Abstract
Background
Response to treatment may be useful for diagnostic confirmation of childhood tuberculosis (TB). We aimed to evaluate time to symptom resolution in children treated for pulmonary TB.
Methods
We compared pulmonary TB cases and non-cases, classified by a published diagnostic algorithm, in South African children younger than two years of age. TB treatment was prescribed independently on clinical grounds. We analyzed independent determinants of baseline symptom resolution by Cox regression.
Results
191 symptomatic children, median age 12 months, were prescribed TB treatment. Chest radiograph features of TB were associated with longer time to resolution of cough (adjusted hazard ratio, AHR 0.31), wheeze (AHR 0.26) and failure to thrive (FTT) (AHR 0.41), (all p<0.05). However, median duration of baseline cough (63 vs. 70 days, p=0.98), wheeze (62 vs. 68 days, p=0.87) and FTT (76 vs. 66 days, p=0.59) did not differ in TB cases (n=48) vs. non-cases (n=46).
Conclusions
Baseline symptoms take longer than 60 days to resolve in the majority of young children after starting TB treatment. Further, since time to resolution does not differentiate TB cases from non-cases, clinical response to treatment is not an appropriate diagnostic criterion for pediatric trials of TB diagnostics, drugs and vaccines.
Keywords: Resolution, tuberculosis, children, symptoms, treatment
BACKGROUND
Childhood tuberculosis (TB) is a significant contributor to the overall global burden of TB disease, with an estimated 6% of total TB cases per year occurring in children <15 years of age [1]. Children, less than two years of age, have increased risk of TB disease progression after infection and increased TB morbidity and mortality after disease, compared with adults [2–4]. Whileintra-thoracic TB is the most common pathology in children, definitive diagnosis is difficult, since childhood disease is pauci-bacillary [5–7]. This diagnostic challenge has implications for definition of endpoints in pediatric TB research, including studies of new TB diagnostics, vaccines, and drugs [8]. Since microbiologic confirmation is often not possible, composite endpoints for TB diagnosis are sometimes based on clinical diagnostic algorithms [2, 8–10].
Given the lack of a standardized case definition for childhood TB [2, 8, 11], a recent expert consensus statement on diagnostic endpoints for TB research suggested that clinical response to anti-tuberculosis treatment should be one of the diagnostic criteria in a composite TB endpoint [4]. The expert consensus statement defined response to treatment as resolution of clinical symptoms within two months from start of TB treatment [4], but it was acknowledged that there is little evidence on which to base this recommendation. Objective data on duration of symptoms and treatment response in childhood TB are lacking, and the panel highlighted the need for further research in this area [4].
We aimed to measure the time to resolution of baseline symptoms following initiation of TB treatment, and to describe the independent determinants of symptom resolution, in a group of Bacillus Calmette–Guérin (BCG) vaccinated children younger than two years of age who were diagnosed and treated for incident pulmonary TB, in the context of a clinical trial of TB surveillance methodology [12, 13]. We hypothesized that: first, resolution of baseline symptoms would require longer than 60 days in young children treated for pulmonary TB; second, that time to resolution of symptoms would differentiate children with TB disease from those without TB disease; and third, that clinical, radiographic and microbiological factors might be identified that are independent predictors of TB symptom duration.
METHODS
A randomized trial to determine the impact of active or passive surveillance methodology on the two-year cumulative incidence of pulmonary TB in healthy, BCG vaccinated infants, was conducted between 2005–2008 near Cape Town, South Africa [12]. The trial was approved by the University of Cape Town Human Research Ethics Committee (HREC) (064/2005).
Study Design and Population
We present data from the active surveillance trial arm, in which children had follow-up visits scheduled at three-monthly intervals, from birth until at least two years of age. We analyzed the subgroup of symptomatic children who were investigated and treated for pulmonary TB, which was diagnosed using a standardized, published [9] diagnostic algorithm, during the course of the trial. Children were included in the analysis if: a) they had at least one baseline symptom compatible with pulmonary TB; b) they had received treatment for TB disease diagnosed on clinical grounds; and c) they had completed at least one follow-up visit within 210 days from date of starting TB treatment. Children without baseline TB symptom data; or who did not receive TB treatment; or who were HIV ELISA positive, were excluded from this analysis.
