Abstract
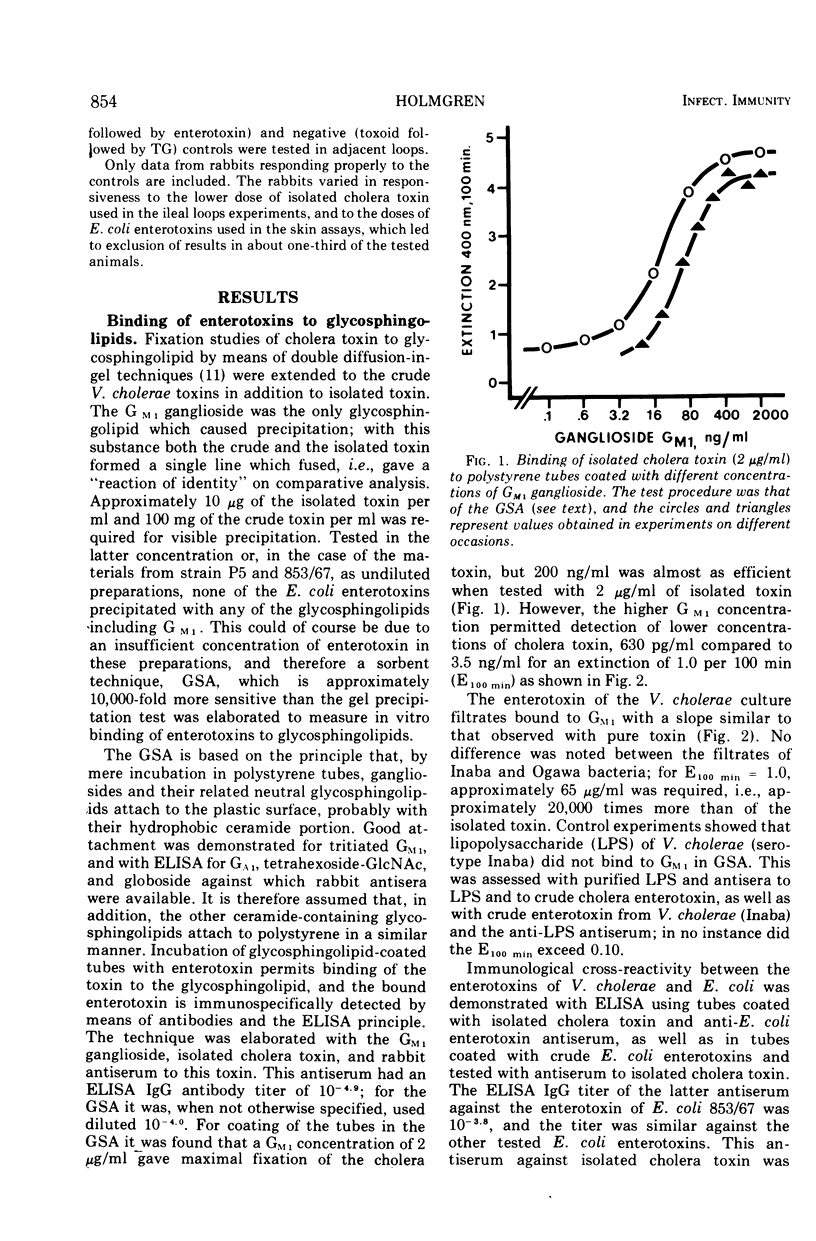
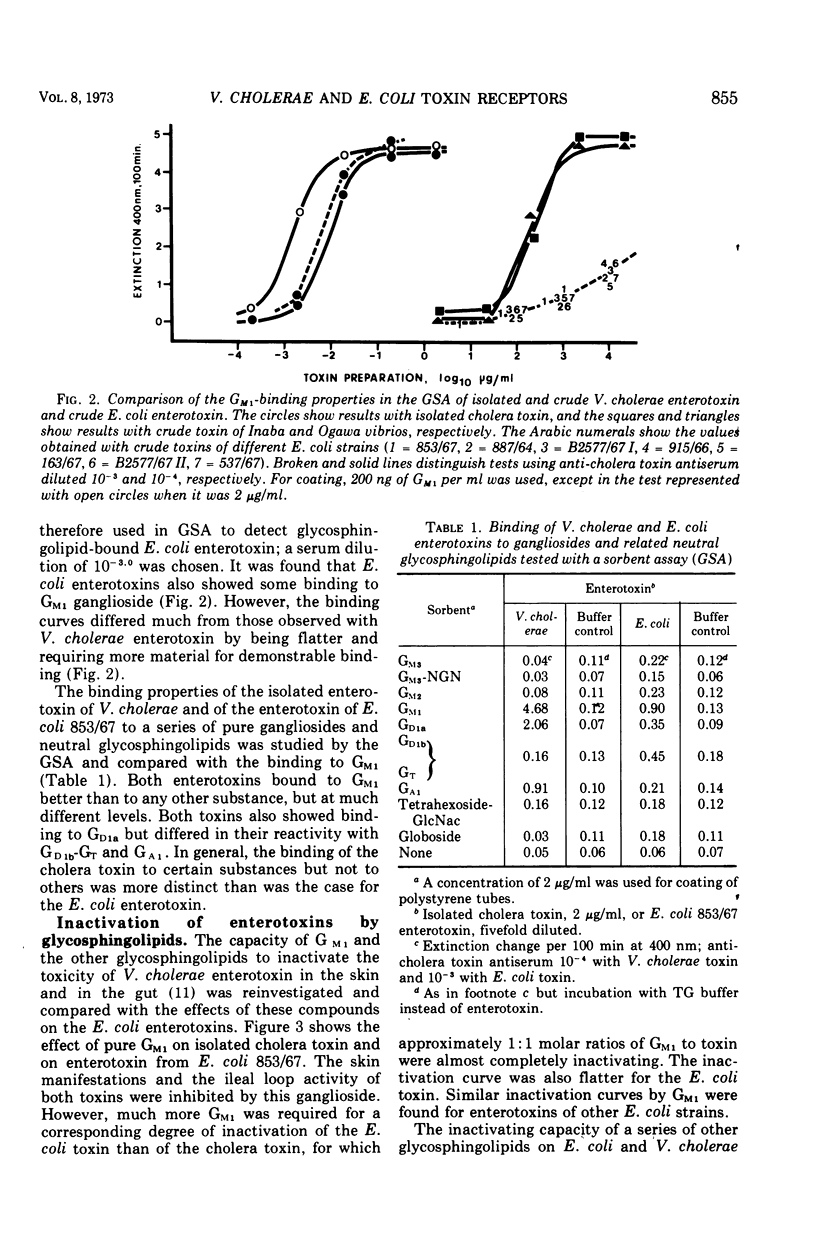
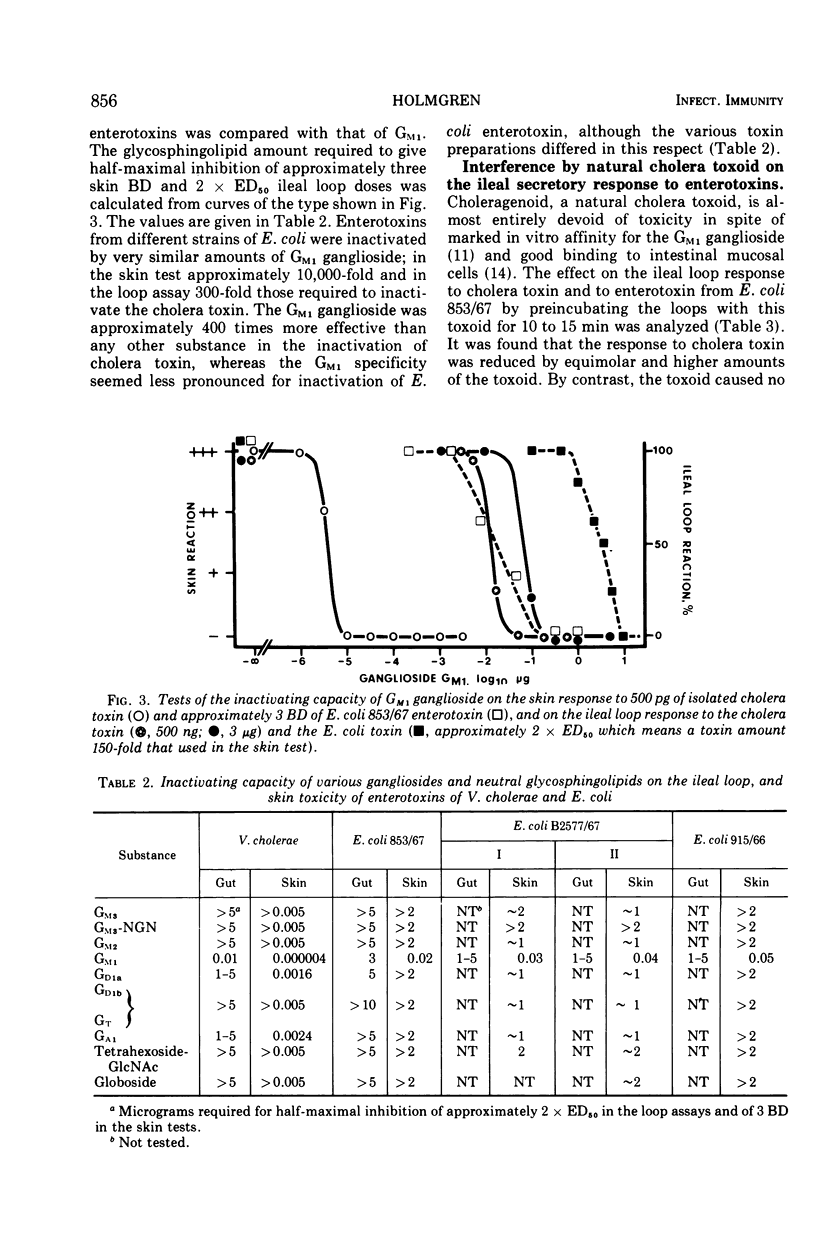
The in vitro binding properties of enterotoxins of Vibrio cholerae and Escherichia coli to different pure gangliosides and related neutral glycosphin-golipids were analyzed with a sorbent assay utilizing plastic tubes to which the glycolipid substances had been coupled. It was found that the cholera toxin bound to GM1 ganglioside better than to the other tested substances GM3, GM3-NGN, GM2, GD1a, GD1b, GT, GA1, tetrahexoside-GlcNac and globoside. With this assay using GM1-coated tubes it is possible to measure cholera toxin even at concentrations below 1 ng/ml. Also enterotoxin of various E. coli strains bound to GM1, but the affinity was much less than for cholera toxin. The GM1 ganglioside, in contrast to the other glycosphingolipids, effectively inactivated cholera toxin as determined with the intradermal and the ileal loop assays; approximately equimolar concentrations of the ganglioside in relation to toxin sufficed. Also, the skin and ileal loop activities of E. coli enterotoxins could be inhibited by GM1; however, several orders more of the ganglioside were required for such inhibition than for inactivation of the cholera toxin, and the differences