Abstract
Purpose of review
Depression in pediatric inflammatory bowel disease (IBD) is increasingly recognized to be a heterogeneous condition with diverse underlying predisposing and precipitating factors. Although there is a growing awareness regarding the benefits of integrating behavioral health into medical care, the way psychiatric treatments can best target different aspects of depression and related dysfunction has not been systematically explored.
Recent findings
This review discusses neurobiological risk factors for depression in IBD including inflammation, associated anti-inflammatory treatment with corticosteroids, pain, and sleep disturbance, as well as psychosocial factors including reactions to illness, illness perception, and disease and environmental stressors with emphasis of how these factors can influence treatment decisions. Empirically-supported psychosocial and psychopharmacological interventions are discussed within this context.
Summary
Understanding the diverse pathways that can lead to depression in youth with IBD can lead to the development of more targeted interventions and better integration of psychosocial care into the medical treatment of IBD.
Keywords: depression, inflammatory bowel disease, psychotherapy, antidepressants
Introduction
Youth with inflammatory bowel disease (IBD) have high rates of depression and a higher risk of developing depression in comparison to community controls and even those with other chronic diseases (i.e. cystic fibrosis, diabetes, chronic headache, or cancer. (1, 2)) Crohn’s disease (CD) and ulcerative colitis (UC), the most common subtypes, are lifelong debilitating conditions resulting from chronic and episodic worsening of gastrointestinal inflammation. Symptoms include abdominal pain, fever, fatigue, weight loss, diarrhea, and bloody stools.(3) With chronic disease activity, osteopenia, skin and joint disease, growth retardation and pubertal delay are possible physical manifestations. Psychosocial consequences can include social and academic challenges often related to the relapsing physical course. (4) Currently there is no overall cure for IBD, though colectomy can be curative for UC. Treatment includes immunosuppressive agents, biologics, and for refractory disease, surgery.(5)
The pediatric IBD population is of special clinical interest as approximately a quarter of patients with IBD are diagnosed before age 20 years with peak onset in adolescence.(6) This developmental period is one of particular psychiatric vulnerability; it represents a time of brain maturation and neuroplasticity as well as development of behavioral repertoires which may lend themselves to behavioral intervention.(7) This review will focus on neurobiological and psychological risk factors associated with depression in pediatric IBD and consideration of varying underlying etiologies to guide treatment options. The term youth will be used to encompass both children and adolescents.
Diagnosis of Depression
Diagnosis of major depression is based on criteria for a threshold number of depressive symptoms occurring for at least two weeks and associated marked functional impairment.(8) In patients with IBD, functional impairment includes adherence to medical treatment.(9) Depressive phenotypes of individual patients can be quite heterogeneous; however, distinct clusters of depressive symptoms have been identified.(10, 11) In patients with IBD, such depressive subtypes can have many different etiological underpinnings,(12) In a recent study, we conducted latent profile analysis to evaluate the relationship between systemic inflammation and depressive symptom profiles in 226 depressed youth with IBD.(13) Three distinct depressive profiles were identified: (1) youth with diverse milder depressive symptoms in the relative absence of systemic inflammation (75%); (2) youth with (13) high levels of somatic depressive symptoms in the presence of elevated inflammation (19%); and (3) youth with high levels of cognitive despair, including suicidal ideation, with relatively low inflammation (6%). These clusters of depressive symptoms may have unique etiological underpinnings including neurobiological effects of inflammation, gastrointestinal symptoms (e.g., abdominal pain), medication side effects and/or a psychological reaction to a new diagnosis, hospital admission, poor disease course, or other life stressor.(12)
Neurobiological factors
Neurobiological risk factors associated with depression in this population include IBD-related inflammation, abdominal pain, and sleep disturbance.(14, 15) The relationship between inflammation in pediatric IBD patients and depression is supported by growing evidence for a relationship between inflammation and depression in other pediatric samples.(16) In pediatric IBD, somatic depressive symptoms (e.g., fatigue, sleep disturbance) have most correlated with IBD activity.(13) From a phylogenetic survival perspective, these depressive symptoms may play a protective role during threat states such as severe acute IBD flares by shunting energy resources from the brain to the gastrointestinal tract to facilitate healing.(17) Thus, in some patients, rest and medical management of their IBD may sufficiently facilitate improvement in depressive symptoms. In adults with IBD, reduced IBD activity and tumor necrosis factor (TNF)-alpha after infliximab treatment for their IBD had the added benefit of improving depression.(18) In screening 765 youth (ages 9–27) with IBD for depression, those on anti-TNF agent infliximab were found to have less severe depression than those not on infliximab.(19) In contrast, a prediction model for depression demonstrated IBD activity and socioeconomic class, but not infliximab use, as significant predictors of depressive severity. These results suggest that youth with IBD and depression may benefit from more than just aggressive medical treatment, even during active disease.
