Abstract
BACKGROUND
Although human red blood cell (RBC) units may be refrigerator stored for up to 42 days, transfusion of older RBCs acutely delivers a large bolus of iron to mononuclear phagocytes. Similarly, iron dextran circulates in plasma for hours to days and is progressively cleared by mononuclear phagocytes, which return iron to plasma. Finally, malaria infection continuously delivers iron to macrophages by intra- and extravascular hemolysis. Studies suggest that iron administration increases infectious risk.
STUDY DESIGN AND METHODS
To assess the effects of increased iron availability on susceptibility to infection, we infected mice with model Gram-negative intracellular or extracellular pathogens (Salmonella typhimurium or Escherichia coli, respectively), accompanied by RBC transfusion, iron dextran administration, or malarial coinfection.
RESULTS
In our mouse models, transfusion of older RBCs exacerbates infection with both Gram-negative pathogens. Although iron dextran exacerbates E. coli infection to a similar extent as transfusion of corresponding amounts of iron, higher iron doses are required to produce comparable effects with S. typhimurium. Coinfection of mice with Plasmodium yoelii and S. typhimurium produces overwhelming Salmonella sepsis. Finally, treating mice with antibiotics abrogates the enhancing effect on E. coli infection of both older RBC transfusion and iron dextran administration.
CONCLUSIONS
Transfusion of older RBCs exacerbates Gram-negative infection to a similar extent as malaria coinfection or iron dextran administration. Appropriate antibiotic therapy abrogates the effect of older RBC transfusions on infection with E. coli. Iron delivery to macrophages may be an underappreciated mechanism mediating, at least some, adverse effects of RBC transfusions.
Administration of iron supplements, particularly to children in developing nations, increases the risk of infection and mortality.1–4 For example, the risk of Gram-negative sepsis increased by sixfold in a trial of iron dextran administration to newborns in Polynesia.2 Furthermore, in a meta-analysis of randomized studies of intravenous (IV) iron therapy, although iron administration reduced the need for red blood cell (RBC) transfusions, this practice is associated with a significantly increased risk of infection.5 Human tissues are iron restricted, and there is fierce competition between host and pathogen for this essential nutrient.6 In a meta-analysis of controlled trials,7 a restrictive transfusion strategy reduced the relative risk of infection (0.76; 95% confidence interval, 0.60–0.97). By US Food and Drug Administration (FDA) criteria, RBCs destined for transfusion can be refrigerator stored for up to 42 days before transfusion. Furthermore, in many observational studies,8–15 transfusions of RBCs stored for longer durations are an independent risk factor for infectious complications. Thus, both transfusion per se and iron therapy used to prevent RBC transfusions are associated with increased infectious complications.
We previously showed in mice,16 dogs,17 and humans18 that transfused, storage-damaged RBCs are rapidly cleared from the circulation by mononuclear phagocytes; the accompanying hemoglobin (Hb) is rapidly catabolized, and the associated iron is returned to plasma at a pace that can exceed the rate of uptake by transferrin, the physiologic iron transporter, thereby producing circulating non–transferrin-bound iron.16,18 After administration of iron dextran, most of the colloid circulates in plasma for several hours to days and is progressively cleared by mononuclear phagocytes that split the complex and then return the iron to plasma.19,20 Malaria infection continuously delivers iron to macrophages by intra- and extravascular hemolysis.21 Interestingly, certain pathogens, such as Salmonella sp., exhibit seasonality along with malaria infection, and coinfection with malaria increases morbidity and mortality.22–24 Thus, we hypothesize that iron administration, whether by older, stored RBC transfusions, by iron dextran, or by malaria infection, exacerbates infection with model Gram-negative pathogens (i.e., Salmonella typhimurium and Escherichia coli).
