Abstract
Objective
In vitro superoxide activates pulmonary endothelial TRPM2 channels and increases the capillary filtration coefficient (Kf). We hypothesized that pulmonary capillary Kf is increased in a model of type I diabetes due to elevated vascular superoxide and resultant TRPM2 channel activation.
Methods
Type I diabetes was induced in Zucker rats using streptozotocin. Half of the streptozotocin animals were treated with apocynin, a NOX inhibitor. After 4 weeks, lung Kf was measured in the isolated lung in the presence or absence of TRPM2 inhibitors (2-APB and flufenamic acid). In an additional set of experiments, Kf was measured in non-diabetic Zucker rats after applying the superoxide donor (PMS).
Results
As compared to control rats, hyperglycemic rats exhibited increased vascular superoxide and Kf, along with decreased lung vascular TRPM2-L expression. Apocynin treatment reduced superoxide and Kf in hyperglycemic rats with no effect in control rats. TRPM2 channel inhibition decreased Kf in hyperglycemic rats with no effect in control rats. PMS increased the lung Kf in control rats, with TRPM2 inhibition attenuating this response.
Conclusion
Diabetic rats exhibit a TRPM2-mediated increase in lung Kf, which is associated with increased TRPM2 activation and increased vascular superoxide levels.
Keywords: Hyperglycemia, superoxide, lung permeability, TRPM2
INTRODUCTION
Type I diabetic humans and animals exhibit increased vascular oxidative stress and lung complications attributed to elevated capillary filtration coefficient (Kf) (16, 17, 23, 25, 26, 29, 30, 34). However, the mechanisms for the elevated lung vascular permeability and the possible role of ROS on Kf have not been determined in a setting type I diabetes.
Superoxide has been shown to increase endothelial permeability in different vascular beds (7, 10, 15, 41) through an increase in intracellular calcium concentration and the resultant cell retraction (43). Three transient receptor potential (TRP) channels, including TRP vanilloid (TRPV), TRP canonical (TRPC), and TRP melastatin (TRPM), are known to increase calcium entry and thus pulmonary Kf (4, 37, 38). TRPM2 is a major target of superoxide and hydrogen peroxide (H2O2) (19, 20). An over-expression of TRPM2 enhances H2O2-mediated calcium entry in human pulmonary endothelial cells (19, 20). However, whether an elevated vascular superoxide in type I diabetics increases pulmonary Kf via activation TRPM2 has not been determined.
The STZ-induced type I diabetic model is characterized by chronic hyperglycemia and increased NOX-mediated vascular oxidative stress (8). We determined if pulmonary capillary Kf is increased in STZ-treated hyperglycemic rats due to elevated TRPM2 channel activation. Since NOX is a major source of vascular superoxide (27, 28), we also determined pulmonary permeability in STZ-treated rats with or without treatment with apocynin, a NOX inhibitor. We hypothesized that pulmonary endothelial permeability is increased during chronic hyperglycemia due to superoxide-induced activation of TRPM2 channels.
METHODS
Male lean Zucker rats (LZ, 8-9 weeks old) were acquired from Harlan Laboratories (Indianapolis, IN). Experimental protocols for this study were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were carried out according to both the National Institutes of Health Guide for the Care and Use of Laboratory Animals and guidelines of the Animal Welfare Act.
STZ (50mg/kg) was injected once i.p. to induce chronic hyperglycemia. Half of the STZ-treated rats were treated with apocynin, a NOX inhibitor, in the drinking water (2 mM for 4 wks, approximately 46 mg/kg/d) from the time the STZ was injected. All animals were 12-13 weeks old at the time of the experiments.
Pulmonary Kf
Animals were anesthetized with pentobarbital (65mg/kg, i.p.), and the lung and heart were isolated and surgically removed. Pulmonary Kf was measured as previously described (39). Briefly, we measured pulmonary arterial pressure (Pa) and pulmonary venous pressure (Pv) by using a PowerLab system (Model: ML 118, Colorado Springs, CO). The lung weights were measured at 3.5 mmHg (Pv) and then again after 10 min at 8.5 mmHg (Pv). Pressure was controlled by changing the height of the perfusate reservoir. The weight increases (ΔW) were calculated. Capillary pressure (Pc) was determined by the equilibrated pressure after the occlusion of both the arterial and venous sides at 3.5 mmHg (Pv) and 8.5 mmHg (Pv). Kf was calculated by using the following formula: Kf = ΔW / ΔPc / lung dry weight. ΔPc is the delta change of Pc from the 3.5 mmHg (Pv) to 8.5 mmHg (Pv).
