion was detected during the protocol treatment. All of the DTH-positive patients showed at least ≥4 mm diameter of erythema, which was a length that was easy enough to measure. Next, for evaluation of associations between survival and DTH, the patients were classified into 4 groups according to survival time: Superior (>12 mo), good (8–12 mo), moderate (4–8 mo), and poor (≤4 mo) responders. These categories were based on the following findings: (i) MST for best supportive care only is no more than 3–4 months1; (ii) MST of our patients was 8.1 months; and (iii) survival time of >12 months generally indicates that the treatment has been beneficial. DTH-positivity of superior and good responders was 68.7% (11/16), whereas that of poor responders was 0% (0/7). The association between DTH-positivity and longer survival time was statistically significant (χ2=15.908, P=0.0012) (Table 4). Therefore, survival was retrospectively reanalyzed in terms of DTH-positivity or DTH-negativity. MST was 3.9 and 10.9 months for DTH-negative (N=13) and DTH-positive (N=17) patients, respectively, with a statistically significant difference (P=0.0030) (Fig. 2C and Table 3).
TABLE 4.
Association Between DTH and Survival
The number of WT1-CTLs and the percentages of naive (CD45RA+CCR7+), memory (CD45RA−CCR7+ and CD45RA−CCR7−), and effector (CD45RA+CCR7−) phenotypes in WT1-CTLs did not show any significant changes during the protocol treatment by the analysis using all patients (Supplementary Table 1, Supplemental Digital Content 3, http://links.lww.com/JIT/A319 and Supplementary Table 2, Supplemental Digital Content 4, http://links.lww.com/JIT/A320). Next, these immunologic parameters were compared between patients showing DTH-positivity and DTH-negativity. The difference in the number of WT1-CTLs was not statistically significant (Supplementary Table 1, Supplemental Digital Content 3, http://links.lww.com/JIT/A319). Phenotype analysis of WT1-CTLs showed that the percentage of naive-phenotype was higher in DTH-positive than in DTH-negative patients at baseline (Fig. 3A). After treatment, DTH-positive patients showed a significantly higher percentage of memory-phenotype and consequently a lower percentage of effector-phenotype WT1-CTLs than did their DTH-negative counterparts (Fig. 3A and Supplementary Table 2, Supplemental Digital Content 4, http://links.lww.com/JIT/A320). Furthermore, the percentage of memory-phenotype WT1-CTLs for the superior responders seemed to be relatively higher than that of effector-phenotype WT1-CTLs (Fig. 3B), whereas this tendency was quite the opposite for the poor responders (Fig. 3B and Supplementary Table 3, Supplemental Digital Content 5, http://links.lww.com/JIT/A321).