Abdominal pain commonly co-occurs with depression as seen in pediatric studies. (20–23)It is often unrelated to the degree of IBD inflammation and termed “functional” abdominal pain. From a clinical perspective, depressive coping style is more predictive than medical variables for disease-related concerns (including reports of abdominal pain) providing insight into mechanisms of depression leading to abdominal pain.(24) Furthermore, psychological dysfunction is known to amplify symptom severity, particularly abdominal pain perception in adults with IBD and functional pain syndromes.(25–27) The direction of this relationship needs elucidation. If depression does indeed contribute to the development of abdominal pain, then treatment targeting depression may be first approach to managing pain. If depression results from chronic abdominal pain, it may be practical to target pain first.
Sleep disturbance is also highly correlated with depression both in the presence and absence of inflammation in both children and adults.(28, 29) Sleep disturbance includes difficulty falling asleep, disruption in sleep continuity, early morning awakenings, or hypersomnia in the absence of feeling refreshed. Poor sleep is linked to worsening mental and physical health, particularly immune dysregulation. In screening depressed youth with IBD for sleep disturbance using the Pittsburgh Sleep Quality Index (PSQI), 42% met the threshold score for sleep disturbance. In depressed youth with CD, sleep disturbance was significantly greater than in healthy controls. A correlation between sleep disruption and disease severity was also noted, however, sleep disturbance was still present in those with inactive disease. In remission, depression was more related to sleep disturbance than in active disease, during which inflammatory biomarkers were more related. Anxiety and pain predicted subjective aspects of sleep disturbance. In adults, the PSQI was highly predictive of histological inflammatory activity,(30) suggesting that sleep disturbance is an early sign of inflammation and thus a possible indicator for IBD treatment. The difference in sleep disturbance among different IBD groups may suggest that treatment options can be targeted based on severity of disease.
Other often under-recognized neurobiological contributors to depressive symptoms are potential side effects from IBD pharmacotherapy. For example, corticosteroids have been linked to irritability, inattention and reduction in sleep time. (31–33) Youth with IBD treated with at least 20 mg per day prednisone equivalent for at least one week had greater depressive symptoms, attention and memory difficulties, and sleep disturbance than controls not on corticosteroids.(31) In longitudinal follow-up, mood and attentional symptoms improved with steroid taper however memory problems persisted.(34) These results are consistent with recent findings showing that adolescents with acute IBD had significantly higher incidence of mild verbal memory problems but not major cognitive deficits in comparison to adolescents with juvenile idiopathic arthritis. The presence of mild verbal memory problems did not correlate with depressive symptoms.(35)
Psychosocial Factors
Reactive depressive symptoms are also important to consider as predisposing factors to depression due to IBD-related circumstances (e.g., new IBD diagnosis, ostomy persistence of pain) or life stress. These factors can occur in the presence or absence of active disease.(36) Descriptive studies examining the impact of IBD on everyday function of adolescents identified themes of discomfort from symptoms and treatment, diminished control over life and future, embarrassment, and perception of being different from healthy peers.(37–39)
In our person-centered analyses of depressive subtypes, 6% of depressed youth with IBD manifested hopelessness, suicidal ideation, and weeping.(13) This group had a greater number of ostomies and longest IBD duration, as well as pain in the relative absence of inflammation. Suicidal ideation has been understudied in pediatric IBD. In our depressed cohort, 22% of youth with IBD reported some degree of suicidality on the Children’s Depression Inventory (Szigethy, unpublished observations), with only small correlation with inflammatory biomarkers.
Growing evidence suggests a relationship between IBD health status, psychological outcomes (anxiety, depression) and illness perception and coping in adults with IBD.(40) Adult IBD patients with ostomies showed elevated depression and anxiety with illness perceptions partially mediating the relationship between health status and psychological symptoms.(41) In children with IBD, high rates of illness-related anxiety have been reported (42), and depressive severity was more significantly associated with negative illness perceptions than IBD activity in adolescents with IBD.(43) Finally, cognitive processes such as catastrophizing (negative prediction of the future) and rumination are common in depression and have also been implicated in worse pain perception thus also presenting an opportunity for intervention.(44, 45)
Treatment considerations
Neurobiological and psychosocial factors do not occur in isolation thus it is important to consider all sources of stress (external and internal) that could drive or maintain the depression in treatment-related decision-making (Figure 1).
Figure 1.