Evaluation of TB Disease
Children with suspected TB, on the basis of persistent cough or wheeze for longer than two weeks, or failure to thrive for longer than two months (defined as crossing growth chart centiles) [14], or who were in close household contact with an adult with known TB disease, were investigated using a standard diagnostic approach [12]. Clinical data collection included a history of the presence and duration of symptoms compatible with pulmonary TB at baseline. The symptoms of fever, night sweats, and loss of appetite were excluded from analysis, since these symptoms had been negatively associated with Mycobacterium tuberculosis (MTB) culture-confirmed TB in our previous pediatric studies [4]. A tuberculin skin test (TST); two-paired gastric lavage and induced sputum samples for MTB liquid culture; and a chest radiograph (CXR), were performed. CXRs were reviewed by three independent reviewers, blinded to clinical data, and were categorized as compatible or not compatible with a diagnosis of pulmonary TB, on the basis of two-thirds majority opinion. A post hoc data-driven diagnostic algorithm, using clinical, radiological and microbiological variables, was applied to classify each episode as Definite/Probable TB (TB cases), Possible TB (included here only in sensitivity analyses), or Unlikely/Not TB (non-cases) [12].
Response to Treatment
Treatment decisions were made on clinical grounds by the attending clinician, independent of the final algorithmic TB case classification. Therefore, any child investigated for pulmonary TB might be prescribed the first-line regimen of isoniazid, rifampicin and pyrazinamide (HRZ); or isoniazid preventive therapy (IPT) prophylaxis only; or other antibiotic therapy; or no treatment (observation only). Following investigation, all children resumed their pre-existing three-monthly follow-up visit schedule, independent of the timing of TB treatment start. The study team recorded the presence of persistent symptoms compatible with TB at each subsequent study visit. Resolution of a baseline symptom was defined as absence of that specific symptom at follow-up. Time within which a baseline symptom resolved was defined as the period from start of TB treatment to the first follow-up visit at which resolution was recorded.
Statistical Analysis and Data Presentation
Data are presented as medians, interquartile ranges (IQR) (skewed data), box and whisker plots, proportions, means (normal data) and hazard ratios, with 95% confidence intervals (95% CI). Differences in median symptom duration (days) between TB cases and non-cases were analyzed using the Mann-Whitney rank-sum test. Possible TB cases were excluded from this primary comparison. Sensitivity analyses were performed under the assumption, first, that Possible TB cases were true TB cases (grouped with Definite/Probable TB); and second, that Possible TB cases were non-cases (grouped with Unlikely/Not TB). Factors independently associated with time to resolution of baseline cough, wheeze and FTT, were modeled using Cox proportional hazard regression in TB cases only. The variables age, gender, CXR, birth weight, household TB contact, weight for age z-score, and MTB culture were included in the model. A forward stepwise regression strategy, beginning with a null model, was used to select the most significant variables in the multivariate analysis (p value < 0.1). Adjusted Hazard Ratios (AHR) with 95% CI were computed to analyze determinants of time-to-event (i.e. symptom resolution). Data analysis was performed using STATA version 12.1 (Stata Corp; College Station, Texas).
RESULTS
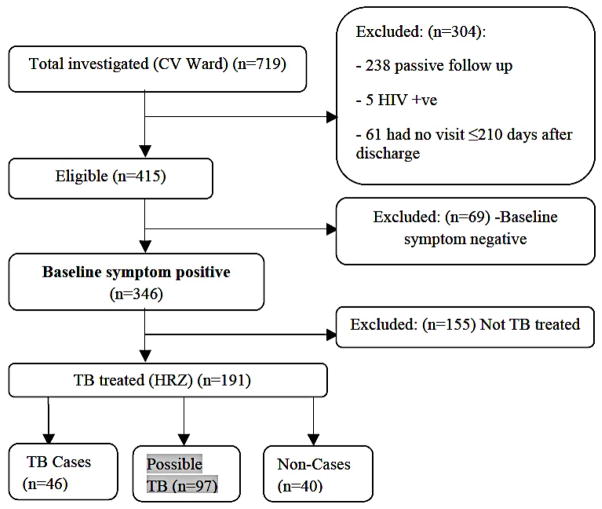
Figure 1 summarizes the flow of children who were investigated, diagnosed, and treated for pulmonary TB, and who are included in this analysis. A total of 719 children were investigated for suspected TB. Two hundred and thirty eight children in the passive surveillance arm, who did not have scheduled three-monthly follow-up visits, were excluded. Sixty-six children were excluded on other grounds, including five children on the basis of HIV infection status (positive or unknown) and 61 children who did not have a follow up visit within 210 days. A further 69 children either had no symptoms at baseline, or had missing symptom data. One hundred and fifty five of the remaining 346 symptomatic children were not treated for TB by the attending clinician. Therefore, a total of 191 symptomatic children, who started a six-month course of HRZ treatment for suspected pulmonary TB disease, are included in this analysis.