FIGURE 3.
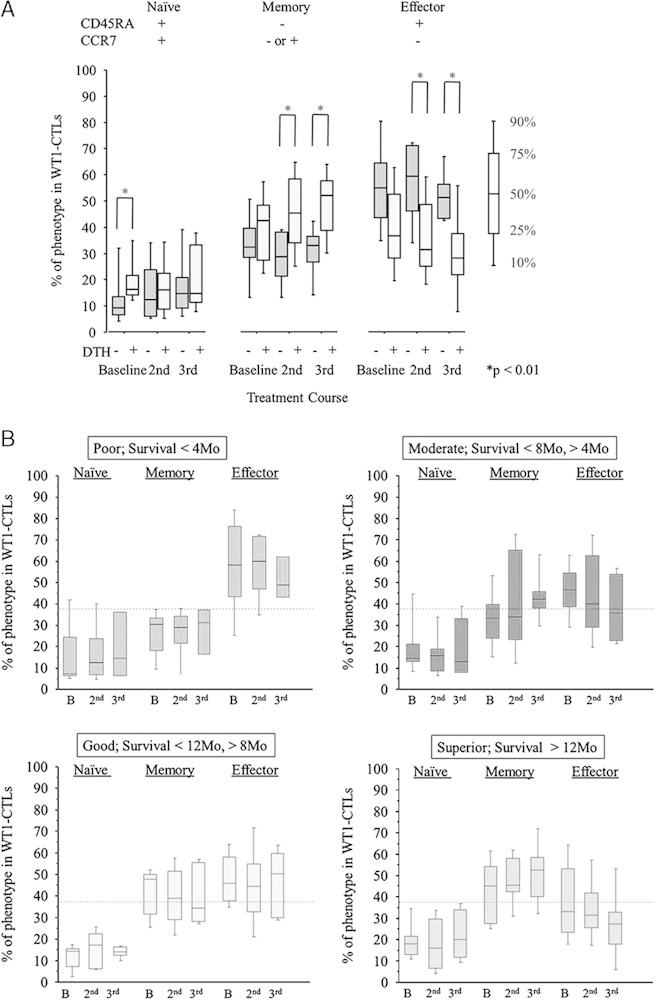
Analysis of WT1-specfic immune response. A, Immunologic monitoring of the phenotype analysis of WT1 tetramer+CD3+CD8+ T lymphocytes (WT1-CTLs) in DTH-positive (light gray columns) and DTH-negative patients (dark gray columns). B, Immunologic monitoring of the phenotype analysis of WT1 tetramer+CD3+CD8+ T lymphocytes (WT1-CTLs) in the patients of 4 groups classified according to overall survival time. The broken line represents the median percentage of memory-phenotype WT1-CTLs at baseline for all patients. WT1 tetramer=PE-conjugated WT1235 tetramer [HLA-A*24:02-restricted natural 9-mer WT1 peptide (CMTWNQMNL)], naive (CD45RA+CCR7+), memory (CD45RA-CCR7+ or CD45RA-CCR7−), and effector (CD45RA+CCR7−). 2nd indicates day 1 in the second course; 3rd, day 1 in the third course; B, baseline; DTH, delayed-type hypersensitivity.
Case Report
A 44-year-old male with a locally advanced pancreatic head cancer (T4N1M0; stage III) received WT1 vaccine in combination with gemcitabine, and achieved PR (Fig. 4A). Five months after the beginning of the treatment, this patient underwent a complete surgical resection. Histopathologic examination of the resected specimen showed an invasive ductal adenocarcinoma with mononuclear cell infiltration around the cancer region and moderate to severe fibrotic change (Fig. 4B). This patient proved to be positive for DTH to WT1 peptide after 1 treatment course (Fig. 4C). The number of WT1-CTLs transiently decreased during the first 2–3 treatment courses but subsequently increased again, while the percentage of memory-phenotype WT1-CTLs remained high during the treatment courses (Fig. 4C). Of note, the percentage of WT1-CTLs in the tumor-infiltrating CD3+CD8+ T lymphocytes was 2.48%, which was about 6 times higher than that in PB (0.39%) (Fig. 4D). This patient had been receiving monthly administration of WT1 vaccine in combination with gemcitabine for 3 years and has maintained a Karnofsky performance status of 100% with no evidence of disease recurrence.
FIGURE 4.
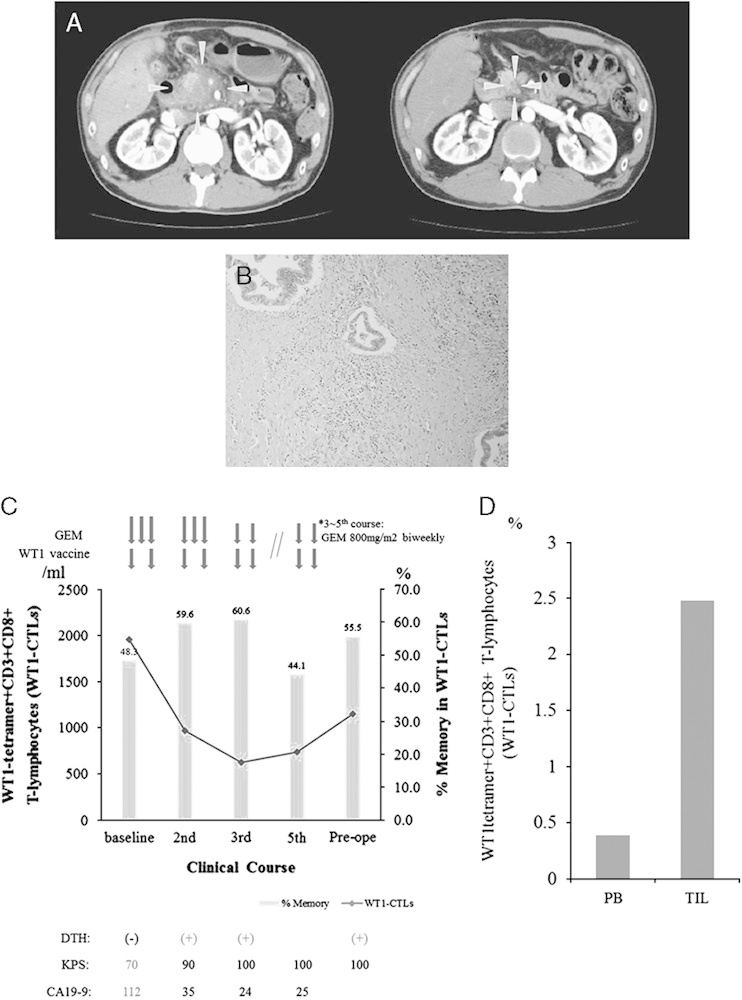
Clinical course and immunologic monitoring of 1 patient. A, Abdominal computed tomography (CT) scan before and after treatment. Left: CT scan at baseline showed a large hypodense lesion in the head of the pancreas, which had also invaded the supramesenteric artery and portal vein. Right: 5 months after treatment (before operation), a follow-up CT scan showed >80% regression of the primary lesion. Gray arrows shows primary lesion of pancreas. B, Microscopic findings of the resected specimen (hematoxylin-eosin stain). C, Clinical