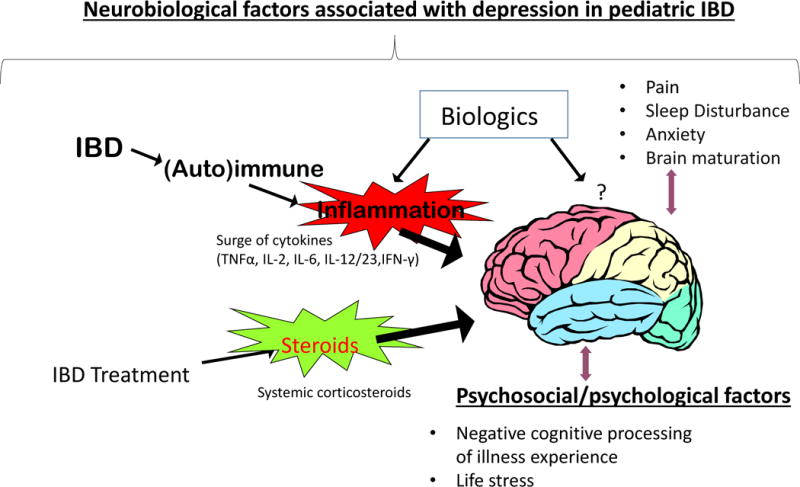
Neurobiological and psychosocial factors that could lead to depression in youth with inflammatory bowel disease (original). Courtesy by C. Mrakotsky
Psychosocial Assessment
IBD is a psychosocially challenging disease and listening to patients’ illness narrative is important.(36) In addition to a thorough medical history, incorporating neurobiological and psychological factors in evaluating youth with IBD is critical. Whereas specific instruments to evaluate emotional distress in patients with IBD are available, not all are openly accessible.(32, 46) Health related quality of life (HRQoL) instruments are becoming more broadly available and correlate with emotional distress.(47, 48) Lower HRQoL was a good predictor of healthcare utilization in the IBD pediatric population; therefore, quality of life screening may be beneficial for efficient resource allocation by clinicians.(49) Evaluation of family factors such as relationships, caregiver stress, and social support can elucidate the role of environmental factors in maintaining depression.
Psychosocial interventions
Gastroenterology providers are first-line agents in identifying and treating depression by offering empathy and education to patients and their families. Encouraging participation in social activities in patients’ school and community settings, including IBD specific support groups and summer camps can provide respite.(36, 50) For more severe or persistent depressive symptoms, however, involvement of behaviorally trained mental health providers (e.g. social workers, psychologists, psychiatrists) is critical.
In most studies of adults with IBD, treatment with psychotherapy has been associated with improved depression and anxiety.(51, 52) In children and adolescents with IBD, treating subsyndromal depressive symptoms with cognitive behavioral therapy (CBT) correlated with significantly greater improvement of overall depressive severity as well as somatic symptoms compared to medical treatment as usual.(53, 54) CBT is a brief, interactive therapy modality based upon the assumption that emotions, behaviors and thoughts are interconnected thus targeting the latter two domains can improve mood. In a longitudinal study assessing the effects of CBT on pediatric IBD patients with a 12 month follow-up, there was significant improvement in self-reported depressive severity and global psychosocial functioning in youths randomized to CBT.(55) For these studies, the CBT model, originally for depression treatment (48), was made feasible for this physically ill population through several modifications: 1) Illness perceptions were identified and targeted by the intervention with an emphasis on improving a sense of control; 2) IBD-related maladaptive behaviors such as medical non-adherence were addressed; 3) Sessions with parents or caretakers were incorporated into individual child therapy sessions in developmentally appropriate ways; and 4) the intervention was accessible with phone sessions and/or face-to-face sessions coordinated with medical care.(56)
Recent findings of another randomized trial comparing cognitive behavioral therapy to supportive therapy for depression in youth (ages 9–17) with IBD showed that although both treatments were associated with improved depression over time, CBT was associated with greater reduction of IBD activity, suggesting that psychotherapy is a useful adjunct to medical management.(57) Similar CBT approaches have also been shown to improve pain and sleep disturbances in other pediatric populations.(58, 59) Additional promising psychosocial interventions for youth with IBD include hypnotherapy(60) and mindfulness-based techniques(61) which both can improve mood, pain and HRQoL.
While the field works toward the development of clinical guidelines for integrated psychosocial care in pediatric IBD, stress management and coping strategies, disease self-management training, and wellness/life style coaching (e.g., sleep hygiene, nutrition, exercise) may help prevent depression and increase resilience in all youth with IBD.(62, 63)
Role of pharmacotherapy
It is important to note that there have been no randomized controlled trials of any antidepressant for children and adolescents with IBD, thus use of these medications in this population is off-label and based on clinical opinion, reviews and case reports in adults.(64, 65) Further, long-term effects of these medications have also not been studied. Thus, particularly in pediatric patients, treating depression, pain and sleep disturbance using psychosocial interventions as first line and pharmacological treatment only if needed is recommended. In adults with IBD, the use of antidepressants has been associated with favorable effects such as reduced relapse rates, steroid use, and endoscopies. In pediatric IBD, serotonin reuptake inhibitors (SSRIs) used concomitantly with psychotherapy have shown positive effects for depression and anxiety.(66) In adults with CD, bupropion, a dopaminergic/noradrenergic reuptake blocker and TNF-alpha inhibitor, has shown to improve depression and inflammation (67, 68) and has also been effective in patients with severe fatigue in other inflammatory diseases (39–42). Both SSRIs and bupropion are relatively well-tolerated in other pediatric cohorts with depression and medically ill adults (69–72) but placebo response can also be present. Many antidepressants have been linked to gastrointestinal side effects (e.g., nausea, pain, diarrhea, and risk of gastrointestinal bleeding) that need to be particularly monitored.(73, 74) For depression comorbid with abdominal pain, several non-opioid medications have shown positive effects, if behavioral interventions have failed.(14, 75) In particular, noradrenergic agents such as tricyclic antidepressants have been found to be effective for pain and other residual symptoms of IBD in adults, but findings are more mixed for children.(76, 77) As is the case for most psychotropic medications, there have been reports of associated increased suicidal ideation. Despite lack of a clear causal relationship, this serious symptom needs to be regularly monitored.(78, 79)
Finally, one often overlooked aspect of treating depression in the context of severe gastrointestinal inflammation or bowel resection is oral antidepressant absorption. Though not studied in pediatric IBD, therapeutic doses may need to be adjusted or use of psychotropic medications via dermal patch or intravenous administration considered.
