Abstract
Aims
To examine the genetic overlap between borderline personality features (BPF) and substance use disorders (SUDs) and the extent to which variation in personality traits contributes to this covariance.
Design
Genetic structural equation modelling was used to partition the variance in and covariance between personality traits, BPF, and SUDs into additive genetic, shared, and individual-specific environmental factors.
Setting
All participants were registered with the Australian Twin Registry.
Participants
A total of 3,127 Australian adult twins participated in the study.
Measurements
Diagnoses of DSM-IV alcohol and cannabis abuse/dependence (AAD; CAD), and nicotine dependence (ND) were derived via computer-assisted telephone interview. BPF and five-factor model personality traits were derived via self-report questionnaires.
Findings
Genetic factors were responsible for 49% (95%CI: 42%–55%) of the variance in BPF, 38–42% (95%CI range: 32%–49%) for personality traits and 47% (95%CI: 17%–77%), 54% (95%CI: 43%–64%), and 78% (67%–86%) for ND, AAD and CAD, respectively. Genetic and individual-specific environmental correlations between BPF and SUDs ranged from .33–.56 (95%CI range: .19–.74) and .19–.32 (95%CI range: .06–.43), respectively. Overall, there was substantial support for genetic influences that were specific to AAD, ND and CAD (31%–69%). Finally, genetic variation in personality traits was responsible for 11% (Extraversion for CAD) to 59% (Neuroticism for AAD) of the correlation between BPF and SUDs.
Conclusions
Both genetic and individual-specific environmental factors contribute to comorbidity between borderline personality features and substance use disorders. A substantial proportion of this comorbidity can be attributed to variation in normal personality traits, particularly Neuroticism.
Introduction
Borderline personality disorder is characterized by unstable relationships, identity disturbance, affective instability and impulsivity. Prevalence rates are estimated at 1.6% – 5.9% in the general population (1) and are considerably higher in outpatient and inpatient clinical settings (i.e., 10% and 20%, respectively). Additionally, it is associated with increased risk for comorbid physical(2) and mental health disorders(3). One particularly prevalent comorbidity is that between borderline personality disorder and substance use disorders (SUDs). Furthermore, individuals with borderline personality disorder are more likely to transition from asymptomatic to symptomatic substance use (4), persist in problematic use over a 3-year period(5), and are 4 to 10 times more likely to meet criteria for SUDs relative to those without a borderline diagnosis(6).
It is estimated that 40% of the variance in borderline personality disorder is accounted for by genetic factors(7), while approximately 50% of the variance in SUDs is heritable(8). Given this genetic underpinning, Bornovalova and colleagues(9) longitudinally examined genetic and environmental contributions to the comorbidity of borderline personality traits and substance use (i.e., mean quantity/frequency across alcohol, nicotine, and marijuana) in adolescent twins and demonstrated that shared environment accounted for all of the association between borderline personality and substance use at age 14 (rc=1.00); however, a moderate genetic correlation accounted for most of this association at age 18 (rg=.38), suggesting that common genes influence these phenotypes. In adult twins, Distel and colleagues(10) examined genetic overlap between substance use and borderline personality features (BPF), which comprises affective instability, identity problems, negative relationships, and self-harm derived from the Personality Assessment Inventory-Borderline subscale(11). They found that the correlation between BPF and both regular smoking and ever use of cannabis was explained by genetic factors (rg=.40 and .51, respectively), whereas unique environmental factors (re=.32) accounted for the overlap between BPF and high alcohol consumption. While these studies allude to shared genetic underpinnings between BPF and substance involvement, particularly during adulthood, neither study examined SUDs, a severe and more heritable manifestation of maladaptive substance involvement (12–15).
An additional gap in this literature is the role of personality traits in explaining the relationship between borderline personality disorder and SUDs. As incorporated into Section III of the Fifth edition of the Diagnostic and Statistical Manual (1), personality disorders can be conceptualized as maladaptive configurations of personality traits and assessed using dimensional trait models, such as the Five Factor Model (16). Meta-analytic findings have demonstrated that FFM Neuroticism (high), Agreeableness (low) and Conscientiousness (low) are most strongly associated with borderline personality disorder(17;18). Furthermore, personality traits are heritable(19), and studies (20;21) have reported strong genetic correlations between BPF and Neuroticism, Conscientiousness, Agreeableness, and in one case, Extraversion(20). More importantly, Distel and colleagues found that, when modeled simultaneously, genetic variation in these traits explains all of the heritable variation in BPF. Therefore, personality traits may harbor important etiological information regarding vulnerability to borderline personality disorder.
Meta-analytic research also shows that Neuroticism, Agreeableness and Conscientiousness are linked to SUDs(22). Specifically, significant effect sizes emerged between Neuroticism and Conscientiousness and alcohol and drug use disorders, as well as mixed SUDs (i.e., both alcohol and drug). Low Agreeableness was significantly associated with drug use and mixed SUDs only. Genetic variation in SUDs is also correlated with personality traits, in particular Conscientiousness-related dimensions, as well as Agreeableness and Extraversion related dimensions (23–25).