course and immunologic monitoring. The black line represents the absolute number of WT1 tetramer+CD3+CD8+ T lymphocytes (WT1-CTLs), and the gray column represents the percentage of memory-phenotype WT1-CTLs. D, Percentages of WT1-CTLs in the peripheral blood (PB) and tumor-infiltrating lymphocytes (TIL). CA19-9 indicates carbohydrate antigen 19-9; CTLs, cytotoxic T lymphocytes; DTH, delayed-type hypersensitivity; GEM, gemcitabine; KPS, Karnofsky performance status.
DISCUSSION
This study was designed with a DLT target rate of 10% during the first 2 treatment courses, but only one of the 21 initial evaluable patients (4.8%) actually experienced DLT. These results confirmed that WT1 vaccine in combination with gemcitabine is acceptable for patients with advanced pancreatic cancer. Cerebrovascular ischemia, reported here as a DLT, could be also caused by pancreatic cancer itself and/or the administration of gemcitabine, both of which are sometimes associated with a high risk of developing thrombotic disease.28,29 Therefore, this adverse event was considered to be multifactorial and judged to be “possibly” related to treatment.
Except for skin reactions at the local injection sites, the toxicity profiles of WT1 vaccine in combination with gemcitabine were consistently similar to those of gemcitabine alone. As the WT1 gene is physiologically expressed in hematopoietic progenitor cells,13 damage to hematopoiesis is one of the major concerns in WT1-peptide–based immunotherapy. The incidence of hematological adverse events in our study, however, was similar to that observed for treatment with gemcitabine alone,30 and these events were easily managed and reversible. These findings suggest that WT1 vaccine does not synergistically intensify hematological adverse events associated with gemcitabine. It seems unlikely that WT1-specific CTLs elicited by WT1 vaccine might damage normal WT1-expressing hematopoietic progenitor cells as well as WT-expressing tumor cells, as following reasons. First, in the previous clinical studies, we and others reported that WT1-specifc CTLs elicited by WT1 vaccine decreased WT1-expressing leukemia cells and suppressed the disease progression of WT-expression cancer cells, but not significantly damaged normal hematopoiesis.19,23–26 Second, it was demonstrated that, using mice in vivo experiments, WT1-targetting immunotherapy gave damage to tumor cells, but not WT1-expressing normal tissue, including hematopoietic cells.31,32 The reason why the normal WT1-expressing hematopoietic cells are able to escape from the attack by WT1-specific CTLs is not well known. Further investigations should be required to address this issue.