Conclusions
Depression has been associated with a more challenging course of IBD as well as medical non-adherence and psychosocial dysfunction, making a diagnosis, treatment, and prevention primary objectives in comprehensive medical care for IBD in pediatric patients. While in some patients depressive symptoms improve with aggressive medical treatment of underlying IBD activity, for others with functionally impairing or persistent depression, concomitant behavioral interventions or symptom-specific medication may be indicated. It is important to note that not all youth with IBD become depressed, particularly during IBD remission.(2, 80) Future research needs to address management of diverse depressive phenotypes and underlying mechanisms as well as potential differences in depressive presentation in CD versus UC.(15) For example, the role of the hypothalamic pituitary adrenal (HPA) axis, autonomic nervous system dysfunction, genetic factors, early childhood adversity and their interactions as predisposing and precipitating factors for depression need to be better understood.(81, 82) Thus, although many areas are not yet explored, the current literature supports depression as a viable therapeutic target in pediatric IBD patients. Comprehensive evaluation of both neurobiological and psychosocial factors associated with depression will facilitate interventions to improve health-related quality of life.
Key points.
Youth with inflammatory bowel disease (IBD) are at increased risk of depression compared to other pediatric populations.
Depression in IBD patients can be associated with neurobiological factors (e.g. inflammation, abdominal pain, sleep disturbance) and psychological/psychosocial factors (e.g. reaction to illness, illness perception, disease and environmental stressors).
Improvement in the IBD illness experience and quality of life may be possible through identification and targeted treatment of factors linked to depression.
Pharmacotherapy towards depression is understudied in pediatric IBD and psychosocial interventions may be an effective and safer treatment option.
Acknowledgments
Work cited by the authors were partially funded by grants NIH R01 MH077770 (Dr. Szigethy) and NIH K23 HD058466 (Dr. Mrakotsky). The authors would like to thank Dr. Arvind Srinath for his critical review.
Funding: National Institute of Mental Health (R01 MH077770, Dr. Szigethy); National Institute for Child Health and Human Development (K23 HD058466, Dr. Mrakotsky)
Footnotes
In addition, Dr. Szigethy’s potential conflict of interest relative to this work includes being an editor for a book on cognitive behavioral therapy for children and adolescents for which she receives royalties and a grant to study sleep from the Crohn’s and Colitis Foundation of America. She is also served as a consultant for a Merck Advisory board. Ms. Keethy has no conflicts of interest to report.
References
- 1.Burke P, Meyer V, Kocoshis S, Orenstein DM, Chandra R, Nord DJ, et al. Depression and anxiety in pediatric inflammatory bowel disease and cystic fibrosis. J Am Acad Child Adolesc Psychiatry. 1989;28(6):948–51. doi: 10.1097/00004583-198911000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Greenley RN, Hommel KA, Nebel J, Raboin T, Li SH, Simpson P, et al. A meta-analytic review of the psychosocial adjustment of youth with inflammatory bowel disease. J Pediatr Psychol. 2010;35(8):857–69. doi: 10.1093/jpepsy/jsp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regueiro MD, Swoger JM. Clinical Challenges and Complications of IBD. Thorofare, NJ: SLACK Inc; 2013. [Google Scholar]
- 4*.Mackner L, Greenley RN, Szigethy E. A Clinical Report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Ped Gastro Hep Nutr. 2013 doi: 10.1097/MPG.0b013e3182841263. This is the first comprehensive clinical report to review research on psychosocial functioning in pediatric inflammatory bowel disease and to provide treatment recommendations to gastroenterologists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kappelman MD, Palmer L, Boyle BM, Rubin DT. Quality of care in inflammatory bowel disease: a review and discussion. Inflammatory bowel diseases. 2010;16(1):125–33. doi: 10.1002/ibd.21028. [DOI] [PubMed] [Google Scholar]
- 6.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflammatory bowel diseases. 2011;17(1):423–39. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 7.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Translational psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric A, American Psychiatric Association. Task Force on D-I. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 9*.Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment adherence in adolescents with inflammatory bowel disease: the collective impact of barriers to adherence and anxiety/depressive symptoms. J Pediatr Psychol. 