Figure 1. Participant Flow.
Abbreviations: TB, tuberculosis; HRZ, isoniazid, rifampin, pyrazinamide
Table 1 summarizes baseline characteristics and diagnostic data. 191 symptomatic TB treated children; median age, 12 months (IQR: 8–15 months), mean birth weight, 2.8 kg (95% CI 2.7 – 2.8 kg) and mean weight for age z-score −1.31 (95% CI −1.56 to −1.06) were analyzed. Forty-three (23%) of the 191 children had a CXR compatible with pulmonary TB and 9 (5%) children had culture-confirmed MTB.
Table 1.
Baseline Characteristics and diagnostic variables [n=191]
| Variable | TB Cases (n = 48) | Possible Cases (n = 97) | Non Cases (n = 46) |
|---|---|---|---|
| N Mean (SD) or % n | N Mean (SD) or % n | N Mean (SD) or % n | |
| Female Gender | 52 % [25] | 53 % [51] | 34 % [16] |
| Household TB Contact | 56 % [27] | 67 % [64] | 72 % [34] |
| Age [months]# | 12.1 [7.4 – 16.5] | 12.4 [7.7 – 16.1] | 12.6 [9.3 – 15.5] |
| WFA Z-score | −1.29 [1.63] | −1.32 [1.59] | − 1.32 [2.1] |
| Birth Wt> 2500g | 29% [14] | 27 % [26] | 32 % [15] |
| Baseline Symptoms | |||
| Cough | 50 % [24] | 65 % [62] | 51 % [24] |
| Wheeze | 47 % [23] | 54 % [52] | 53 % [25] |
| FTT | 65 % [31] | 67 % [64] | 53 % [25] |
| Radiologic Factors (Chest Radiograph) | |||
| Pos Chest Xray | 83 % [40] | 3 % [3] | 0 % [0] |
| Microbiologic Factors | |||
| MTB Culture tve | 19 % [9] | 0 % [0] | 0 % [0] |
| Pos Tuberculin Skin Test (TST) | 40 % [19] | 22 % [21] | 11 % [5] |
Abbreviations: House-hold TB Contact, smear positive TB contact or contact with a recently diagnosed TB case; WFA Z-score, weight for age z-score; Wt, weight; FTT, Failure to Thrive; Pos, positive; MTB, Mycobacterium tuberculosis; Pos Chest radiograph, chest radiograph compatible with TB; TB, tuberculosis
Median [interquartile range]; Data are mean [SD] or % [n] unless otherwise stated
Median time to first post-treatment visit was 61 days (IQR 41–75 days). Forty-seven of the 110 (43%) children with cough, 43 of the 100 (43%) children with wheeze, and 43 of the 120 (36%) children with FTT at baseline, had resolved these symptoms by the first post-treatment visit. Median time to resolution of baseline cough was 66 days (IQR 47–78 days); that of wheeze was 65 days (IQR 50–76 days); and that of FTT was 68 days (IQR 47–97 days).
Forty-eight children (25%) were classified as TB cases and 46 children as non-cases, based on the study diagnostic algorithm. There was no statistical difference in time to resolution of baseline cough, wheeze, or FTT, between TB cases and non-cases. Median duration of baseline cough was (63 vs. 70 days, p=0.98), wheeze (62 vs. 68 days, p=0.87) and FTT (76 vs. 66 days, p=0.59). Sensitivity analyses performed, first, under the assumption that children with Possible TB were true TB cases, and second, under the assumption that children with Possible TB were non-cases, did not alter these findings. Thereafter, Possible TB cases were excluded from subsequent analyses (data not shown).