The clinical efficacy of treatment with WT1 vaccine in combination with gemcitabine, especially in terms of survival, seemed to be better than of that with gemcitabine alone.1,2 About half of patients who had been induced WT1-specific immunity after vaccination showed better clinical outcome with 12 months or longer survival time, suggesting additional or synergistic effects of WT1 vaccine in combination with gemcitabine. Furthermore, the former contributed to pain relief and thus to improvement of QOL. Recently, the result of the phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer (GEST study) conducted in Japan and Taiwan between 2007 and 2009 has been reported.33 Median OS and OS rate at 12 months in the gemcitabine alone group were 8.8 months and 35.4%, respectively. These results seemed a little better than those in our study. One reason for this may be the difference in the proportion of the patients with the locally advanced pancreatic cancer, in which survival data were apparently much better than those in metastatic ones. In our study, this proportion was 18.8%, which was lower than that in GEST study (23.8%). The other reason may be PS at baseline, which was also one of the important prognostic factors. The proportion of the patients with ECOG-PS 0, 1, and 2 at baseline in our study were 46.9%, 31.3%, and 21.9%, respectively, whereas those in GEST study were 65.3%, 34.7%, and 0.0%, respectively. It is apparent that our patients are predicted to worse prognosis than those in GEST study. Despite lower proportion of locally advanced stage and worse PS, however, the survival data gained from the patients with DTH-positivity seemed to be better than those in GEST study. These results suggested additional or synergistic effects of WT1 vaccine. Although the number of patients in our present study was too small to reach any definitive conclusions about clinical efficacy, these findings have been sufficiently encouraging to prompt us to conduct a further clinical study to determine the potency of this combination therapy. No combination chemotherapy, with the exception of FOLFIRINOX,34 has resulted in a significant improvement in survival of patients with pancreatic cancer although some combination therapies are thought to be more effective for several cancers than single-agent treatments.1 The use of FOLFIRINOX, however, may have to be limited to patients with good performance status as this regimen has much higher toxicity that sometimes can impair QOL.34,35 In contrast, as toxicities associated with cancer vaccines are generally mild and acceptable, combination therapies using chemotherapy and cancer vaccine can be expected to exert their clinical benefits without worsening of QOL, which is often impaired by combination chemotherapies using several kinds of cytotoxic agents.
Immunologic monitoring is an important step in the development of evidence-based immunotherapy. Our data provided 2 useful prognostic markers of better clinical outcomes for the combination therapy used in our study. One is DTH to WT1 peptide and the other the frequency of memory-phenotype WT1-CTLs in PB although we did not find the correlation between clinical effects, including survival, and the frequency or absolute numbers of nonphenotypically divided WT1-specific CTLs statistically (data not shown). DTH-positive patients had a notably better prognosis than DTH-negative patients, and the OS curve for DTH-positive patients showed a late separation beyond the median. As DTH has long been used for evaluation of antigen memory for bacterial, viral, and cancer antigens,36 the occurrence of DTH to WT1 peptide may reflect the development and persistence of memory-phenotype WT1-CTLs. This can be inferred from our observation that DTH-positive patients showed a significantly higher frequency of memory-phenotype WT1-CTLs than did DTH-negative patients after WT1 vaccination. Furthermore, patients who survived 12 months or longer (superior responders) seemed to have the highest frequency of memory-phenotype WT1-CTLs in their PB although the number of patients in each subgroup was too small to make a statistically valid comparison. It was reported that long-term survivors who had been treated with mutant K-ras vaccine against pancreatic cancer showed the persistence of vaccinated peptide-recognizing T cells (long-term T-cell memory response) for many years after the last vaccination.37 This report and our results suggest that the development and persistence of TAA-specific CTLs with memory-phenotype resulting from treatment with cancer vaccine contributed to the longer survival. Further investigations are needed to validate these findings in the larger-scale clinical trial.