Determination of lung Kf following TRPM2 channel inhibition
We determined the role of TRPM2 channel activation in regulating Kf in chronic hyperglycemia using the isolated lung preparation. In isolated lungs from control and hyperglycemic animals, we added 2-APB to the perfusate 15 minutes before the increase in Pv. 2-APB is shown to selectively inhibit TRPM2 channels at a concentration lower than 10 μM (36).
Role of superoxide on TRPM2 channel activation and lung Kf
In a group of control (non-hyperglycemic) animals we performed isolated lung experiments in which we added phenazine methosulfate (PMS, 1 μM), a superoxide donor, to the perfusate 15 minutes before the increase in Pv. Kf was determined as described above. To determine if TRPM2 channels were responsible for the superoxide-mediated increased in Kf, we added two TRPM2 channel inhibitors, 2-APB (1 μM) and flufenamic acid (FA, 100 μM), to the perfusate using the protocol described above. Additionally, it has been shown that the TRPC/store-operated calcium channel (SOC) also regulates pulmonary endothelial permeability (1, 19). ROS are reported to activate TRPC/SOC channels (19, 20). To exclude the effect of TRPC/SOC activation on Kf in our study, a TRPC/SOC inhibitor, SKF96365 (SKF, 1 μM), was applied to the perfusate using the protocol as described above.
Superoxide level and NOX activity
Superoxide levels in aortic tissue were measured using dihydroethidium (DHE) fluorescence (28). Aortic segments were rinsed in a physiological salt solution [PSS; containing (in mM) 119.0 NaCl, 4.7 KCl, 1.6 CaCl2, 1.18 NaH2PO4, 1.17 MgSO4, and 24.0 NaHCO3]. These segments were incubated in light-protected PSS (37°C) containing 5 μM DHE for 30 min. Segments were rinsed in DHE-free PSS and split longitudinally and placed endothelium side up on a coverslip. A drop of Fluotrogel with tris buffer (EMS, PA) was applied to keep the tissue moist. The endothelium was visualized, and images were obtained using a laser scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL).
To validate that the PMS treatment increased vascular superoxide, aortas were collected from control animals, and superoxide levels were measured using DHE (37°C, 5 μM) fluorescence, as we previously described (38), in untreated tissue and in tissue incubated for 15 min with PMS (1 μM).
NOX activity was measured in pulmonary arteries using lucigenin chemiluminescence as we have published previously (25). Homogenates were prepared from pulmonary arteries. The homogenates were incubated with lucigenin (5 μM) for chemiluminescence detection using a Berthold luminometer. For measurement of NOX activity, NADPH was added (100 μM), and chemiluminescence was measured. A luminescence reading was obtained for an overall measuring time of 5 min for each sample. Enzyme activity is expressed in relative light units (RLU) per second and normalized by protein concentration from each sample.
TRPM2 channel protein expression
Pulmonary arteries from LZ control and hyperglycemic LZ were collected, homogenized and centrifuged at 13,000 rpm. The supernatant was separated by electrophoresis on a Criterion TGX AnykD Gel (Bio-Rad, Hercules, CA), and transferred to a nitrocellulose membrane for Western blotting. TRPM2-L, the only functional TRPM2 channel isoform (NB110-81601, Novus Biologicals, Littleton, CO) and β-actin antibodies were applied after incubation with an Odyssey Blocking Buffer (LI-COR, Lincoln, NE). The TRPM2-L antibody recognizes a C-terminal portion of the rat TRPM2 protein (within residues 1430-1508), specific for TRPM2-L. The membrane was read by a LI-COR Odyssey scanner (LI-COR, Lincoln, NE).