The above findings suggest that FFM personality traits may be common etiological risk factors for BPF-SUD symptomatology, and that variation in these traits may be partially implicated in the covariation between BPF and SUDs. Using data from a population-based sample of young adult twins, the current study examines (a) the role of overlapping genetic and environmental influences in the comorbidity between BPF and SUDs, including DSM-IV alcohol and cannabis abuse/dependence and nicotine dependence, and further, (b) the extent to which genetic and environmental influences on FFM personality traits contribute to the covariation between BPF and SUDs.
Methods and Materials
Sample
The initial sample consisted of 3,348 twins, ages 21–40 from the Australian Twin Registry interviewed between 2005 and 2009 in a study primarily focusing on cannabis use (26). Participants were interviewed using a computer-assisted telephone interview, and 3,186 of the original sample completed a self-report questionnaire, on average, within two weeks of the interview. The final sample included 3,127 twins with complete data for relevant study variables (i.e., SUDs, BPF, and FFM traits): (a) 948 monozygotic female twins (MZF; 382 pairs, 184 unpaired); (b) 444 monozygotic male twins (MZM; 153 pairs, 138 unpaired); (c) 716 dizygotic female twins (DZF; 288 pairs, 140 unpaired) (d) 330 dizygotic male twin (DZM; 95 pairs, 140 unpaired) and (e) 689 dizygotic opposite sex twins (DZOS; 198 pairs, 293 unpaired). Mean age in the current study was 31.84 (sd=2.48).
Measures
Substance Use Disorders (SUDs)
SUDs were assessed using the Australian version of the Semi-Structured Assessment of the Genetics of Alcoholism (Australian version; SSAGA-OZ), which utilizes a computer-administered telephone interview. The SSAGA(27) has demonstrated good reliability and validity as a measure of standardized DSM-IV diagnostic criteria for a range of conditions including SUDs(28). For the current analyses, binary variables were created for DSM-IV diagnoses of alcohol and cannabis abuse/dependence (AAD and CAD, respectively) and nicotine dependence (ND). For AAD and CAD, a “1” was coded if participants met criteria for either abuse or dependence and a “0” for neither abuse nor dependence. For AAD, CAD, and ND, prevalence rates in the current sample were 47.2%, 15.6% and 25%, respectively.
Borderline Personality Features (BPF)
BPF were assessed by the 24-item, self-reported Personality Assessment Inventory– Borderline subscale (PAI-BOR;11). The PAI-BOR generates a total score and scores on four subscales. In the current study, only the total score was used (α=.88). PAI-BOR scores were divided into five approximately equal categories and tested for multivariate normality prior to phenotypic and twin modeling analyses.
Five-Factor Model Personality Traits (FFM)
FFM personality traits were assessed using the 60-item, self-report NEO Five-Factor Inventory (NEO-FFI;29). Items are scored on a 1 (Strongly Disagree) to 5 (Strongly Agree) scale, and domain scores were generated by computing the mean of items from each subscale. Alphas for Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness were .89, .80, .73, .76, and .84, respectively. Each domain score was divided into quartiles and tested for multivariate normality prior to phenotypic and twin modeling analyses.
Data Analysis
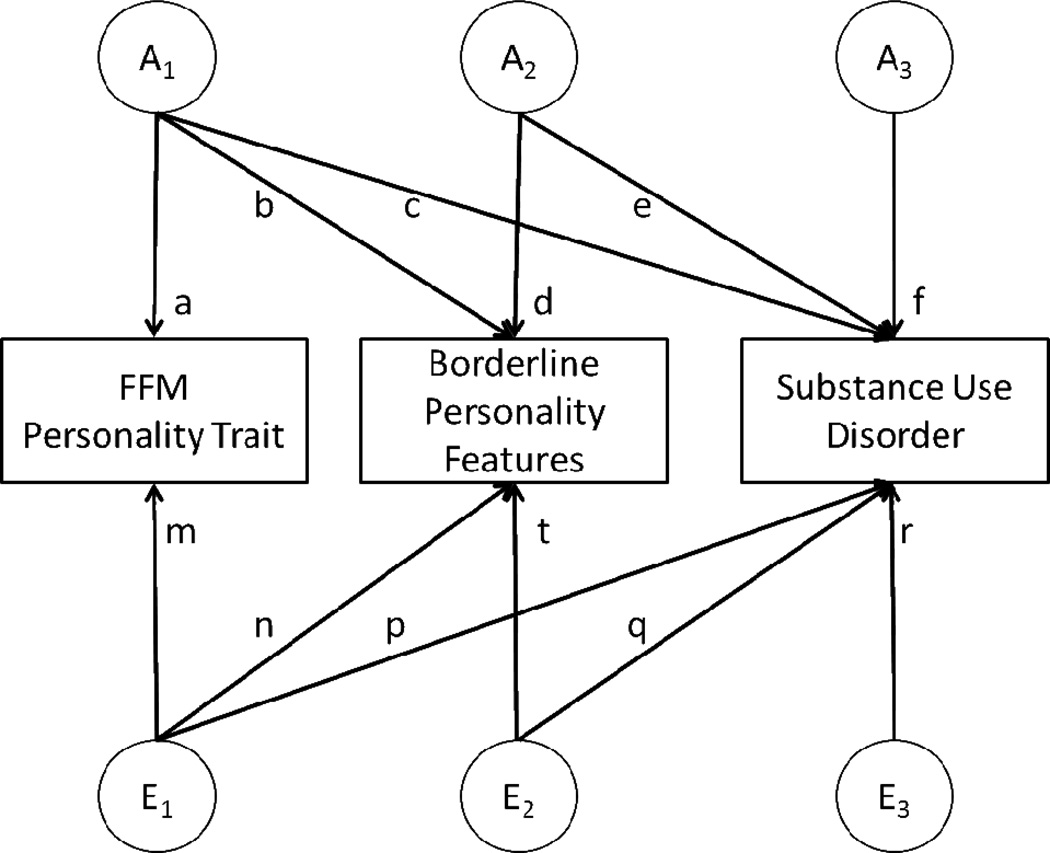
Phenotypic analyses (i.e., correlations between BPF, FFM personality traits, and SUDs) were conducted in SAS. Only FFM traits statistically significantly correlated with both BPF and SUDs (at p<0.05) were included in twin modeling procedures, conducted in Mx (30) using raw ordinal data and full-information maximum-likelihood (FIML) estimation. A series of trivariate Cholesky models were fit to the data (Figure 1). Each FFM personality trait was modeled first, followed by BPF, and then each SUD. Initial analyses allowed for differing thresholds (adjusted for age) and separate estimates for additive genetic (a2), shared environmental (c2) and individual-specific environmental (e2) influences on male and female twins. An omnibus test of sex differences was utilized to investigate whether constraining variance components (a2, c2, and e2) but not thresholds, across sexes resulted in a significant deterioration of fit using the difference in −2 log likelihood fit which is distributed as a chi-square. Similarly, the role of c2 was examined by constraining it to zero. Due to the relatively small sample size and uneven distribution of same- versus opposite-sex DZ pairs, qualitative sex differences (rDZOS <>0.5) were not explored. In addition to attributing variance in FFM personality, BPF and SUDs to a2, c2, and e2, we calculated the extent to which variance in SUDs and covariance between them and BPF was attributable to genetic and environmental influences on FFM personality.