2012;37(3):282–91. doi: 10.1093/jpepsy/jsr092. First study to show anxiety and depressive symptoms moderate the relationship between barriers to medical adherence and adherence in adolescents with inflammatory bowel disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamers F, Burstein M, He JP, Avenevoli S, Angst J, Merikangas KR. Structure of major depressive disorder in adolescents and adults in the US general population. Br J Psychiatry. 2012;201:143–50. doi: 10.1192/bjp.bp.111.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RD, Craig AE, Mrakotsky C, Bousvaros A, Demaso DR, Szigethy E. Using the Children’s Depression Inventory in youth with inflammatory bowel disease: support for a physical illness-related factor. Compr Psychiatry. 2012 doi: 10.1016/j.comppsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benhayon D, Szigethy EM. Psychiatric Complications of Inflammatory Bowel Disease. In: Incorporated S, editor. Clinical Challenges and Complications of IBD. 2012. [Google Scholar]
- 13**.Szigethy EM, Youk AO, Benhayon D, Fairclough DL, Newara MC, Kirshner MA, et al. Depression subtypes in pediatric inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2014;58(5):574–81. doi: 10.1097/MPG.0000000000000262. First study to evaluate depression subtypes using patient-centered analysis and show predictors of subtype membership by various clinical variables such as inflammation, steroid use, and IBD-related complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinath AI, Walter C, Newara MC, Szigethy EM. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap Adv Gastroenterol. 2012;5(5):339–57. doi: 10.1177/1756283X12446158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donovan A. Inflammation and depression: unraveling the complex interplay in inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2014;58(5):541–2. doi: 10.1097/MPG.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 16.Rao U. Biomarkers in pediatric depression. Depression and anxiety. 2013;30(9):787–91. doi: 10.1002/da.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Molecular psychiatry. 2013;18(1):15–37. doi: 10.1038/mp.2012.2. An important review of studies on risk alleles for depression to support the contention that these alleles were retained to promote pathogen host defense in the face of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 19.Clark JG, Srinath AI, Youk AO, Kirshner MA, McCarthy FN, Keljo DJ, et al. Predictors of depression in youth with crohn disease. Journal of pediatric gastroenterology and nutrition. 2014;58(5):569–73. doi: 10.1097/MPG.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman LA, Srinath AI, Goyal A, Bousvaros A, Ducharme P, Szigethy E, et al. The overlap of functional abdominal pain in pediatric Crohn’s disease. Inflammatory bowel diseases. 2013;19(4):826–31. doi: 10.1097/MIB.0b013e3182802a0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinath AI, Goyal A, Zimmerman LA, Newara MC, Kirshner MA, McCarthy FN, et al. Predictors of abdominal pain in depressed pediatric inflammatory bowel disease patients. Inflammatory bowel diseases. 2014 doi: 10.1097/MIB.0000000000000104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piche T, Ducrotte P, Sabate JM, Coffin B, Zerbib F, Dapoigny M, et al. Impact of functional bowel symptoms on quality of life and fatigue in quiescent Crohn disease and irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(6):626–e174. doi: 10.1111/j.1365-2982.2010.01502.x. [DOI] [PubMed] [Google Scholar]
- 23.Bryant RV, van Langenberg DR, Holtmann GJ, Andrews JM. Functional gastrointestinal disorders in inflammatory bowel disease: impact on quality of life and psychological status. Journal of gastroenterology and hepatology. 2011;26(5):916–23. doi: 10.1111/j.1440-1746.2011.06624.x. [DOI] [PubMed] [Google Scholar]
- 24.Mussell M, Bocker U, Nagel N, Singer MV. Predictors of disease-related concerns and other aspects of health-related quality of life in outpatients with inflammatory bowel disease. European journal of gastroenterology & hepatology. 2004;16(12):1273–80. doi: 10.1097/00042737-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA. Abuse, trauma, and GI illness: is there a link? The American journal of gastroenterology. 2011;106(1):14–25. doi: 10.1038/ajg.2010.453. [DOI] [PubMed] [Google Scholar]
- 26.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain, behavior, and immunity. 2011;25(3):386–94. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697–707. doi: 10.1097/00054725-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 28*.Benhayon D, Youk A, McCarthy FN, Davis S, Keljo DJ, Bousvaros A, et al. Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. Journal of pediatric gastroenterology and nutrition. 2013;57(3):335–42. doi: 10.1097/MPG.0b013e31829641df. This is the first study to compare sleep disturbance in depressed youth with Crohn’s disease to healthy controls and to show that different aspects of sleep disturbance differentially correlated with disease activity, pain and anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnucan JA, Rubin DT, Ali T. Sleep and inflammatory bowel disease: exploring the relationship between sleep disturbances and inflammation. Gastroenterology & hepatology. 2013;9(11):718–27. [PMC free article] [PubMed] [Google Scholar]