Despite its potent cytotoxicity, gemcitabine reportedly has immune-modulating functions, such as increase in antigen cross-presentation,38 and inhibition of B-cells,39 myeloid-derived suppressive cells,40 and regulatory T cells,41 resulting in enhancement of the antigen-specific CTL function. Recently, we reported that gemcitabine enhanced the WT1 expression on human pancreatic cancer cells thus sensitizing the cancer cells to WT1-specific CTL.11 Furthermore, it was reported that lymphopenia-induced memory-phenotype WT1-CTLs from naive-phenotype WT1-CTLs without self-antigen-induced tolerance.42 Transient mild to moderate lymphopenia induced by gemcitabine and immediate recovery of T cells could thus promote both the differentiation of naive-phenotype WT1-CTLs into memory-phenotype WT1-CTLs and their proliferation in the clinical application of the combination therapy of gemcitabine and WT1 vaccine. In view of these immunostimulatory properties of gemcitabine, this combination therapy can be expected to generate additional or synergistic effects.
In conclusion, the combination of WT1 vaccine with the standard gemcitabine therapy was well tolerated for patients with advanced pancreatic cancer. WT1 vaccine might have additional effects on gemcitabine to improve survival benefit. An increase in memory-phenotype WT1-CTLs could be a useful predictive marker for a favorable clinical outcome. To determine the clinical efficacy of this combination therapy, we have started a phase 2 randomized clinical study (UMIN000005248).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the member of the nursing team for their care for the patients in this trial, Hiroko Nakajima, and Fumihiro Fujiki for their technical supports, Tomoe Umeda for her coordination of clinical research, Takushi Okusaka and Yuji Heike (National Cancer Center in Japan) for their advices about the planning of this study.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
This study was supported by the Japanese Ministries of Education, Culture, Sports, Science and Technology, and of Health, Labor and Welfare; the National Cancer Center Research and Development Fund; the Takeda Science Foundation; and Mitsui Life Social Welfare Foundation, Promotion of Cancer Research.
All authors have declared there are no financial conflicts of interest with regard to this work.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.immunotherapy-journal.com.
S.N. and S.K. contributed equally.
Trial registration ID: UMIN000001187.
REFERENCES
- 1.Stathis A, Moore MJ.Advanced pancreatic carcinoma: current treatment and future challenges.Nat Rev Clin Oncol. 2010;7:163–172. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial.J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group.J Clin Oncol. 2007;25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 4.Disis ML, Bernhard H, Jaffee EM.Use of tumour-responsive T cells as cancer treatment.Lancet. 2009;373:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellman I, Coukos G, Dranoff G.Cancer immunotherapy comes of age.Nature. 2011;480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Restifo NP.Cancer immunotherapy: moving beyond current vaccines.Nat Med. 2004;10:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxevanis CN, Perez SA, Papamichail M.Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy.Cancer Immunol Immunother. 2009;58:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak AK, Lake RA, Robinson BWS.Combined chemoimmunotherapy of solid tumours: improving vaccines?Adv Drug Deliv Rev. 2006;58:975–990. [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy.Nat Rev Immunol. 2008;8:59–73. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice.J Clin Invest. 2010;120:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahara A, Koido S, Ito M, et al. Gemcitabine enhances Wilms’ tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response.Cancer Immunol Immunother. 2011;60:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huff V.Wilms’ tumours: about tumour suppressor genes, an oncogene and a chameleon gene.Nature Rev Cancer. 2011;11:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiyama H.WT1 (Wilms’ tumor gene 1): biology and cancer immunotherapy.Jpn J Clin Oncol. 2010;40:377–387. [DOI] [PubMed] [Google Scholar]
- 14.Elisseeva OA, Oka Y, Tsuboi A, et al. Humoral immune responses against Wilms’ tumor gene WT1 product in patients with hematopoietic malignancies.Blood. 2002;99:3272–3279. [DOI] [PubMed] [Google Scholar]