Figure 1. Trivariate Cholesky decomposition model.
A1=genetic influences shared in common by five-factor model (FFM) trait, borderline personality features (BPF) and substance use disorder (SUD). A2=genetic influences shared in common by BPF and SUD. A3=genetic influences specific to SUD. E1=individual-specific environmental influences shared in common by five-factor model (FFM) trait, borderline personality features (BPF) and substance use disorder (SUD). E2= individual-specific environmental influences shared in common by BPF and SUD. E3= individual-specific environmental influences specific to SUD.
Results
Phenotypic Correlations
Polychoric and tetrachoric correlations between BPF, FFM personality traits and SUDs are shown in Table 1 (N=3,127). Strong correlations were noted between BPF and FFM traits, particularly Neuroticism (.75) and Agreeableness (−.48). The correlation between Openness and BPF was nonsignificant, and Openness was excluded from subsequent genetic analyses. BPF was moderately correlated with all three SUD variables (AAD=.25, CAD=.30, ND=.36). The SUDs were moderately to strongly intercorrelated (.40–.60). All correlations between SUDs and FFM traits were significant except for Openness and ND (.03) and Extraversion and AAD (.04). The latter relationship was therefore not explored from a genetic perspective. The remaining correlations ranged from |.07| (Openness and AAD) to |.25| (Conscientiousness and CAD). Based on these phenotypic associations, we examined 11 genetic models (i.e. the extent to which each of the 4 FFM personality traits accounted for the correlation between BPF and the three SUD measures, excluding Extraversion and AAD). This trivariate model is illustrated in Figure 1.
Table 1.
Phenotypic correlations and 95% confidence limits between borderline personality features, five-factor model personality traits, and substance use variables
| BPF | N | E | O | A | C | AAD | CAD | ND | |
|---|---|---|---|---|---|---|---|---|---|
| BPF | - | ||||||||
| N | .75* (.73–.77) | - | |||||||
| E | −.36*(−.40–−.33) | −.51*(−.54–−.47) | - | ||||||
| O | .00(−.04–.04) | −.02(−.06−.02) | .15*(.11–.18) | ||||||
| A | −.48*(−.52–−.46) | −.38*(−.41–−.35) | .30*(.26−.33) | .09*(.05−.13) | - | ||||
| C | −.36*(−.40–−.32) | −.41*(−.45–−.38) | .33*(.29–.36) | −.02(−.06−.03) | .30*(.27–.34) | - | |||
| AAD | .25*(.20–.29) | .15*(.11–.20) | .04(−.01–.09) | .07*(.03–.12) | −.19*(−.24–−.14) | −.15*(−.20–−.10) | - | ||
| CAD | .30*(.25–.36) | .15*(.09–.21) | −.10*(−.16–−.04) | .14*(.08–.20) | −.21*(−.26–−.15) | −.25*(−.30–−.19) | .48*(.42–.53) | - | |
| ND | .36*(.32–.41) | .23*(.18–.28) | −.12*(−.17–−.07) | .03(−.02–.09) | −.20*(−.25–−.15) | −.20*(−.25−.15) | .40*(.34–.45) | .60*(.54–.65) | - |
Note:
=p<.05; BPF=borderline personality features; N=Neuroticism; E=Extraversion; O=Openness; A=Agreeableness; C=Conscientiousness; AAD=Lifetime Alcohol Abuse or Dependence; CAD = Lifetime Cannabis Abuse or Dependence; ND=Lifetime Nicotine Dependence; N=3127;
Twin Modeling
All polychotomous variables satisfied the assumption of multivariate normality (ps > 0.05). All results are shown for models where parameters were constrained to be equal across male and female twins as there was no significant evidence for sex differences (p>0.05). Univariate heritability estimates (a2), ranging from .38 (Neuroticism) to .78 (CAD), for BPF, FFM traits and the SUDs are shown in Table 2 (N=3,127). The remainder of the variance in each measure, except for ND, could be attributed to individual-specific environmental factors (e2). While shared environmental influences could not be constrained to zero for ND, for all other measures such a constraint did not produce a significant deterioration in model-fit (χ2 ranging from 3.173–4.985, df=6, p>0.05).