- 30.Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflammatory bowel diseases. 2013;19(11):2440–3. doi: 10.1097/MIB.0b013e3182a0ea54. [DOI] [PubMed] [Google Scholar]
- 31.Mrakotsky C, Forbes PW, Bernstein JH, Grand RJ, Bousvaros A, Szigethy E, et al. Acute cognitive and behavioral effects of systemic corticosteroids in children treated for inflammatory bowel disease. Journal of the International Neuropsychological Society : JINS. 2013;19(1):96–109. doi: 10.1017/S1355617712001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szigethy E, Levy-Warren A, Whitton S, Bousvaros A, Gauvreau K, Leichtner AM, et al. Depressive symptoms and inflammatory bowel disease in children and adolescents: a cross-sectional study. Journal of pediatric gastroenterology and nutrition. 2004;39(4):395–403. doi: 10.1097/00005176-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013;4(Suppl 1):S94–8. doi: 10.4103/0976-500X.120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mrakotsky C, Waber DP, Bousvaros A, Grand RJ. Corticosteroids, inflammation and memory in pediatric Crohn’s disease. Inflammatory bowel diseases. 2011;17:S9–S10. [Google Scholar]
- 35.Castaneda AE, Tuulio-Henriksson A, Aronen ET, Marttunen M, Kolho KL. Cognitive functioning and depressive symptoms in adolescents with inflammatory bowel disease. World journal of gastroenterology : WJG. 2013;19(10):1611–7. doi: 10.3748/wjg.v19.i10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar G. Depression and IBD. Journal of pediatric gastroenterology and nutrition. 2014;58(5):543–4. doi: 10.1097/MPG.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 37.Nicholas DB, Otley A, Smith C, Avolio J, Munk M, Griffiths AM. Challenges and strategies of children and adolescents with inflammatory bowel disease: a qualitative examination. Health Qual Life Outcomes. 2007;5:28. doi: 10.1186/1477-7525-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholas DB, Swan SR, Gerstle TJ, Allan T, Griffiths AM. Struggles, strengths, and strategies: an ethnographic study exploring the experiences of adolescents living with an ostomy. Health Qual Life Outcomes. 2008;6:114. doi: 10.1186/1477-7525-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabbett H, Elbadri A, Thwaites R, Northover H, Dady I, Firth D, et al. Quality of life in children with Crohn’s disease. Journal of pediatric gastroenterology and nutrition. 1996;23(5):528–33. doi: 10.1097/00005176-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Knowles SR, Wilson JL, Connell WR, Kamm MA. Preliminary examination of the relations between disease activity, illness perceptions, coping strategies, and psychological morbidity in Crohn’s disease guided by the common sense model of illness. Inflammatory bowel diseases. 2011;17(12):2551–7. doi: 10.1002/ibd.21650. [DOI] [PubMed] [Google Scholar]
- 41.Knowles SR, Cook SI, Tribbick D. Relationship between health status, illness perceptions, coping strategies and psychological morbidity: A preliminary study with IBD stoma patients. Journal of Crohn’s & colitis. 2013 doi: 10.1016/j.crohns.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Reigada LC, Bruzzese JM, Benkov KJ, Levy J, Waxman AR, Petkova E, et al. Illness-specific anxiety: implications for functioning and utilization of medical services in adolescents with inflammatory bowel disease. Journal for specialists in pediatric nursing : JSPN. 2011;16(3):207–15. doi: 10.1111/j.1744-6155.2011.00292.x. [DOI] [PubMed] [Google Scholar]
- 43.McLafferty LCA, Levine A, Jones N, Becker A, Szigethy E. Thematic Analysis of Physical Illness Perceptions in Depressed Youth with Inflammatory Bowel Disease. Inflammatory bowel diseases. 2011;17(Supplement 1):S54. [Google Scholar]
- 44.Wilkinson PO, Croudace TJ, Goodyer IM. Rumination, anxiety, depressive symptoms and subsequent depression in adolescents at risk for psychopathology: a longitudinal cohort study. BMC psychiatry. 2013;13:250. doi: 10.1186/1471-244X-13-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wojtowicz AA, Greenley RN, Gumidyala AP, Rosen A, Williams SE. Pain severity and pain catastrophizing predict functional disability in youth with inflammatory bowel disease. Journal of Crohn’s & colitis. 2014 doi: 10.1016/j.crohns.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 46*.Kappelman MD, Long MD, Martin C, Dewalt DA, Kinneer PM, Chen W, et al. Evaluation of the Patient-Reported Outcomes Measurement Information System in a Large Cohort of Patients With Inflammatory Bowel Diseases. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013 doi: 10.1016/j.cgh.2013.10.019. This is the first study to use Patient Reported Outcome Measurement Information System (PROMIS) to evaluated psychosocial domains in 10,634 adults with inflammatory bowel disease compared to the general population. Significant differences were found in several domains such as depression, anxiety, fatigue, sleep, pain, and social satisfaction; in each level of impairment associated with disease activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflammatory bowel diseases. 2009;15(11):1755–64. doi: 10.1002/ibd.20919. [DOI] [PubMed] [Google Scholar]