S. typhimurium is a facultative intracellular pathogen that survives and replicates inside mononuclear phagocytes.25 Salmonella infection is also associated with hemolytic disorders, such as malaria26–29 and sickle cell disease.30,31 In addition, in Africa, nontyphoidal Salmonella is frequently isolated, representing 18% of all isolates in children more than 60 days old.32 Although the prevalence of coinfection varies by region,24 nontyphoidal Salmonella is the most frequent,27–29 or among the most frequent,32 isolates from malaria-infected children. Finally, coinfections of malaria and nontyphoidal Salmonella are associated with increased morbidity and mortality in humans and animal models.27,33,34
Infection is a leading cause of morbidity and mortality in hospitalized patients. E. coli, an extracellular Gram-negative bacterium, frequently causes infection in intensive care units.35 Furthermore, animal models of hemolysis suggest that, like S. typhimurium, E. coli infections are exacerbated by hemolysis.36 Thus, using murine models,16,37 we examined the effects of RBC transfusion, iron dextran administration, or malaria on infections with model intracellular and extracellular bacterial pathogens: S. typhimurium and E. coli, respectively. Finally, to provide a potential explanation of why the effect of transfusion on infection risk is not more striking in epidemiologic studies of hospitalized patients, many of whom are on antibiotics, we treated mice with empiric antibiotic therapy and infected them with E. coli with or without RBC transfusion or iron dextran therapy.
MATERIALS AND METHODS
Mice
Wild-type male C57BL/6 and CD-1 mice were purchased (Charles River Laboratories, Wilmington, MA). Mice were used at 8 to 12 weeks of age. Procedures were approved by the Columbia University Medical Center Institutional Animal Care and Use Committee.
Mouse RBCs: collection, storage, and derivatives
Blood banks were prepared, as described.16 Briefly, C57BL/6 or CD-1 mice were bled aseptically by cardiac puncture into CPDA-1 obtained directly from di-(2-ethylhexyl)phthalate-plasticized, polyvinyl chloride, human primary collection packs (Code 4R3611, Baxter, Deerfield, IL). The final CPDA-1 concentration used for storage was 14%. Whole blood collected from 15 to 40 mice was pooled and leukoreduced using a neonatal high-efficiency leukoreduction filter (Purecell Neo, Pall Corporation, Port Washington, NY), centrifuged (400 × g for 15 min), and volume reduced to a final hematocrit of 60% to 80% and Hb of 16 to 19 g/dL. The RBCs (approx. 10 mL) were placed in 50-mL tubes (Falcon, BD Biosciences, San Jose, CA) and stored at 4°C for up to 14 days. On the day of transfusion, a 500-µL aliquot of stored RBCs was inoculated into a blood culture bottle (Peds Plus/F, BD Diagnostic Systems, San Jose, CA) and loaded into a blood culture system (BACTEC 9000, BD Diagnostic Systems), a continuous monitoring blood culture system, for up to 5 days or until bacterial growth was detected (this method detects at least 10 colony-forming units [CFUs]/mL with a sensitivity of 97%).38 Hemolysis rate was calculated, as follows:39
Hemolysis rate (%) = [(100 – RBC volume) (%) × fHb (g/dL)]/[Total Hb (g/dL)].
Washed stored RBCs were prepared with three washes using 10 volumes of phosphate-buffered saline (PBS) and centrifugation at 400 × g. After the final wash, the washed stored RBCs were resuspended in PBS to a final Hb concentration similar to the unwashed fresh unit (i.e., Hb of 16–19 g/dL). Supernatant was obtained after 400 × g centrifugation of stored RBCs, and 400 µL of this solution was infused undiluted. RBC ghosts were obtained by hypotonic lysis of twice the volume of stored RBCs (i.e., for 400 µL of ghosts, 800 µL of stored RBCs was lysed) with PBS:distilled water (1:15), followed by multiple washes with the same buffer and centrifugation at 30,000 × g until a white pellet was obtained; the latter was then resuspended in PBS.
Bacteria and malaria pathogens
S. typhimurium, strain LT2 (American Type Culture Collection, Manassas, VA) and bioluminescent enteropathogenic E. coli, Strain Xen 14 (PerkinElmer, Waltham, MA), were used. For each experiment, a sample from a frozen stock of bacteria was inoculated into lysogeny broth (Becton Dickinson) and grown to mid-log phase (approx. 3 hr). Bacteria were then washed twice in PBS and resuspended to the appropriate number of CFUs/µL in PBS by correlating absorbance at 600 nm to CFUs. Frozen stocks (200 µL) of Plasmodium yoelii 17XL in glycerolyte 57 solution (Fenwal, Lake Zurich, IL) at 5% to 15% parasitemia were inoculated into C57BL/6 donor mice. When parasitemia reached 5% to 15% (i.e., in 2–4 days), donor mice were euthanized and blood was obtained by aseptic cardiac puncture. After the parasitemia of pooled blood was quantified, the sample was diluted with normal saline and each test mouse was inoculated intraperitoneally with 200,000 infected RBCs (100 µL). Parasitemia was calculated by microscopic evaluation of 100 Wright-Giemsa (Sigma, St Louis, MO) stained RBCs.