Table 2.
Standardized estimates of additive genetic (a2), shared (c2) and individual-specific (e2) environmental variance for borderline personality features, five-factor model personality traits, and substance use disorders
| a2 (95% CI) | c2 (95% CI) | e2 (95% CI) | |
|---|---|---|---|
| Borderline Personality Traits (BPF) | .49 (.42 – .55) | - | .51 (.45 – .58) |
| Neuroticism (N) | .38 (.30 – .45) | - | .62 (.55 – .70) |
| Extraversion (E) | .41 (.33 – .48) | - | .59 (.52 – .67) |
| Agreeableness (A) | .40 (.32 – .48) | - | .60 (.52 – .68) |
| Conscientiousness (C) | .42 (.34 – .49) | - | .58 (.50 – .66) |
| Alcohol abuse/dependence (AAD) | .54 (.43 – .64) | - | .46 (.36 – .57) |
| Cannabis abuse/dependence (CAD) | .78 (.67 – .86) | - | .22 (.14 – .33) |
| Nicotine dependence (ND) | .47 (.17 – .77) | .27 (.00 – .51) | .26 (.18 – .36) |
Note: All estimates are statistically significant at p < 0.05; N=3127
Genetic (rg) and environmental (re) correlations between BPF and FFM traits as well as SUDs are shown in Table 3 (N=3,127). The strongest genetic and environmental overlap with BPF emerged for Neuroticism, followed by Agreeableness. The phenotypic correlation between BPF and AAD, ND and CAD was attributable to genetic and environmental influences as well, with genetic correlations ranging from .33 to .56 and individual-specific environmental correlations ranging from .19 to .32. Genetic correlations between FFM traits and SUDs (not shown in Table 3) ranged from |.11| (Extraversion and CAD) to |.46| (Agreeableness and ND); individual-specific environmental correlations ranged from |.03| (Agreeableness and AAD) to |.29| (Neuroticism and CAD).
Table 3.
Genetic (rg) and individual-specific environmental correlations (re) between borderline personality features (BPF) and substance use disorders (SUDs), accounting for correlations between BPF and five-factor model personality traits (shown) and FFM and SUDs (not shown)
| Borderline Personality Features | ||
|---|---|---|
| rg [95% CI] | re [95% CI] | |
| Neuroticism (N) | .87 (.80 – .93) | .68 (.62 –.73) |
| Extraversion (E) | −.52 (−.63 – −.40) | −.22 (−.31 – −.13) |
| Agreeableness (A) | −.65 (−.74 – −.54) | −.39 (−.46 – −.30) |
| Conscientiousness (C) | −.45 (−.51 – −.34) | −.26 (−.34 – −.17) |
| Alcohol abuse/dependence (AAD) | .33 (.19 – .47) | .19 (.06 – .32) |
| Cannabis abuse/dependence (CAD) | .34 (.20 – .46) | .32 (.14 – .37) |
| Nicotine dependence (ND) | .56 (.39 – .74) | .28 (.13 – .43) |
Note: All estimates are statistically significant at p < 0.05; N=3127
Importantly, we were interested in the proportion of variance in SUDs attributable to genetic and environmental influences on BPF and FFM personality traits – we provide proportions of the total variance in Table 4 (N=3,127) and, individually, proportions of genetic and individual-specific environmental variance separately in supplemental Table 1 (Table S1). Overall, there was substantial support for genetic influences specific to AAD, ND and CAD that were unshared with BPF and FFM personality traits (30.96–68.60%). Genetic influences on FFM traits contributed from 2.21% (Neuroticism and CAD; the overlap between Extraversion and CAD was not significant) to 9.75% (Agreeableness and ND) of the total variance in SUDs (approximating 2.97–21.14% of the genetic variance; Table S1). Even after accounting for that overlap and genetic overlap between FFM traits and BPF (Table 3), approximately 1.71% (Conscientiousness and AAD; the overlap between Neuroticism and AAD was not significant) to 12.48% (Neuroticism and ND) of the total variance in SUDs was attributable to BPF (approximating 3.28–22.61% of the genetic variance; Table S1). Broadly, speaking, environmental influences on FFM personality traits did not consistently overlap with those impacting SUDs (with a few exceptions, i.e., Neuroticism for CAD and ND) but there was consistent yet modest overlap in individual-specific environmental influences on BPF and SUDs.
Table 4.