Reagents and chemicals
Apocynin, STZ, PMS, 2-APB, FA, and SKF were purchased from Sigma-Aldrich Corporation (St. Louis, MO). Apocynin was given in the tap water. STZ was given i.p. in saline. 2-APB, FA, and SKF were dissolved in 100% ethanol and diluted as 1:1000-10000 in the isolated lung perfusate.
Quantitative and statistical analyses
Superoxide levels before and after PMS treatments were compared using t-test. The superoxide levels and NOX activity in non-hyperglycemic, hyperglycemic, and apocynin-treated hyperglycemic animals were compared using one-way ANOVA. The Kf under baseline, PMS-treated, PMS+SKF-treated conditions were compared by one-way ANOVA. TRPM2 channel expression in LZ and hyperglycemic LZ were compared using a t-test. All of the other data were compared by using two-way ANOVA. Where significant effects occurred, individual groups were compared using the Holm-Sidak method. All of the data are presented as means ± SEM. A probability of p < 0.05 was accepted as statistically significant for all comparisons.
RESULTS
STZ induces chronic hyperglycemia in lean Zucker rats
As compared with control LZ, the STZ-treated LZ exhibited significantly higher fasting glucose levels from the third day after STZ injection (Table 1). Four weeks after STZ injection the LZ (12-13 wks old) had significantly lower body weights than their age-matched controls (Table 1). Apocynin treatment had no effect on glucose levels or body weights (Table 1).
Table 1.
Body weight and blood glucose levels in control, STZ-treated, and STZ+apocynin-treated LZ.
| Control | STZ | STZ+apocynin | |
|---|---|---|---|
| Body weight (g) | 354±21 | 274±18* | 265±27* |
| Glucose (mg/dL) | 100±12 | 444±44* | 457±27* |
Values are means±SEM
p<0.05 vs. control; there is no significant difference in body weight and glucose levels between STZ and STZ+apocynin group. n=12 for control group, n=16 for STZ group, and n=18 for STZ+apocynin group.
TRPM2 channel expression decreased in STZ-treated hyperglycemic LZ
There was a significantly lower pulmonary artery TRPM2-L channel expression in hyperglycemic LZ as compared to control LZ (Figure 1).
Figure 1. Pulmonary arterial TRPM2-L channel expression in control LZ and STZ-treated LZ.
TRPM2-L channel is down-expressed in STZ-treated type I diabetic LZ (*, p<0.05 vs. Control). n=6 in Control LZ; n=6 in STZ-treated LZ.
Superoxide levels and NOX activity are elevated in type I diabetic rats
STZ-treated LZ, as compared with control LZ, exhibited significantly higher aortic superoxide levels indicated by DHE fluorescence (Figure 2A) and higher NOX activity in pulmonary arteries measured by RLU of chemiluminescence (Figure 2B). Apocynin treatment in hyperglycemic LZ significantly decreased both vascular superoxide levels and NOX activity (Figures 2A and 2B, respectively).
Figure 2. Aortic superoxide levels and pulmonary arterial NADPH oxidase activity in LZ with and without STZ/apocynin treatment.
A) The top is the example of confocal images obtained using a confocal microscopy from DHE-treated aorta. STZ treatment significantly increases superoxide level in aorta compared with control group (*, p<0.05 vs. control). Apocynin treatment partially normalizes superoxide in STZ-treated group (*, p<0.05 vs. control; #, p<0.05 vs. STZ group). n=6 for control group, n=5 for STZ group, n=6 for STZ+apocynin group. B) STZ treatment significantly increases NOX activity in pulmonary arteries compared with control group (*, p<0.05 vs. control). Apocynin treatment partially normalizes NOX activity in STZ-treated group (*, p<0.05 vs. control; #, p<0.05 vs. STZ group). n=6 for control group, n=6 for STZ group, n=6 for STZ+apocynin group.