RBC transfusion and bacterial infection
RBC suspensions (400 µL; equivalent to 2 human units), iron dextran (0.5–4 mg; INFeD, Watson Pharma, Inc., Parsippany, NJ), dextran (30 mg, 70,000 MW USP; Polysciences, Inc., Warrington, PA), or normal saline (400 µL) was transfused or infused through the retroorbital plexus of isoflurane-anesthetized mice. Concurrently, cohorts of mice were infected intraperitoneally with 1000 CFUs of S. typhimurium or 1 × 108 CFUs of E. coli (in 400 µL of PBS). The dose of S. typhimurium was selected based on initial escalating dose–response studies to achieve 50% mortality in 28 days. The dose of E. coli was selected based on the maximal dose of bacteria required to produce more than 90% survival in 48 hours in control mice. Mouse survival was assessed by a blinded observer at least once per day for the course of the experiment. The decision to euthanize, based on a clinical severity score, was made by the blinded observer.
Imaging luciferase activity in vivo
At specified times following infection with E. coli Xen 14, in vivo bioluminescent imaging was performed using an in vivo imaging system (IVIS Spectrum, PerkinElmer). CD-1 mice were used for these studies because of the increased sensitivity of imaging due to their white fur. Mice were anesthetized with isoflurane and imaged for 1 to 60 seconds. Bioluminescent flux, measured in photons per second emitted from specific regions, was quantified using computer software (LivingImage, Caliper Life Sciences, Hopkinton, MA); luciferase activity is expressed as photons per second.
Histology and immunohistochemistry
At necropsy, the liver and spleen were removed, fixed overnight with 10% neutral buffered formalin, and embedded in paraffin. Sections were stained with hematoxylin and eosin. Images were captured using a microscope (Model BX40, Olympus America, Center Valley, PA) and a digital camera (SPOT INSIGHT, Diagnostic Instruments, Sterling Heights, MI).
Statistical analysis
Significance among means was calculated using one-way analysis of variance with the Tukey multiple comparison test or using the Kruskal-Wallis test with Dunn’s multiple comparison test, as appropriate. Comparison of Kaplan-Meier survival curves was performed using a log-rank (Mantel-Cox) test. A p value of less than 0.05 was considered significant. Statistical analyses were performed using computer software (Prism 5, GraphPad Software, Inc., La Jolla, CA).
RESULTS
Mouse RBC storage procedures are analogous to human procedures
In this study, only blood from syngeneic donors was transfused into recipients to avoid the possibility of RBC alloimmunization and subsequent immune-mediated hemolysis. RBCs were leukoreduced before storage (>3-log leukoreduction [Fig. S1A, available as supporting information in the online version of this paper]) and blood cultures from poststorage aliquots had no microbial growth after incubation for 5 days. For the experiments presented hereafter, all “old,” stored RBCs were transfused after 2 weeks of storage and “fresh” RBCs were transfused within 24 hours of collection and leukoreduction. Two weeks of storage of C57BL/6 RBCs is substantially comparable to the FDA maximum-allowable-storage period of 6 weeks in humans;37 however, CD-1 RBCs store less well, with a hemolysis rate of 2.3% and a mean 24-hour posttransfusion RBC recovery of 40.1 ± 8.6% (Fig. S1B).