Percentage of total variance in substance use disorders attributable to genetic and environmental variation in five-factor model personality traits, borderline personality features, and specific to substance use disorder
| Additive genetic factors | |||
| Personality | BPF | Specific | |
| Neuroticism | % | % | % |
| AAD | 6.58 | 0.10ns | 46.38 |
| CAD | 2.28 | 11.40 | 63.01 |
| ND | 6.35 | 10.99 | 30.76 |
| Extraversion | |||
| AAD | - | - | - |
| CAD | 1.00ns | 7.88 | 68.54 |
| ND | 2.21 | 12.48 | 32.22 |
| Agreeableness | |||
| AAD | 3.88 | 2.17 | 47.22 |
| CAD | 3.40 | 5.51 | 68.60 |
| ND | 9.75 | 5.41 | 30.96 |
| Conscientiousness | |||
| AAD | 6.09 | 1.71 | 44.41 |
| CAD | 3.65 | 5.14 | 68.06 |
| ND | 7.06 | 8.56 | 32.13 |
| Individual-specific environmental factors | |||
| Personality | BPF | Specific | |
| Neuroticism | % | % | % |
| AAD | 0.32ns | 1.60 | 45.04 |
| CAD | 1.95 | 0.69ns | 20.67 |
| ND | 1.65 | 0.62ns | 24.19 |
| Extraversion | |||
| AAD | - | - | - |
| CAD | 0.38ns | 2.09 | 20.11 |
| ND | 0.11ns | 2.03 | 24.58 |
| Agreeableness | |||
| AAD | 0.06ns | 1.74 | 44.93 |
| CAD | 0.05ns | 2.50 | 19.92 |
| ND | 0.14ns | 3.20 | 23.76 |
| Conscientiousness | |||
| AAD | 0.17ns | 2.44 | 45.19 |
| CAD | 0.71ns | 2.12 | 20.31 |
| ND | 0.03ns | 2.32 | 24.17 |
Note:
: not significant at p < 0.05; all other estimates are significant at p < 0.05;
BPF = borderline personality features; AAD=Lifetime Alcohol Abuse or Dependence; CAD = Lifetime Cannabis Abuse or Dependence; ND=Lifetime Nicotine Dependence; From Figure 1, total variance in SUDs = c2+e2+f2+p2+q2+r2 (e.g., genetic % personality = c2/c2+e2+f2+p2+q2+r2; environmental % personality = p2/c2+e2+f2+p2+q2+r2;); For ND, added genetic and individual-specific percentages do not equal 100% due to significant contribution of shared environmental factors, which are not shown in this table; N=3127
Table 5 shows similar estimates but for the proportion of total covariance between BPF and SUDs due to FFM traits (N=3,127). Proportions of covariance between BPF and SUD accounted for by personality computed individually for genetic and individual-specific environmental covariance are presented in supplemental Table 2 (Table S2). FFM personality traits were responsible for 14.55% (Extraversion for ND) to 59.30% (Neuroticism for AAD) of the total correlation/covariance between BPF and SUDs. With the exception of Neuroticism (10.67–21.34%), the total covariance between BPF and SUDs was largely independent of individual-specific environmental influences on personality, in that the covariance accounted for by Extraversion, Agreeableness, and Conscientiousness ranged from 0.81% (Conscientiousness and ND) to 4.89% (Conscientiousness and CAD).
Table 5.
Proportion of total covariance between BPF and SUD explained by genetic and environmental variation in five-factor model personality traits and specific to variation shared by BPF and SUD
| A | E | |||
|---|---|---|---|---|
| % personality |
% specific |
% personality |
% specific |
|
| Neuroticism | ||||
| AAD | 59.30 | 4.29 | 10.67 | 25.75 |
| CAD | 28.54 | 36.39 | 21.34 | 13.74 |
| ND | 40.81 | 31.00 | 16.98 | 11.20 |
| Extraversion | ||||
| AAD | - | - | - | - |
| CAD | 11.46 | 53.54 | 3.09 | 31.92 |
| ND | 14.55 | 57.14 | 1.43 | 26.87 |
| Agreeableness | ||||
| AAD | 34.15 | 30.23 | 2.50 | 33.12 |
| CCAD | 26.15 | 39.58 | 1.94 | 32.34 |
| ND | 35.72 | 31.88 | 2.63 | 29.78 |
| Conscientiousness | ||||
| AAD | 28.70 | 29.23 | 2.73 | 39.34 |
| CAD | 18.89 | 44.40 | 4.89 | 31.83 |
| ND | 22.65 | 48.22 | 0.81 | 28.32 |
Note: BPF=Borderline Personality Features; SUD=Substance Use Disorder; AAD=Lifetime Alcohol Abuse or Dependence; CAD = Lifetime Cannabis Abuse or Dependence; ND=Lifetime Nicotine Dependence A=genetic covariance; E=individual-specific environmental covariance; A=genetic covariance; E=individual-specific environmental covariance; From Figure 1, A % personality = bc/(bc+de+np+tq); A % specific = de/(bc+de+np+tq); E=individual-specific environmental covariance; From Figure 1, E % personality = np/(bc+de+np+tq); E % specific = tq/(bc+de+np+tq); N=3127
As we were most interested in the extent to which genetic influences contributed to the relationship between individual SUDs, BPF and FFM personality, our genetic results (Tables S1 and S2) are summarized by SUD below:
AAD: Genetic variance in AAD was partially derived from Neuroticism, Agreeableness and Conscientiousness, as well as from BPF but only in the absence of Neuroticism. In fact, the genetic covariance between AAD and BPF was entirely attributable to genetic overlap with Neuroticism. However, Agreeableness and Conscientiousness only explained a proportion of the genetic covariance between BPF and AAD.