- 48.Engelmann G, Erhard D, Petersen M, Parzer P, Schlarb AA, Resch F, et al. Health-Related Quality of Life in Adolescents with Inflammatory Bowel Disease Depends on Disease Activity and Psychiatric Comorbidity. Child psychiatry and human development. 2014 doi: 10.1007/s10578-014-0471-5. [DOI] [PubMed] [Google Scholar]
- 49.Ryan JL, Mellon MW, Junger KW, Hente EA, Denson LA, Saeed SA, et al. The clinical utility of health-related quality of life screening in a pediatric inflammatory bowel disease clinic. Inflammatory bowel diseases. 2013;19(12):2666–72. doi: 10.1097/MIB.0b013e3182a82b15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shepanski MA, Hurd LB, Culton K, Markowitz JE, Mamula P, Baldassano RN. Health-related quality of life improves in children and adolescents with inflammatory bowel disease after attending a camp sponsored by the Crohn’s and Colitis Foundation of America. Inflammatory bowel diseases. 2005;11(2):164–70. doi: 10.1097/00054725-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 51.McCombie AM, Mulder RT, Gearry RB. Psychotherapy for inflammatory bowel disease: A review and update. Journal of Crohn’s & colitis. 2013 doi: 10.1016/j.crohns.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Knowles SR, Monshat K, Castle DJ. The Efficacy and Methodological Challenges of Psychotherapy for Adults with Inflammatory Bowel Disease: A Review. Inflammatory bowel diseases. 2013 doi: 10.1097/MIB.0b013e318296ae5a. [DOI] [PubMed] [Google Scholar]
- 53.Szigethy E, Kenney E, Carpenter J, Hardy DM, Fairclough D, Bousvaros A, et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1290–8. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 54.Szigethy E, Craig AE, Iobst EA, Grand RJ, Keljo D, DeMaso D, et al. Profile of depression in adolescents with inflammatory bowel disease: implications for treatment. Inflammatory bowel diseases. 2009;15(1):69–74. doi: 10.1002/ibd.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson RD, Craig A, Crawford EA, Fairclough D, Gonzalez-Heydrich J, Bousvaros A, et al. Longitudinal results of cognitive behavioral treatment for youths with inflammatory bowel disease and depressive symptoms. Journal of clinical psychology in medical settings. 2012;19(3):329–37. doi: 10.1007/s10880-012-9301-8. [DOI] [PubMed] [Google Scholar]
- 56.Szigethy E, Thompson R, Turner S, Delaney P, Beardslee W, W JR. Cognitive behavioral therapy for general medical conditions. In: S E, Weisz JR, Findling RL, editors. Cognitive Behavioral Therapy for Children and Adolescents. Washington DC: American Psychiatric Publishing; 2012. pp. 331–82. [Google Scholar]
- 57**.Szigethy E, Bujoreanu SI, Youk A, Weisz J, Benhayon D, Fairclough D, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc. 2014 doi: 10.1016/j.jaac.2014.04.014. in press. This study is first randomized trial comparing two active psychotherapies, cognitive behavioral versus supportive, to evaluate effects on depression, quality of life, and disease activity in youth with inflammatory bowel disease (IBD). While both interventions were associated with reduced depression over time, cognitive behavioral therapy was associated with significantly greater improvement in IBD activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clarke G, Harvey AG. The complex role of sleep in adolescent depression. Child and adolescent psychiatric clinics of North America. 2012;21(2):385–400. doi: 10.1016/j.chc.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Veek SM, Derkx BH, Benninga MA, Boer F, de Haan E. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics. 2013;132(5):e1163–72. doi: 10.1542/peds.2013-0242. [DOI] [PubMed] [Google Scholar]
- 60.Shaoul R, Sukhotnik I, Mogilner J. Hypnosis as an adjuvant treatment for children with inflammatory bowel disease. Journal of developmental and behavioral pediatrics : JDBP. 2009;30(3):268. doi: 10.1097/DBP.0b013e3181a7eeb0. [DOI] [PubMed] [Google Scholar]
- 61.Schoultz M, Atherton IM, Hubbard G, Watson AJ. The use of mindfulness-based cognitive therapy for improving quality of life for inflammatory bowel disease patients: study protocol for a pilot randomised controlled trial with embedded process evaluation. Trials. 2013;14:431. doi: 10.1186/1745-6215-14-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werkstetter KJ, Ullrich J, Schatz SB, Prell C, Koletzko B, Koletzko S. Lean body mass, physical activity and quality of life in paediatric patients with inflammatory bowel disease and in healthy controls. Journal of Crohn’s & colitis. 2012;6(6):665–73. doi: 10.1016/j.crohns.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 63.van Groningen J, Ziniel S, Arnold J, Fishman LN. When independent healthcare behaviors develop in adolescents with inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2310–4. doi: 10.1002/ibd.22937. [DOI] [PubMed] [Google Scholar]