Effect of RBC transfusions on S. typhimurium infection
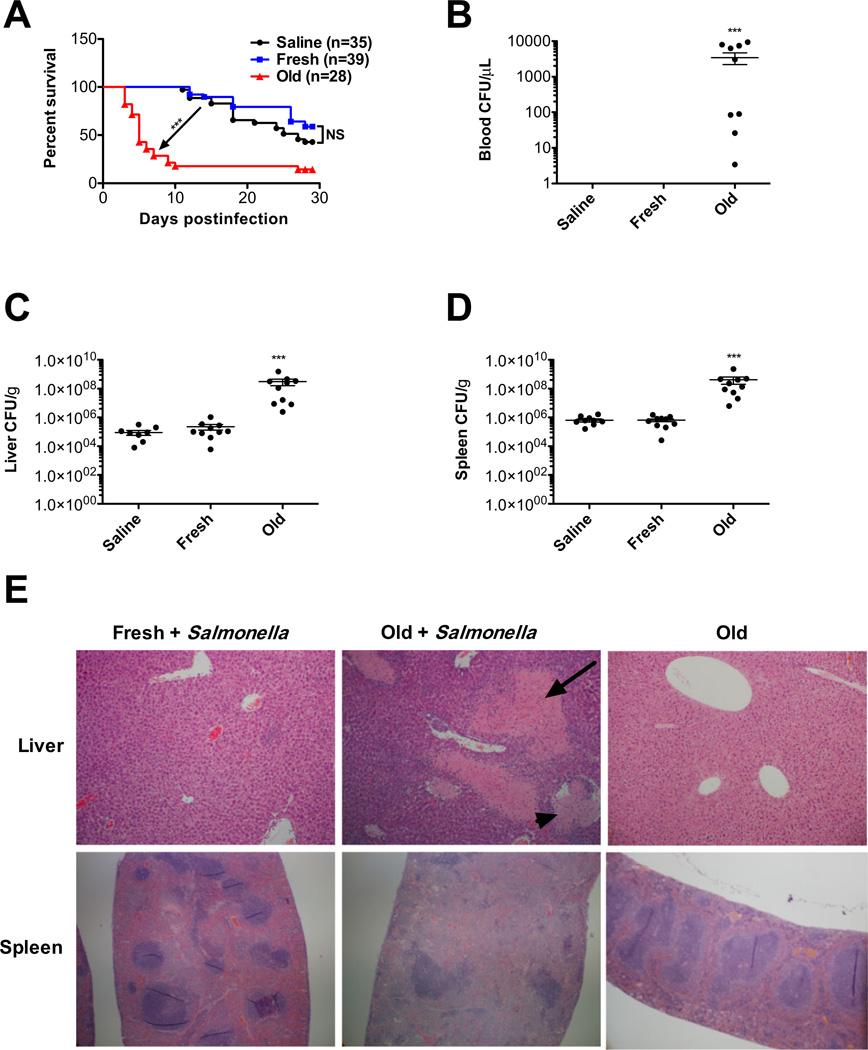
For C57BL/6 mice infected with 1000 CFUs of S. typhimurium, those infused with saline had a median survival of 27 days (Fig. 1A). Similarly, mice transfused with fresh RBCs had a median survival of more than 28 days (not significantly different than saline infusion; Fig. 1A). In contrast, transfusions of old RBCs reduced the median survival to 5 days (p < 0.001, Fig. 1A). Old RBC transfusions in the absence of infection did not affect overall mouse survival (not shown). To assess the cause of death and to obtain bacterial counts in the liver, spleen, and blood of recipients, mice were similarly treated and euthanized on the fifth day after infection. Old RBC transfusions resulted in at least a two orders of magnitude increase in the median bacterial count in blood (Fig. 1B), liver (Fig. 1C), and spleen (Fig. 1D) at 5 days after infection. Furthermore, histologic examination of the liver and spleen at 5 days after infection showed a normal architecture in mice infused with saline (not shown) or transfused with fresh RBCs (Fig. 1E). In contrast, mice transfused with old RBCs had large areas of liver necrosis with evidence of thrombosis and expansion of the splenic red pulp (Fig. 1E).
Fig. 1.
Older, stored RBC transfusions exacerbate sepsis and decrease survival in S. typhimurium–infected mice. Transfusion recipients were male C57BL/6 mice. (A) Mice were infected intraperitoneally with 1000 CFUs of S. typhimurium strain LT2 and infused with saline (n = 35; black circles); fresh syngeneic RBCs (<24-hr storage; n = 39; blue squares); or older, stored RBCs (2 weeks of storage; n = 28; red triangles). Kaplan-Meier survival estimates of mice are shown. (B–D) Salmonella infected mice (n = 10 per group) were infused with saline, fresh RBCs, or old RBCs, as above, and euthanized 5 days later. Bacterial load was measured by plate dilution in (B) blood, (C) liver, and (D) spleen. Results are presented as mean ± SEM; no bacteria were detected in the blood of mice infused with saline or transfused fresh RBCs. (E) Representative images of hematoxylin and eosin–stained histologic sections of the liver and spleen, 5 days after transfusion of S. typhimurium –infected mice with fresh or old RBCs or mice transfused old RBCs without infection. The arrow denotes tissue necrosis; the arrowhead denotes a blood vessel. Original magnification was 400× (liver) and 40× (spleen). ***p < 0.001 compared to fresh RBC transfusions.