ND: The genetic variance in ND overlapped most considerably with BPF and Agreeableness, with the latter responsible for 53% of the covariance between BPF and ND. The personality traits of Extraversion and Conscientiousness contributed genetic influences to ND and the covariance between BPF and ND to a more modest degree. However, while Neuroticism only contributed to 13% of the genetic variance in ND, it was responsible for over half the covariance between BPF and ND.
CAD): While BPF contributed modestly to the variance in CAD, the proportion of variance in CAD attributable to FFM personality traits was very small (0.4–4.8%). The traits of Neuroticism and Agreeableness contributed more substantially (40–43%) to the covariance between BPF and CAD than Extraversion and Conscientiousness (29–30%).
Discussion
The current study sought to parse the genetic and environmental contributions to comorbid BPF and SUDs, as well as the extent to which variation in FFM traits explains this overlap. As expected, BPF was correlated with all three SUD variables, and BPF and SUDs were most consistently associated with Neuroticism, Agreeableness, and Conscientiousness. The correlation between BPF and Neuroticism (r=.75) is somewhat higher than that reported in meta-analytic research (18). However, a strong correlation is expected given that personality disorders are conceptualized as maladaptive configurations of normal traits, and Neuroticism is most relevant to the borderline diagnosis. This high correlation could reflect common method variance or semantic overlap, although Distel and colleagues(20) indicated that “removing systematically one of the 12 items of the neuroticism scale does not result in a large change in the correlation with the PAI-BOR, thus the influence of semantic overlap on the association is unlikely to be large” (p.1136). The correlation between BPF and Extraversion was also significant (−.36), which is inconsistent with meta-analytic research (17;18); however, the latter did not include studies using the PAI to assess borderline pathology, and previous research using the PAI has shown that individuals scoring high on BPF report significantly lower scores on FFM Extraversion (31). The nonsignificant correlation between Extraversion and AAD might also be considered surprising, given that sensation seeking is a core component of this personality domain and consistently linked to alcohol use (32;33). This may reflect a limitation of the NEO-FFI, in that only one item assesses the excitement seeking facet. However, meta-analysis has revealed nonsignificant effect sizes between Extraversion and substance use pathology(22).
In general, the magnitude of additive genetic and individual-specific environmental contributions to BPF, SUDs and FFM traits were highly consistent with the published literature (7;8;19). Though we used ordinal FFM variables, estimates of heritability were nearly identical to studies that have used continuously distributed scores (19). The lack of the role of shared environment also mirrors published work. We were, however, unable to constrain shared environmental influences on ND to zero, which departs from previous findings (15;34).
The primary aim was to examine genetic and environmental contributions to the comorbidity between BPF and SUDs. Moderate genetic correlations emerged between BPF and SUDs (i.e., .33, .34, and .56 for AAD, CAD, and ND, respectively). Other studies examining comorbid heritability of BPF and substance involvement also reported moderate genetic influences for lifetime cannabis use and regular tobacco use in adults, as well as with tobacco, alcohol, and cannabis use at age 18(9;10). However, Distel and colleagues reported that only the individual-specific environmental correlation was significant between BPF and high alcohol consumption (i.e., 21+ drinks/wk for males; 14+ drinks/wk for females), whereas the current results indicate that comorbid AAD and BPF are influenced by both individual-specific environmental and genetic factors. This may reflect differences in the importance of genetic factors at various stages of substance use, and extant research(12–15) supports the notion that later stages of involvement (e.g., SUDs) may be more heavily genetically influenced than earlier stages (e.g., initiation, regular use).
Genetic variation in personality traits has also been implicated in the etiology of substance involvement, albeit to a lesser degree. This study provides the most comprehensive examination of the genetic overlap between FFM traits and SUDs. Small to moderate genetic correlations emerged for the overlap between FFM traits and both AAD and ND (|.22| – |.46|). More modest genetic correlations emerged for CAD and FFM traits (|.17| to |.22|). These findings are generally in line with two studies examining FFM personality traits and alcohol involvement – one found small to modest significant phenotypic correlations between all five FFM domains and problem drinking (35;35). An additional study reported moderate genetic correlations between alcohol use disorder symptoms and Neuroticism, Agreeableness, and Conscientiousness, but reported that drinking motives accounted for this genetic overlap(24). The findings for cannabis are nearly identical to genetic correlations reported previously between CAD and Neuroticism and Extraversion (23). There is a dearth of research, however, regarding the correlation between tobacco involvement and FFM traits. For instance, Heath and colleagues(36) examined the correlation between Neuroticism and Extraversion drawn from the Eysenck Personality Questionnaire and tobacco smoking and found the associations too modest to decompose into genetic and environmental sources. Overall, findings in the current study are consistent with molecular genetic studies suggesting that personality traits and SUDs, in part, share a genetic basis (37;38) and furthermore, that personality traits may partially mediate the relationship between specific genetic polymorphisms and SUDs (39).