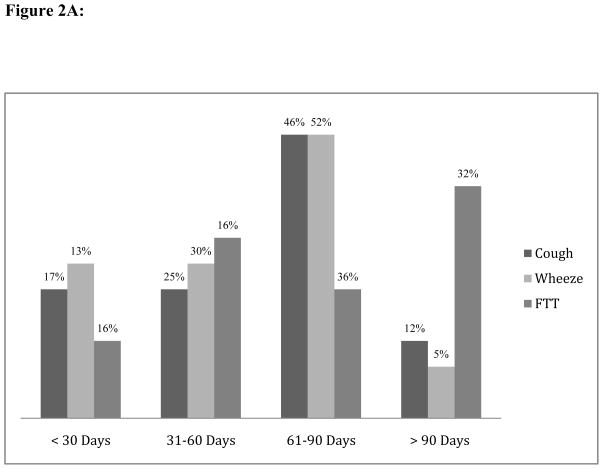
Among TB cases, only twelve of the 24 (50%) children with baseline cough, 11 of the 23 (48%) children with baseline wheeze, and 14 of the 31 (45%) children with baseline FTT, had resolved these symptoms by the first post-treatment visit. Only 10 of 24 (42%) TB cases with baseline cough, 10 of 23 TB cases with baseline wheeze (44%), and 10 of 31 (32%) TB cases with baseline FTT, had resolved these symptoms within 60 days after starting treatment (Figure 2A).
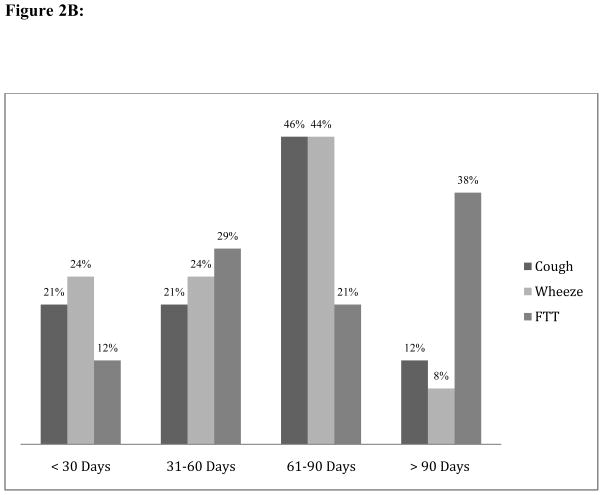
Figure 2.
Figure 2A: Proportion of Symptom Resolution Among TB Cases By Timing Of Follow-up Visit
Abbreviations: FTT, failure to thrive.
Figure 2B: Proportion of Symptom Resolution Among Non-Cases By Timing Of Follow-up Visit
Abbreviations: FTT, failure to thrive.
Univariate associations between demographic factors, other possible predictors, and median time to baseline symptom resolution were modeled using Cox proportional hazards regression and summarized in Table 2. Only low birth weight was a significant predictor of time to resolution of FTT. The relative ‘hazard’ for resolution of FTT increased nearly three-fold (i.e. shorter time to resolution) in newborns of normal birth weight, compared to low birth weight newborns.
Table 2.
Hazard Ratios and 95% Confidence Intervals: Univariate Model Estimating Associations Between Predictors and Time to Symptom Resolution in TB cases [n=48]
| Predictor | Cough | Wheeze | FTT | |||
|---|---|---|---|---|---|---|
| HR [95% CI] | P. value | HR [95% CI] | P. value | HR [95% CI] | P. value | |
| Gender (F) | 0.79 [0.33–1.91] | 0.60 | 1.43 [0.61–3.36] | 0.42 | 0.92 [0.42–2.01] | 0.83 |
| Base Age | 1.06 [0.98–1.15] | 0.12 | 1.01 [0.93–1.09] | 0.86 | 0.99 [0.93–1.07] | 0.88 |
| BW > 2500g | 1.13 [0.43–2.93] | 0.80 | 1.75 [0.61–5.02] | 0.29 | 2.71 [1.09–6.77] | 0.03* |
| HhTB contact | 0.57 [0.24–1.39] | 0.22 | 1.09 [0.45–2.68] | 0.84 | 0.99 [0.46–2.19] | 0.99 |
| Z–score | 0.95 [0.78–1.16] | 0.64 | 0.72 [0.46–1.14] | 0.16 | 0.84 [0.64–1.11] | 0.22 |
| MTB culture | 0.95 [0.22–4.18] | 0.96 | 0.72 [0.16–3.16] | 0.67 | 0.70 [0.24–2.07] | 0.52 |
| CXR +ve | 0.51 [0.19–1.31] | 0.16 | 0.51 [0.21–1.27] | 0.15 | 0.56 [0.24–1.27] | 0.16 |
Abbreviations: HR, hazard ratio; FTT, failure to thrive; F, female gender; Base Age, base line age; BW, birth weight; HhTB, household tuberculosis contact; MTB, Mycobacterium tuberculosis; CXR +ve, chest radiograph compatible with TB.