between GM1 and the other substances were less pronounced for E. coli toxins. Preincubation of rabbit ileal loops with choleragenoid, a natural toxoid of V. cholerae which has binding properties to the GM1 ganglioside similar to cholera toxin, made the loops resistant to subsequently added enterotoxin of V. cholerae. The responsiveness to enterotoxin of E. coli was not reduced by this toxoid. A likely interpretation of these data is that the GM1 ganglioside constitutes or at least contains the structure of functional tissue receptors for the cholera toxin, whereas the weak binding to GM1 by E. coli enterotoxins is probably a pathogenetically insignificant reflection of structural similarities between these toxins and cholera toxin. Consequently, the cholera toxoid by occupying functional intestinal GM1 receptors for the cholera toxin could inhibit the ileal response to this toxin, but not the response to E. coli enterotoxin since the intestinal receptors for the latter toxin are not affected by the cholera toxoid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hakomori S. I., Siddiqui B., Li Y. T., Li S. C., Hellerqvist C. G. Anomeric structure of globoside and ceramide grihexoside of human erythrocytes and hamster fibroblasts. J Biol Chem. 1971 Apr 10;246(7):2271–2277. [PubMed] [Google Scholar]
- Holmgren J., Andersson A., Wallerstrom G., Ouchterlony O. Experimental studies on cholera immunization. II. Evidence for protective antitoxic immunity mediated by serum antibodies as well as local antibodies. Infect Immun. 1972 May;5(5):662–667. doi: 10.1128/iai.5.5.662-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Ouchterlony O. Immunochemical Studies of Two Cholera Toxin-Containing Standard Culture Filtrate Preparations of Vibrio cholerae. Infect Immun. 1971 Jun;3(6):747–755. doi: 10.1128/iai.3.6.747-755.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand J Infect Dis. 1973;5(1):77–78. doi: 10.3109/inf.1973.5.issue-1.15. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Li S. C. Anomeric configuration of galactose residues in ceramide trihexosides. J Biol Chem. 1971 Jun 10;246(11):3769–3771. [PubMed] [Google Scholar]
- Peterson J. W., LoSpalluto J. J., Finkelstein R. A. Localization of cholera toxin in vivo. J Infect Dis. 1972 Dec;126(6):617–628. doi: 10.1093/infdis/126.6.617. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]
- Van Heyningen W. E., Carpenter C. C., Pierce N. F., Greenough W. B., 3rd Deactivation of cholera toxin by ganglioside. J Infect Dis. 1971 Oct;124(4):415–418. doi: 10.1093/infdis/124.4.415. [DOI] [PubMed] [Google Scholar]