Finally, we examined the extent to which variation in personality traits explains the comorbidity between BPF and SUDs. Overall, genetic variation in FFM traits accounted for a substantial portion of the genetic covariance between BPF and SUDs, and in one case, variation in Neuroticism completely explained the genetic comorbidity between BPF and AAD. This is consistent with theoretical and empirical work conceptualizing personality disorders as maladaptive configurations of normal personality traits(40) and provides support for the notion that personality trait variation may explain patterns of psychiatric comorbidity(41). Specifically, researchers have suggested that traits such as affective lability and impulsivity are a common diathesis for borderline personality disorder and SUDs and thus contribute to their co-occurrence(42). However, there is somewhat limited coverage of these traits within the NEO-FFI given that it assesses only the FFM domains (and not facet-level traits) which likely attenuated the proportion of genetic covariance in BPF-SUD accounted for by the FFM traits. Generally, these findings have important implications, because identifying etiological mechanisms of risk for psychopathology can inform more effective treatment. Specifically, these data suggest that personality traits may represent an intermediate phenotype in the risk for comorbid BPF and SUD. Therefore, greater attention in addiction genetics research to identification of genes conferring vulnerability for personality traits may be fruitful. Also, interventions targeting more basic traits that give rise to various forms of psychopathology (e.g., affective instability), rather than disorder-specific interventions, may be more effective in prevention and treatment.
Limitations beyond the lack of facet-level FFM trait assessment include the use of self-reported BPF rather than interview-derived data. Additionally, the study sample size limited the ability to model FFM traits simultaneously in relation to BPF and SUDs. For instance, the FFM personality trait domains share genetic underpinnings and a multivariate model that accounts, simultaneously, for this overlap as well as that between these traits, BPF and SUDs may be quite informative in a larger sample. Likewise, simultaneously modeling the genetic overlap across the three SUDs could be conducted and may be warranted given the high intercorrelations among the SUD variables. A larger sample would also allow disentangling the extent to which the genetic overlap between BPF, FFM personality traits and SUDs is due to covariation with earlier stages of substance experimentation and regular use versus later stages of abuse/dependence.
In conclusion, results of the current study indicate that both genetic and individual-specific environmental factors contribute to comorbid BPF and SUDs. More importantly, genetic variation in normal personality traits accounts for a substantial proportion of the genetic covariance between BPF and SUDs, particularly for Neuroticism and AAD. These findings support the notion that personality traits confer genetic vulnerability to comorbid BPF and SUDs. This is consistent with theoretical and empirical work that influenced the inclusion of a dimensional personality trait model in Section III of the DSM-5(1). Not only can this trait model be utilized in the assessment of personality disorders, but it could be used as a hierarchical framework for classifying specific disorders in the DSM(41). Specifically, Krueger and colleagues suggest that personality traits (e.g., Disinhibition) represent the core of various spectra of psychopathology that could be organized in the DSM based on commonly observed patterns of comorbidity (e.g., externalizing disorders). These are important considerations for future iterations of the DSM, and moving forward, research examining borderline personality disorder and comorbid psychopathology should account for the role of personality traits.
Supplementary Material
Acknowledgements
We thank Anjali Henders, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, and Adele Somerville, who worked on this project and the twins and their siblings for participating.
Declarations of Interest: This research was funded by National Institute on Drug Abuse (NIDA) grants. DA18267 (ML; data collection); DA23668 & K02DA32573 (AA; analysis) and T32DA007313 (LF); and facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health & Medical Research Council. Dr. Agrawal has received peer-reviewed grant funding, travel reimbursements and an honorarium from ABMRF/Foundation for Alcohol Research, which receives some of its funds from brewers.
Reference List
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.El-Gabalawy R, Katz LY, Sareen J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosom Med. 2010 Sep;72(7):641–647. doi: 10.1097/PSY.0b013e3181e10c7b. [DOI] [PubMed] [Google Scholar]
- 3.Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of Borderline Personality Disorder in a Community Sample: Comorbidity, Treatment Utilization, and General Functioning. J Pers Disord. 2013 Feb 27; doi: 10.1521/pedi_2012_26_093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton WM, Dawson DA, Conway KP, Brodsky M, Grant BF. Transitions in illicit drug use status over 3 years: a prospective analysis of a general population sample. Am J Psychiatry. 2013 Jun 1;170(6):660–670. doi: 10.1176/appi.ajp.2012.12060737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasin D, Fenton MC, Skodol A, Krueger R, Keyes K, Geier T, et al. Personality disorders and the 3-year course of alcohol, drug, and nicotine use disorders. Arch Gen Psychiatry. 2011 Nov;68(11):1158–1167. doi: 10.1001/archgenpsychiatry.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. J Pers Disord. 2010 Aug;24(4):412–426. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amad A, Ramoz N, Thomas P, Jardri R, Gorwood P. Genetics of borderline personality disorder: Systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014 Mar;40C:6–19. doi: 10.1016/j.neubiorev.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, et al. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornovalova MA, Hicks BM, Iacono WG, McGue M. Longitudinal twin study of borderline personality disorder traits and substance use in adolescence: developmental change, reciprocal effects, and genetic and environmental influences. Personal Disord. 