- 15.Gaiger A, Carter L, Greinix H, et al. WT1-specific serum antibodies in patients with leukemia.Clin Cancer Res. 2001;7:761s–765s. [PubMed] [Google Scholar]
- 16.Oji Y, Kitamura Y, Kamino E, et al. WT1 IgG antibody for early detection of nonsmall cell lung cancer and as its prognostic factor.Int J Cancer. 2009;125:381–387. [DOI] [PubMed] [Google Scholar]
- 17.Oka Y, Elisseeva OA, Tsuboi A, et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms’ tumor gene (WT1) product.Immunogenetics. 2000;51:99–107. [DOI] [PubMed] [Google Scholar]
- 18.Rezvani K, Brenchley JM, Price DA, et al. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms’ tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization.Clin Cancer Res. 2005;11:8799–8807. [DOI] [PubMed] [Google Scholar]
- 19.Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression.Proc Natl Acad Sci USA. 2004;101:13885–13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita S, Oka Y, Tsuboi A, et al. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data.Jpn J Clin Oncol. 2006;36:231–236. [DOI] [PubMed] [Google Scholar]
- 21.Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme.J Neurosurg. 2008;108:963–971. [DOI] [PubMed] [Google Scholar]
- 22.Miyatake T, Ueda Y, Morimoto A, et al. WT1 peptide immunotherapy for gynecologic malignancies resistant to conventional therapies: a phase II trial.J Cancer Res Clin Oncol. 2013;139:457–463. [DOI] [PubMed] [Google Scholar]
- 23.Tsuboi A, Oka Y, Kyo T, et al. Long-term WT1 peptide vaccination for patients with acute myeloid leukemia with minimal residual disease.Leukemia. 2012;26:1410–1413. [DOI] [PubMed] [Google Scholar]
- 24.Hashii Y, Sato-Miyashita E, Matsumura R, et al. WT1 peptide vaccination following allogeneic stem cell transplantation in pediatric leukemic patients with high risk for relapse: successful maintenance of durable remission.Leukemia. 2012;26:530–532. [DOI] [PubMed] [Google Scholar]
- 25.Keilholz U, Letsch A, Busse A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS.Blood. 2009;113:6541–6548. [DOI] [PubMed] [Google Scholar]
- 26.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies.Blood. 2008;111:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure.J Clin Oncol. 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 28.Khorana AA, Fine RL.Pancreatic cancer and thromboembolic disease.Lancet Oncol. 2004;5:655–663. [DOI] [PubMed] [Google Scholar]
- 29.Otten HM, Mathijssen J, ten Cate H, et al. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: an underestimated phenomenon.Arch Intern Med. 2004;164:190–194. [DOI] [PubMed] [Google Scholar]
- 30.Okada S, Ueno H, Okusaka T, et al. Phase I trial of gemcitabine in patients with advanced pancreatic cancer.Jpn J Clin Oncol. 2001;31:7–12. [DOI] [PubMed] [Google Scholar]
- 31.Gaiger A, Reese V, Disis ML, et al. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia.Blood. 2000;96:1480–1489. [PubMed] [Google Scholar]
- 32.Gao L, Bellantuono I, Elsasser A, et al. Selective elimination of leukemic CD34+ progenitor cells by cytotoxic T lymphocytes specific for WT1.Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 33.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study.J Clin Oncol. 2013;31:1640–1648. [DOI] [PubMed] [Google Scholar]
- 34.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer.N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 35.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial.J Clin Oncol. 2013;31:23–29. [DOI] [PubMed] [Google Scholar]
- 36.Disis ML.Immunologic biomarkers as correlates of clinical response to cancer immunotherapy.Cancer Immunol Immunother. 2011;60:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedén S, Klemp M, Gladhaug IP, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras.Int J Cancer. 2011;128:1120–1128. [DOI] [PubMed] [Google Scholar]
- 38.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells.J Immunol. 2003;170:4905–4913. [DOI] [PubMed] [Google Scholar]
- 39.Nowak AK, Robinson BWS, Lake RA.Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy.Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 40.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity.Clin Cancer Res. 2005;11:6713–6721. [DOI] [PubMed] [Google Scholar]
- 41.Rettig L, Seidenberg S, Parvanova I, et al. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells.Int J Cancer. 2010;129:832–838. [DOI] [PubMed] [Google Scholar]
- 42.Pospori C, Xue SA, Holler A, et al. Specificity for the tumor-associated self-antigen WT1 drives the development of fully functional memory T cells in the absence of vaccination.Blood. 2011;117:6813–6824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