Chronic hyperglycemia increases pulmonary Kf via superoxide-mediated TRPM2 activation
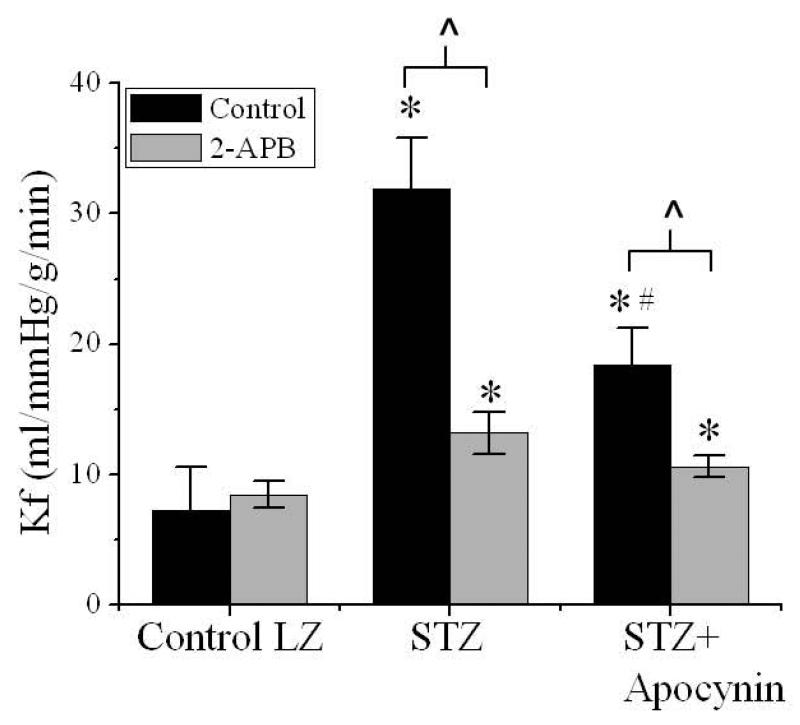
Figure 3 presents the isolated lung Kf from control, hyperglycemic, and apocynin-treated hyperglycemic LZ with or without TRPM2 channel inhibition. Kf was significantly elevated in the LZ with chronic hyperglycemia. This increase in Kf was attenuated by apocynin treatment (Figure 3). 2-APB had no effect on the Kf in non-hyperglycemic LZ but significantly inhibited pulmonary Kf in both STZ-treated and apocynin-treated hyperglycemic LZ (Figure 3).
Figure 3. Pulmonary capillary Kf in control, STZ-treated, and STZ+apocynin-treated LZ with and without 2-APB application.
STZ treatment significantly increase Kf compared with control group (*, p<0.05 vs. control). Apocynin treatment partially normalizes Kf in STZ-treated group (*, p<0.05 vs. control; #, p<0.05 vs. STZ group).2-APB (1 μM) has no effect on control group, but partially normalizes Kf in STZ group (*, p<0.05 vs. 2-APB-treated control; ^, p<0.05 vs. STZ group), and in STZ+apocynin group (*, p<0.05 vs. 2-APB-treated control; ^, p<0.05 vs. STZ+apocynin group). n=5 for control, n=5 for 2-APB-treated control, n=5 for STZ, n=5 for 2-APB-treated STZ, n=6 for STZ+apocynin, n=6 for 2-APB-treated STZ+apocynin.
TRPM2 inhibition prevents superoxide-induced increases in pulmonary Kf
Figures 4 presents the pulmonary Kf changes in normoglycemic LZ (control) after application of the superoxide donor, PMS, with and without treatment with two TRPM2 channel inhibitors. PMS application significantly increased pulmonary Kf (Figure 4). 2-APB (1 μM) significantly inhibited the PMS-induced Kf increase but had no effect on basal Kf (Figure 4). The effects of FA (100 μM), another TRPM2 channel inhibitor, were similar to 2-APB (Figure 4). Inhibition of TRPC/SOC by SKF had no effect on pulmonary Kf after PMS application (Figure 5). PMS significantly increased superoxide levels in the aorta (data not shown).
Figure 4. Pulmonary capillary Kf in control and PMS-treated LZ with and without 2-APB or FA application.