Statistically significant [p < 0.05, HR 95% confidence interval do not overlap HR =1]
Table 3 presents the multivariate model for independent associations between predictor variables and median time to baseline symptom resolution, after adjusting for covariates. A CXR compatible with pulmonary TB was the most consistent predictor of longer time to symptom resolution. Children with a positive CXR had 69%, 74%, and 59% decreased relative ‘hazard’ for resolution (i.e. longer time to resolution) of baseline cough, wheeze, and FTT, respectively. Older age was associated with increased relative ‘hazard’ for resolution of cough (i.e. shorter time to resolution).
Table 3.
Adjusted Hazard Ratio (AHR) and 95% Confidence Intervals: Multivariate Model Estimating Associations Between Predictors and Time to Symptom Resolution in TB cases [n=48]
| Outcome | Predictor Variable | AHR | [95% CI] | P value |
|---|---|---|---|---|
| Cough | CXR +ve | 0.31 | 0.10–0.94 | 0.039 * |
| Baseline Age | 1.11 | 1.01–1.22 | 0.033 * | |
| Wheeze | CXR +ve | 0.26 | 0.07–0.96 | 0.042 * |
| Baseline Age | 1.15 | 0.99–1.34 | 0.060 | |
| Gender [F] | 2.58 | 0.84–7.95 | 0.098 | |
| FTT | CXR +ve | 0.41 | 0.17–0.99 | 0.048 * |
| Birth Weight >2500g | 3.70 | 1.37–9.98 | 0.010 * |
Abbreviations: AHR, adjusted hazard ratio; FTT, failure to thrive; CXR +ve, chest radiograph compatible with TB; F, female gender
Statistically significant [p < 0.05, AHR 95% confidence interval do not overlap AHR =1]
DISCUSSION
We have shown that the majority of young children with a diagnosis of pulmonary TB, in the setting of a clinical trial, took longer than 60 days after starting TB treatment for baseline cough, wheeze, and failure to thrive to resolve. It follows that adoption of a two-month threshold to define a positive clinical response to treatment for retrospective confirmation of TB diagnosis, would exclude at least half of pulmonary TB cases in this age group. These findings conflict with a recent expert consensus statement, which recommends symptom resolution within two months on TB treatment as a diagnostic criterion in young children with pulmonary TB [4].
We have also shown that time to resolution of these clinical features did not differentiate between pulmonary TB cases, including cases with radiological and microbiological confirmation, and non-cases, using a robust clinical trial algorithm. This important finding suggests that the timing of clinical response to TB treatment, characterized by resolution of baseline cough, wheeze, or FTT, should not be included in the TB endpoint definition for clinical trials of new diagnostics, drugs, and vaccines[4, 8].
Experienced clinicians might feel that response to TB treatment is useful for retrospective confirmation of a TB diagnosis in a clinical setting. The contrary findings of our study serve to underline the lack of evidence to support this apparently logical view, which hinges on two linked assumptions: first, that children with true TB disease will show clinical improvement on TB treatment, but no clinical improvement in the absence of TB treatment; and second, that children without true TB disease will show no clinical improvement on TB treatment, but will show clinical improvement in the absence of TB treatment. However, consideration of all possible diagnosis-and-treatment scenarios reveals several reasons why a child with TB might fail to respond to TB treatment, including: poor treatment adherence, inadequate dosage, drug resistance, co-infection and co-morbidities, including undernutrition, and post-viral and chronic lung disease [4, 8, 15]. It follows that delayed or inadequate response to treatment should not necessarily exclude a TB diagnosis. Similarly, children without true TB disease might show partial or even full clinical improvement for reasons unrelated to anti-TB drug therapy. For example, viral and bacterial pulmonary infections other than TB may cause similar symptoms that resolve coincidentally, while a child is receiving TB treatment [4, 8, 15]. Growth failure may respond to social and nutritional interventions instigated in parallel with TB treatment, and persistent cough and wheeze might resolve periodically in children with undiagnosed asthma. Any of these factors might have contributed to the fact that time to resolution of baseline symptoms did not discriminate TB cases from non-cases.