- 64.Goodhand JR, Greig FI, Koodun Y, McDermott A, Wahed M, Langmead L, et al. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflammatory bowel diseases. 2012;18(7):1232–9. doi: 10.1002/ibd.21846. [DOI] [PubMed] [Google Scholar]
- 65.Mikocka-Walus AA, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ. Antidepressants and inflammatory bowel disease: a systematic review. Clin Pract Epidemiol Ment Health. 2006;2:24. doi: 10.1186/1745-0179-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szigethy E, Carpenter J, Baum E, Kenney E, Baptista-Neto L, Beardslee WR, et al. Case study: longitudinal treatment of adolescents with depression and inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2006;45(4):396–400. doi: 10.1097/01.chi.0000198591.45949.a4. [DOI] [PubMed] [Google Scholar]
- 67.Kane S, Altschuler EL, Kast RE. Crohn’s disease remission on bupropion. Gastroenterology. 2003;125(4):1290. doi: 10.1016/j.gastro.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Kast R. Anti- and pro-inflammatory considerations in antidepressant use during medical illness: bupropion lowers and mirtazapine increases circulating tumor necrosis factor-alpha levels. Gen Hosp Psychiatry. 2003;25(6):495–6. doi: 10.1016/s0163-8343(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 69.Glod CA, Lynch A, Flynn E, Berkowitz C, Baldessarini RJ. Open trial of bupropion SR in adolescent major depression. Journal of child and adolescent psychiatric nursing : official publication of the Association of Child and Adolescent Psychiatric Nurses, Inc. 2003;16(3):123–30. doi: 10.1111/j.1744-6171.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 70.Morris DW, Budhwar N, Husain M, Wisniewski SR, Kurian BT, Luther JF, et al. Depression treatment in patients with general medical conditions: results from the CO-MED trial. Annals of family medicine. 2012;10(1):23–33. doi: 10.1370/afm.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner KD, Ambrosini P, Rynn M, Wohlberg C, Yang R, Greenbaum MS, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA : the journal of the American Medical Association. 2003;290(8):1033–41. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]
- 72.Wagner KD, Jonas J, Findling RL, Ventura D, Saikali K. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry. 2006;45(3):280–8. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- 73.Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, Leontiadis GI. Risk of Upper Gastrointestinal Bleeding With Selective Serotonin Reuptake Inhibitors With or Without Concurrent NonSteroidal Anti-Inflammatory Use: A Systematic Review and Meta-Analysis. The American journal of gastroenterology. 2014 doi: 10.1038/ajg.2014.82. [DOI] [PubMed] [Google Scholar]
- 74.Choe CJ, Emslie GJ, Mayes TL. Depression. Child and adolescent psychiatric clinics of North America. 2012;21(4):807–29. doi: 10.1016/j.chc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367–78. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 76.Kaminski A, Kamper A, Thaler K, Chapman A, Gartlehner G. Antidepressants for the treatment of abdominal pain-related functional gastrointestinal disorders in children and adolescents. The Cochrane database of systematic reviews. 2011;(7):CD008013. doi: 10.1002/14651858.CD008013.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iskandar HN, Cassell B, Kanuri N, Gyawali CP, Gutierrez A, Dassopoulos T, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. Journal of clinical gastroenterology. 2014;48(5):423–9. doi: 10.1097/MCG.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gibbons RD, Mann JJ. Strategies for quantifying the relationship between medications and suicidal behaviour: what has been learned? Drug Saf. 2011;34(5):375–95. doi: 10.2165/11589350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Isacsson G, Rich CL. Antidepressant drugs and the risk of suicide in children and adolescents. Paediatric drugs. 2014;16(2):115–22. doi: 10.1007/s40272-013-0061-1. [DOI] [PubMed] [Google Scholar]
- 80.Reed-Knight B, Lobato D, Hagin S, McQuaid EL, Seifer R, Kopel SJ, et al. Depressive symptoms in youth with inflammatory bowel disease compared with a community sample. Inflammatory bowel diseases. 2014;20(4):614–21. doi: 10.1097/01.MIB.0000442678.62674.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackner LM, Clough-Paabo E, Pajer K, Lourie A, Crandall WV. Psychoneuroimmunologic factors in inflammatory bowel disease. Inflammatory bowel diseases. 2011;17(3):849–57. doi: 10.1002/ibd.21430. [DOI] [PubMed] [Google Scholar]
- 82**.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144(1):36–49. doi: 10.1053/j.gastro.2012.10.003. This review explores evidence from both animal and human studies supporting the psychoneurological basis of intestinal inflammation and implications for current and future treatments. [DOI] [PubMed] [Google Scholar]