PMS significantly increases Kf in LZ (*, p<0.05 vs. control LZ). 2-APB (1 μM) has no effect on control LZ but normalized Kf in PMS-treated group (#, p<0.05 vs. PMS-treated LZ). Similarly, FA (100 μM) has no effect on control LZ but partially normalized Kf in PMS-treated group (#, p<0.05 vs. PMS-treated group; *, p<0.05 vs. control LZ treated with FA). n=5 in all groups.
Figure 5. Pulmonary capillary Kf in PMS-treated group with and without SKF application.
SKF (1 μM) has no effect on Kf in PMS-treated lungs. n=5 in all groups.
In Figures 3 and 4, the control group with or without treatment of 2-APB are from the same animals. In Figures 4 and 5, the PMS-treated LZ group represents data from the same animals.
DISCUSSION
The major findings of this work are: 1) type I diabetic LZ exhibited increased vascular oxidative stress, pulmonary Kf, and decreased vascular TRPM2-L channel expression; 2) inhibition of NOX with apocynin treatment decreased vascular oxidative stress and pulmonary Kf in the diabetic rats; 3) inhibition of TRPM2 channel decreased lung Kf in diabetic rats, with this inhibitory effect attenuated in the apocynin-treated diabetic LZ; and 4) a superoxide donor increased the pulmonary Kf in non-hyperglycemic LZ, with this increase blunted following TRPM2 channel inhibition.
Pulmonary Kf in diabetic rats is increased due to oxidative stress
Chronic hyperglycemia has been shown to increase inter-endothelial gaps in the lung capillary endothelium (2, 22, 30, 32). We found that rats with chronic hyperglycemic exhibited an increased pulmonary Kf (Figure 3) in the isolated lungs perfused with a physiological saline solution (PSS) with normal glucose concentration (5.5 mM). Chronic hyperglycemia is known to increase vascular superoxide levels (7, 10, 15, 41), and NOX is one of the major sources of endothelial superoxide generation during chronic hyperglycemia (27, 28). In the current study, we found significant increases in vascular NOX activity and superoxide levels in diabetic rats (Figure 1). The lung vasculature is vulnerable to oxidative stress (11), and the pathological changes are correlated with an increased pulmonary Kf (3, 40). We found that, in diabetic rats, inhibition of superoxide after apocynin treatment significantly reduced lung Kf. These results suggest that NOX-mediated oxidative stress increases pulmonary endothelial permeability in the context of chronic hyperglycemia.
Superoxide increases pulmonary endothelial Kf via activation of the TRPM2 channel
Calcium entry through TRP channels, including TRPC, TRPV, and TRPM channels, increases endothelial permeability (4, 9, 19, 20, 18, 24). 2-APB has been shown to selectively inhibit TRPM2 channels at a concentration lower than 10 μM (36). In current study, we found that 2-APB decreased Kf in STZ-treated rats, suggesting that TRPM2 plays an important role in lung Kf during chronic hyperglycemia. We have previously shown that treatment with PMS, a superoxide donor, can increase the capillary Kf in the isolated lung (39). Since the TRPM2 channel is an oxidant-activated calcium channel, we applied PMS to verify if the superoxide-mediated increase in lung Kf is TRPM2 channel-dependent. Because there is a lack of commercial extracellular agonists, we used two different TRPM2 inhibitors, 2-APB and FA. We found that the PMS-induced increase in lung Kf was blunted by treatment with either 2-APB or FA (Figure 4), suggesting that TRPM2 channel activation by superoxide may be responsible for the increase in lung Kf during chronic hyperglycemia. Furthermore, the inhibitory effect of 2-APB on Kf was attenuated in the diabetic rats following apocynin treatment, suggesting that the increased basal lung Kf is due, at least in part, to NOX-mediated superoxide production. 2-APB still decreased the Kf in the diabetic rats treated with apocynin, suggesting that other factors may also contribute to the TRPM2-mediated increase in Kf.