It is also possible that diagnostic use of symptom resolution assumes a higher degree of specificity for symptom-based TB diagnosis than is truly the case. The use of symptoms for clinical diagnosis of childhood TB is understandable, since microbiological confirmation is rarely achieved in pauci-bacillary disease and radiological features may be difficult to interpret [11, 16, 17]. However, symptom-based approaches for diagnosis of childhood pulmonary TB yield varying results [18–20], may lack validation [11], and may include poorly-defined symptoms that are common in children with conditions other than TB [21]. Previous studies have shown that symptoms commonly associated with TB may be of limited diagnostic value in children from endemic settings, unless these symptoms are well defined [22]. Clinical features in this study were recorded as compatible with TB at baseline if persistent for longer than two weeks, in the case of cough and wheeze, or longer than two months in the case of failure to thrive. Our definitions of symptoms, and time to resolution of symptoms, appear sufficiently precise to detect differences within and between groups, since we have shown that CXR features of pulmonary TB were associated with longer duration of cough, wheeze, and FTT, after adjusting for other covariates. These findings agree with data from other studies, in which severity of radiographic pathology was associated with time to sputum smear and MTB culture conversion, an objective measure of response to TB treatment [15]. Previous studies have also reported a relationship between baseline cough and covariates, including birth weight, age, and CXR, and symptom duration [23].
This study has certain limitations. We performed a retrospective analysis of prospectively collected data. The original data collection instruments were not specifically designed for this study and therefore, we were unable to analyze TB drug adherence, dosage, and susceptibility; or antibiotic treatment of co-morbidities. However, data were collected under clinical trial conditions and we believe that the diagnostic data and TB case classifications are robust. We did not analyze time to resolution of symptoms among children who were not treated for TB, since we chose to focus on a valid comparison only among children who received TB treatment. We analyzed only HIV uninfected children in order to standardize interpretation of the diagnostic data. We acknowledge that our definition of symptom resolution is limited, since only presence or absence was recorded at follow-up, and therefore we were unable to analyze partial changes in symptom severity over time. Accordingly, we were not able to interpret clinical responses that might have occurred within the early weeks of TB treatment, or symptoms which might have resolved, but then recurred.
However, we note that the timing of follow-up visits was ideal to test whether clinical response to treatment occurred within a two-month threshold, as per the recent recommendations for diagnosis of childhood TB[4]. The fact that more than half of symptoms in TB cases had not resolved within two months emphasizes that clinical response to TB treatment requires longer than has been suggested [4]. Further, since we were able to demonstrate an association between CXR pathology, a potential marker of TB disease severity [24], and time to resolution of symptoms, we suggest that our definition of symptom resolution should have been sufficiently sensitive and specific to allow observation of any difference in clinical response to treatment between TB cases and non-cases, if such a difference truly existed [4]. Finally, we acknowledge that these findings might not be applicable to children treated for pulmonary TB in other clinical settings, in older children, HIV infected children, or in children with a different spectrum of TB disease.
Acknowledgments
Source of Funding
The South African Tuberculosis Vaccine Initiative (SATVI) and a National Institute of Health (NIH) Fogarty Operational Research Fellowship supported this work (5 D43 TW 007115-06).
We thank the Neonatal Cohort Study team of the South African Tuberculosis Vaccine Initiative (SATVI); and Professor Linda-Gail Bekker of the Desmond Tutu HIV Center, University of Cape Town.
Footnotes
Conflicts of Interest: No conflicts of interest were declared by any of the authors. Conflicts that the Editors consider relevant to the content of the manuscript are disclosed.