2013 Jan;4(1):23–32. doi: 10.1037/a0027178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Distel MA, Trull TJ, de Moor MM, Vink JM, Geels LM, van Beek JH, et al. Borderline personality traits and substance use: genetic factors underlie the association with smoking and ever use of cannabis, but not with high alcohol consumption. J Pers Disord. 2012 Dec;26(6):867–879. doi: 10.1521/pedi.2012.26.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morey LC. Personality Assessment Inventory: Professional manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 12.Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010 Mar;105(3):417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000 Mar;57(3):261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 14.Sartor CE, Lynskey MT, Bucholz KK, Madden PA, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: evidence of common genetic influences. Addiction. 2009 Sep;104(9):1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005 Jul;35(4):397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- 16.Miller JD. Five-Factor Model personality disorder prototypes: a review of their development, validity, and comparison to alternative approaches. J Pers. 2012 Dec;80(6):1565–1591. doi: 10.1111/j.1467-6494.2012.00773.x. [DOI] [PubMed] [Google Scholar]
- 17.Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: A meta-analytic review. Clin Psychol Rev. 2004 Jan;23(8):1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Samuel DB, Widiger TA. A meta-analytic review of the relationships between the five-factor model and DSM-IV-TR personality disorders: a facet level analysis. Clin Psychol Rev. 2008 Dec;28(8):1326–1342. doi: 10.1016/j.cpr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: a twin study. J Pers. 1996 Sep;64(3):577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 20.Distel MA, Trull TJ, Willemsen G, Vink JM, Derom CA, Lynskey M, et al. The five-factor model of personality and borderline personality disorder: a genetic analysis of comorbidity. Biol Psychiatry. 2009 Dec 15;66(12):1131–1138. doi: 10.1016/j.biopsych.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Kendler KS, Myers J, Reichborn-Kjennerud T. Borderline personality disorder traits and their relationship with dimensions of normative personality: a web-based cohort and twin study. Acta Psychiatr Scand. 2011 May;123(5):349–359. doi: 10.1111/j.1600-0447.2010.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotov R, Gamez W, Schmidt F, Watson D. Linking "big" personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull. 2010 Sep;136(5):768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A, Jacobson KC, Prescott CA, Kendler KS. A twin study of personality and illicit drug use and abuse/dependence. Twin Res. 2004 Feb;7(1):72–81. doi: 10.1375/13690520460741462. [DOI] [PubMed] [Google Scholar]
- 24.Littlefield AK, Agrawal A, Ellingson JM, Kristjansson S, Madden PA, Bucholz KK, et al. Does variance in drinking motives explain the genetic overlap between personality and alcohol use disorder symptoms? A twin study of young women. Alcohol Clin Exp Res. 2011 Dec;35(12):2242–2250. doi: 10.1111/j.1530-0277.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002 Feb;111(1):124–133. [PubMed] [Google Scholar]
- 26.Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PA, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Res Hum Genet. 2012 Oct;15(5):631–641. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994 Mar;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 28.Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, et al. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004 Nov;34(8):1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- 29.Costa PTJ, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 30.Neale MC. Statistical Modeling with Mx. 2004 Ref Type: Unpublished Work. [Google Scholar]
- 31.Trull TJ. Borderline personality disorder features in nonclinical young adults: 1. Identification and validation. Psychological Assessment. 1995;7(1):33–41. [Google Scholar]
- 32.Hittner JB, Swickert R. Sensation seeking and alcohol use: a meta-analytic review. Addict Behav. 2006 Aug;31(8):1383–1401. doi: 10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Stautz K, Cooper A. Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clin Psychol Rev. 2013 Jun;33(4):574–592. doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004 Oct;34(7):1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz MA, Pincus AL, Dickinson KA. NEO PI-R predictors of alcohol use and alcohol-related problems. J Pers Assess. 2003 Dec;81(3):226–236. doi: 10.1207/S15327752JPA8103_05. [DOI] [PubMed] [Google Scholar]
- 36.Heath AC, Madden PA, Slutske WS, Martin NG. Personality and the inheritance of smoking behavior: a genetic perspective. Behav Genet. 1995 Mar;25(2):103–117. doi: 10.1007/BF02196921. [DOI] [PubMed] [Google Scholar]
- 37.Luo X, Zuo L, Kranzler H, Zhang H, Wang S, Gelernter J. Multiple OPR genes influence personality traits in substance dependent and healthy subjects in two American populations. Am J Med Genet B Neuropsychiatr Genet. 2008 Oct 5;147B(7):1028–1039. doi: 10.1002/ajmg.b.30701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang KS, Liu X, Aragam N, Mullersman JE, Jian X, Pan Y, et al. Polymorphisms in ABLIM1 are associated with personality traits and alcohol dependence. J Mol Neurosci. 2012 Feb;46(2):265–271. doi: 10.1007/s12031-011-9530-6. [DOI] [PubMed] [Google Scholar]
- 39.Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, et al. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PLoS One. 2012;7(6):e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widiger TA, Costa PT. Personality disorders and the Five Factor Model of Personality. 3rd ed. Washington, DC: APA; 2013. [Google Scholar]
- 41.Krueger RF, Eaton NR, Derringer J, Markon KE, Watson D, Skodol AE. Personality in DSM-5: helping delineate personality disorder content and framing the metastructure. J Pers Assess. 2011 Jul;93(4):325–331. doi: 10.1080/00223891.2011.577478. [DOI] [PubMed] [Google Scholar]
- 42.Trull TJ, Solhan MB, Brown WC, Tomko RL, Schaefer L, Jahng S. Substance use disorders and personality disorders. In: Sher K, editor. Oxford Handbook of Personality Disorders. New York: Oxford; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.