TRPC channels, acting through SOC and receptor-operated calcium entry, are involved in the regulation of vascular permeability (1, 4). Oxidants are also known to induce calcium entry through TRPC/SOC channels (14, 18). SKF is used to inhibit SOC and TRPC channels (13, 33). Although SKF is not a selective inhibitor for SOCs, in our isolated lung experiment, SKF has no effect on the PMS-induced Kf increase (Figure 5). This indicates that the channels which are inhibited by SKF, including SOC, play a minor role in mediating the increase in Kf. In addition, inhibition of TRPM2 channel by 2-APB significantly decreased but failed to normalized the Kf in STZ rats compared to the control group. This could be due to the activation of other calcium channels in STZ rats which are not inhibited by 2-APB, such as TRPV4 channels. Townsley's laboratory reported that activation of TRPV4, a mechanically activated receptor, also increases pulmonary endothelial permeability (4, 38). However, TRPM2 channel inhibitors significantly decreased the lung Kf in hyperglycemic rats without affecting the Kf in control animals, suggesting that TRPM2 channels play a major role in the increased lung Kf in diabetic rats. In addition to the increased superoxide-mediated activation of TPRM2, we found a down-regulation of TRPM2-L channel expression in the lung vasculature from diabetic rats (Figure 1). The mechanisms responsible for this are unclear. Besides TRPM2-L, 3 isoforms of TRPM2 channels have been determined: TRPM2-S (short), TRPM2-ΔN (a deletion of 20 amino acids in the N-terminus), and TRPM2-ΔC (a deletion of 34 amino acids in the CAP domain) (20, 42). TRPM2-L is the only fully functional isoform. Thus, we determined the TRPM2-L expression level in our model. The decreased TRPM2-L expression during chronic hyperglycemia might be due to the overexposure of superoxide and a resultant negative feedback-mediated down regulation (31). Nevertheless, these results collectively suggest that the function of TRPM2 channels in regulating lung Kf is minor under control conditions, but is increased in response to the elevated superoxide in diabetics. Further studies are clearly needed to determine the pulmonary TRPM2 channel sensitivity in control and diabetic animal models by using pharmacological and electrophysiological approaches.
In conclusion, these results suggest that, in chronic hyperglycemic rats, oxidative stress activates TRPM2 channels, leading to an increase in pulmonary capillary Kf. This TRPM2 channel-mediated elevation in Kf may contribute to the increased susceptibility to lung complications seen in patients with type I diabetes. This study provides novel findings that point to possible TRPM2 channel blockade-based therapeutics in type I diabetic patients with lung injury.
PERSPECTIVES.
Compared with normal individuals, diabetic and obese patients are at a higher risk of developing acute lung injury (ALI) after acute illness such as trauma and sepsis. An increased pulmonary capillary permeability is an important factor contributing to lung edema during ALI. The mechanisms responsible for the increased susceptibility are unclear. In this study, we determined the role of the TRMP2 channel activation in pulmonary permeability regulation in a type I diabetic rat model. Our data suggest that, in hyperglycemic rats, increased oxidative stress activates the TRPM2 channel and subsequently elevates lung Kf. This increased basal lung permeability in type I diabetes may be the potential mechanism responsible for the high susceptibility to ALI after acute illness in the diabetic and obese population.
Acknowledgments
The study was supported by AHA-12POST12060126, AHA-12SDG12050525, NIH HL-89581, HL-51971, NIH-P20GM104357, and an Orthopedic Trauma Association Grant.
List of Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- ALI
acute lung injury
- DHE
dihydroethidium
- FA
flufenamic acid
- H2O2
hydrogen peroxide
- Kf
lung capillary filtration coefficient
- LZ
lean Zucker rats
- NOX
nicotinamide adenine dinucleotide phosphate-oxidase
- PMS
phenazine methosulfate
- ROS
reactive oxygen species
- SOC
store-operated calcium channel
- STZ
streptozotocin
- TRPM2
transient receptor potential melastatin 2
- TRPM2-L
full length TRPM2 channel isoform
Footnotes
CONTRIBUTIONS
S. L. designed the study, performed experiments, analyzed data, and wrote the manuscript. All authors contributed to data interpretation and critical revision of the manuscript.
DISCLOSURES
We have no conflicts to disclose.