References
- 1.World Health Organization. Global tuberculosis report 2012 (in IRIS) Geneva: World Health Organization; 2012. p. viii.p. 272. [Google Scholar]
- 2.Hatherill M, Hanslo M, Hawkridge T, et al. Structured approaches for the screening and diagnosis of childhood tuberculosis in a high prevalence region of South Africa. Bull World Health Organ. 2010;88:312–20. doi: 10.2471/BLT.09.062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. [PubMed] [Google Scholar]
- 4.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205 (Suppl 2):S199–208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev. 2011;12:16–21. doi: 10.1016/j.prrv.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harries AD, Hargreaves NJ, Graham SM, et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis. 2002;6:424–31. [PubMed] [Google Scholar]
- 7.Marais BJ, Hesseling AC, Gie RP, et al. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–63. [PubMed] [Google Scholar]
- 8.Hatherill M, Verver S, Mahomed H. Consensus statement on diagnostic end points for infant tuberculosis vaccine trials. Clin Infect Dis. 2012;54:493–501. doi: 10.1093/cid/cir823. [DOI] [PubMed] [Google Scholar]
- 9.Hawkridge A, Hatherill M, Little F, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. Bmj. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesseling AC, Schaaf HS, Gie RP, et al. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6:1038–45. [PubMed] [Google Scholar]
- 12.Moyo S, Verver S, Hawkridge A, et al. Tuberculosis case finding for vaccine trials in young children in high-incidence settings: a randomised trial. Int J Tuberc Lung Dis. 2012;16:185–91. doi: 10.5588/ijtld.11.0348. [DOI] [PubMed] [Google Scholar]
- 13.Moyo S, Verver S, Mahomed H, et al. Age-related tuberculosis incidence and severity in children under 5 years of age in Cape Town, South Africa. Int J Tuberc Lung Dis. 2010;14:149–54. [PubMed] [Google Scholar]
- 14.Cole SZ, Lanham JS. Failure to thrive: an update. Am Fam Physician. 2011;83:829–34. [PubMed] [Google Scholar]
- 15.Horne DJ, Johnson CO, Oren E, et al. How soon should patients with smear-positive tuberculosis be released from inpatient isolation? Infect Control Hosp Epidemiol. 2010;31:78–84. doi: 10.1086/649022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int J Tuberc Lung Dis. 2001;5:594–603. [PubMed] [Google Scholar]
- 17.Osborne CM. The challenge of diagnosing childhood tuberculosis in a developing country. Arch Dis Child. 1995;72:369–74. doi: 10.1136/adc.72.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houwert KA, Borggreven PA, Schaaf HS, et al. Prospective evaluation of World Health Organization criteria to assist diagnosis of tuberculosis in children. Eur Respir J. 1998;11:1116–20. doi: 10.1183/09031936.98.11051116. [DOI] [PubMed] [Google Scholar]
- 19.Salazar GE, Schmitz TL, Cama R, et al. Pulmonary tuberculosis in children in a developing country. Pediatrics. 2001;108:448–53. doi: 10.1542/peds.108.2.448. [DOI] [PubMed] [Google Scholar]
- 20.Schaaf HS, Beyers N, Gie RP, et al. Respiratory tuberculosis in childhood: the diagnostic value of clinical features and special investigations. Pediatr Infect Dis J. 1995;14:189–94. [PubMed] [Google Scholar]
- 21.Marais BJ, Obihara CC, Gie RP, et al. The prevalence of symptoms associated with pulmonary tuberculosis in randomly selected children from a high burden community. Arch Dis Child. 2005;90:1166–70. doi: 10.1136/adc.2004.060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marais BJ, Gie RP, Obihara CC, et al. Well defined symptoms are of value in the diagnosis of childhood pulmonary tuberculosis. Arch Dis Child. 2005;90:1162–5. doi: 10.1136/adc.2004.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hales CM, Heilig CM, Chaisson R, et al. The Association between Symptoms and Microbiologically Defined Response to Tuberculosis Treatment. Ann Am Thorac Soc. 2013;10:18–25. doi: 10.1513/AnnalsATS.201207-038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiseman CA, Gie RP, Starke JR, et al. A proposed comprehensive classification of tuberculosis disease severity in children. Pediatr Infect Dis J. 2012;31:347–52. doi: 10.1097/INF.0b013e318243e27b. [DOI] [PubMed] [Google Scholar]