References
- 1.Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005 Oct;451(1):131–42. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997 Sep;46(9):1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 3.Allison RC, Hernandez EM, Prasad VR, Grisham MB, Taylor AE. Protective effects of O2 radical scavengers and adenosine in PMA-induced lung injury. J Appl Physiol (1985) 1988 May;64(5):2175–82. doi: 10.1152/jappl.1988.64.5.2175. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med. 2005 Nov 1;172(9):1153–60. doi: 10.1164/rccm.200506-847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck A, Kolisek M, Bagley LA, Fleig A, Penner R. Nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose regulate TRPM2 channels in T lymphocytes. FASEB J. 2006 May;20(7):962–4. doi: 10.1096/fj.05-5538fje. [DOI] [PubMed] [Google Scholar]
- 6.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010 Nov;105(6):687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 7.Craven PA, Melhem MF, Phillips SL, DeRubertis FR. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes. 2001 Sep;50(9):2114–25. doi: 10.2337/diabetes.50.9.2114. [DOI] [PubMed] [Google Scholar]
- 8.Di Marco E, Gray SP, Chew P, Koulis C, Ziegler A, Szyndralewiez C, Touyz RM, Schmidt HH, Cooper ME, Slattery R, Jandeleit-Dahm KA. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe −/− mice. Diabetologia. 2013 Nov 30; doi: 10.1007/s00125-013-3118-3. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich A, Gudermann T. Another TRP to endothelial dysfunction: TRPM2 and endothelial permeability. Circ Res. 2008 Feb 15;102(3):275–7. doi: 10.1161/CIRCRESAHA.107.170548. [DOI] [PubMed] [Google Scholar]
- 10.Dileepan K, Sharma R, Stechschulte DJ, Savin VJ. Effect of superoxide exposure on albumin permeability of isolated rat glomeruli. J Lab Clin Med. 1993 Jun;121(6):797–804. [PubMed] [Google Scholar]
- 11.Dodd-O JM, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia reperfusion lung injury. Am J Physiol Heart Circ Physiol. 2000 Jul;279(1):H303–12. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 12.Fliegert R, Gasser A, Guse AH. Regulation of calcium signalling by adenine-based second messengers. Biochem Soc Trans. 2007 Feb;35(Pt 1):109–14. doi: 10.1042/BST0350109. [DOI] [PubMed] [Google Scholar]
- 13.Forrest AS, Angermann JE, Raghunathan R, Lachendro C, Greenwood IA, Leblanc N. Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells. Adv Exp Med Biol. 2010;661:31–55. doi: 10.1007/978-1-60761-500-2_3. [DOI] [PubMed] [Google Scholar]
- 14.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013 Feb 1;123(2):887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010 Apr 30;106(8):1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med. 2008 Aug;36(8):2407–13. doi: 10.1097/CCM.0b013e318180b3ba. [DOI] [PubMed] [Google Scholar]
- 17.Hansen LA, Prakash UB, Colby TV. Pulmonary complications in diabetes mellitus. Mayo Clin Proc. 1989 Jul;64(7):791–9. doi: 10.1016/s0025-6196(12)61752-2. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010 Aug 9;190(3):391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecquet CM, Ahmmed GU, Malik AB. TRPM2 channel regulates endothelial barrier function. Adv Exp Med Biol. 2010;661:155–67. doi: 10.1007/978-1-60761-500-2_10. [DOI] [PubMed] [Google Scholar]
- 20.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008 Feb 15;102(3):347–55. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 21.Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jüngling E, Lückhoff A. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J. 2006 Sep 1;398(2):225–32. doi: 10.1042/BJ20060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King GL, Berman AB, Bonner-Weir S, Carson MP. Regulation of vascular permeability in cell culture. Diabetes. 1987 Dec;36(12):1460–7. doi: 10.2337/diab.36.12.1460. [DOI] [PubMed] [Google Scholar]
- 23.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009 Jul;32(7):1335–43. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta. 2007 Aug;1772(8):907–14. doi: 10.1016/j.bbadis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004 May;56(5):1058–62. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 26.Lausevic Z, Lausevic M, Trbojevic-Stankovic J, Krstic S, Stojimirovic B. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 2008;51(2):97–102. [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Lopez JG, Moral-Sanz J, Frazziano G, Gomez-Villalobos MJ, Flores-Hernandez J, Monjaraz E, Cogolludo A, Perez-Vizcaino F. Diabetes induces pulmonary artery endothelial dysfunction by NADPH oxidase induction. Am J Physiol Lung Cell Mol Physiol. 2008 Nov;295(5):L727–32. doi: 10.1152/ajplung.90354.2008. [DOI] [PubMed] [Google Scholar]
- 28.Lu S, Xiang L, Clemmer JS, Gowdey AR, Mittwede PN, Hester RL. Impaired vascular KATP function attenuates exercise capacity in obese zucker rats. Microcirculation. 2013 Oct;20(7):662–9. doi: 10.1111/micc.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–41. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 30.Popov D, Simionescu M. Structural and transport property alterations of the lung capillary endothelium in diabetes. Ital J Anat Embryol. 2001;106(2 Suppl 1):405–12. [PubMed] [Google Scholar]
- 31.Roedding AS1, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ. Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord. 2012 Mar;14(2):151–61. doi: 10.1111/j.1399-5618.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 32.Scalia R, Gong Y, Berzins B, Zhao LJ, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of mu-calpain. Diabetes. 2007 Jul;56(7):1842–9. doi: 10.2337/db06-1198. [DOI] [PubMed] [Google Scholar]
- 33.Song M, Chen D, Yu SP. The TRPC channel blocker SKF 96365 Inhibits Glioblastoma Cell Growth by Enhancing Reverse Mode of the Na+ /Ca2+ Exchanger and Increasing Intracellular Ca2+. Br J Pharmacol. 2014 Mar 18; doi: 10.1111/bph.12691. doi: 10.1111/bph.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprung CL, Rackow EC, Fein IA. Pulmonary edema; a complication of diabetic ketoacidosis. Chest. 1980 May;77(5):687–8. doi: 10.1378/chest.77.5.687. [DOI] [PubMed] [Google Scholar]
- 35.Tate RM, Vanbenthuysen KM, Shasby DM, McMurtry IF, Repine JE. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–6. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- 36.Togashi K, Inada H, Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB). Br J Pharmacol. 2008 Mar;153(6):1324–30. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsley MI, King JA, Alvarez DF. Ca2+ channels and pulmonary endothelial permeability: insights from study of intact lung and chronic pulmonary hypertension. Microcirculation. 2006 Dec;13(8):725–39. doi: 10.1080/10739680600930362. [DOI] [PubMed] [Google Scholar]
- 38.Wu S, Jian MY, Xu YC, Zhou C, Al-Mehdi AB, Liedtke W, Shin HS, Townsley MI. Ca2+ entry via alpha1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol. 2009 Oct;297(4):L650–7. doi: 10.1152/ajplung.00015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang L, Lu S, Mittwede P, Clemmer J, Hester RL. Inhibition of NADPH oxidase prevents acute lung injury in obese rats following severe trauma. Am J Physiol Heart Circ Physiol. 2014 Jan 10; doi: 10.1152/ajpheart.00868.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura K, Nakagawa S, Koyama S, Kobayashi T, Homma T. Roles of neutrophil elastase and superoxide anion in leukotriene B4-induced lung injury in rabbit. J Appl Physiol (1985) 1994 Jan;76(1):91–6. doi: 10.1152/jappl.1994.76.1.91. [DOI] [PubMed] [Google Scholar]
- 41.Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, Wu MH. Microvascular Permeability in Diabetes and Insulin Resistance. Microcirculation. 2007 Jun-Jul;14(4-5):363–73. doi: 10.1080/10739680701283091. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W1, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun SC, Penn L, Barber DL, Stahl R, Carey DJ, Cheung JY, Miller BA. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol. 2006 Apr;290(4):C1146–59. doi: 10.1152/ajpcell.00205.2005. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X, Wen K, Yuan D, Ai L, He P. Calcium influx-dependent differential actions of superoxide and hydrogen peroxide on microvessel permeability. Am J Physiol Heart Circ Physiol. 2009 Apr;296(4):H1096–107. doi: 10.1152/ajpheart.01037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]