Effect of malaria coinfection or iron dextran administration on S. typhimurium infection
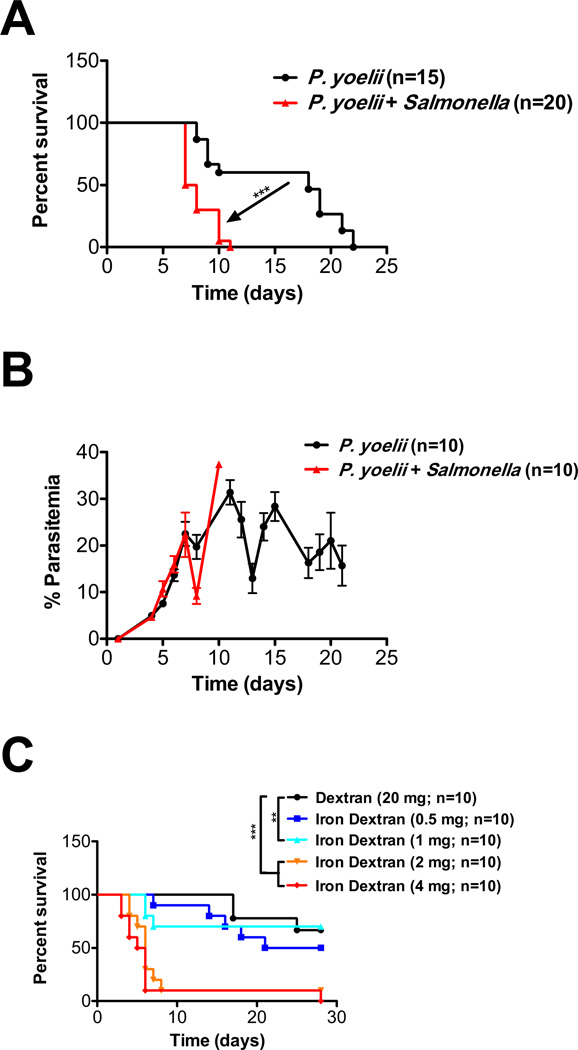
To examine the effects of coinfection on survival, C57BL/6 mice were infused with 200,000 P. yoelii merozoites and injected intraperitoneally with S. typhimurium. Mice coinfected with P. yoelii and S. typhimurium had decreased survival, compared to mice infected with P. yoelii alone (8 days vs. 18 days; p < 0.0001; Fig. 2A). Furthermore, the parasitemia in coinfected mice was similar to that in mice infected with P. yoelii alone (Fig. 2B).
Fig. 2.
S. typhimurium infection is exacerbated by coinfection with malaria or iron delivery to macrophages. (A) C57BL/6 mice were infected intraperitoneally with approximately 100,000 P. yoelii merozoites with or without infection with 1000 CFUs of S. typhimurium strain LT2. Kaplan-Meier survival estimates are shown. (B) Parasitemia, measured by microscopic evaluation of 100 Wright Giemsa–stained RBCs obtained from the tail vein, was determined by a blinded observer at each indicated time point; no significant differences were seen. (C) C57BL/6 mice were infected intraperitoneally with 1000 CFUs of S. typhimurium strain LT2 and infused IV with dextran (70,000 MW; 20 mg) or iron dextran (from 0.5–4 mg iron). Kaplan-Meier survival estimates are shown. **p < 0.01, ***p < 0.001 for the comparisons indicated.
To evaluate the effect of iron delivery to macrophages on S. typhimurium infection, C57BL/6 mice were not only infected with S. typhimurium, but also infused IV with either dextran (as a negative control) or increasing doses of iron dextran. A dose–response effect was observed, with increasing doses of iron resulting in decreasing survival (Fig. 2C). However, the mortality level did not approach that seen with transfusions of old RBCs until the equivalent of 2 mg of iron was infused; this is fivefold higher than the dose of iron delivered in the transfusion model (i.e., 0.4 mg iron per 2-unit RBC transfusion). The doses of iron dextran administered did not affect overall mouse survival in uninfected mice (data not shown).
Effect of transfusion on E. coli infection
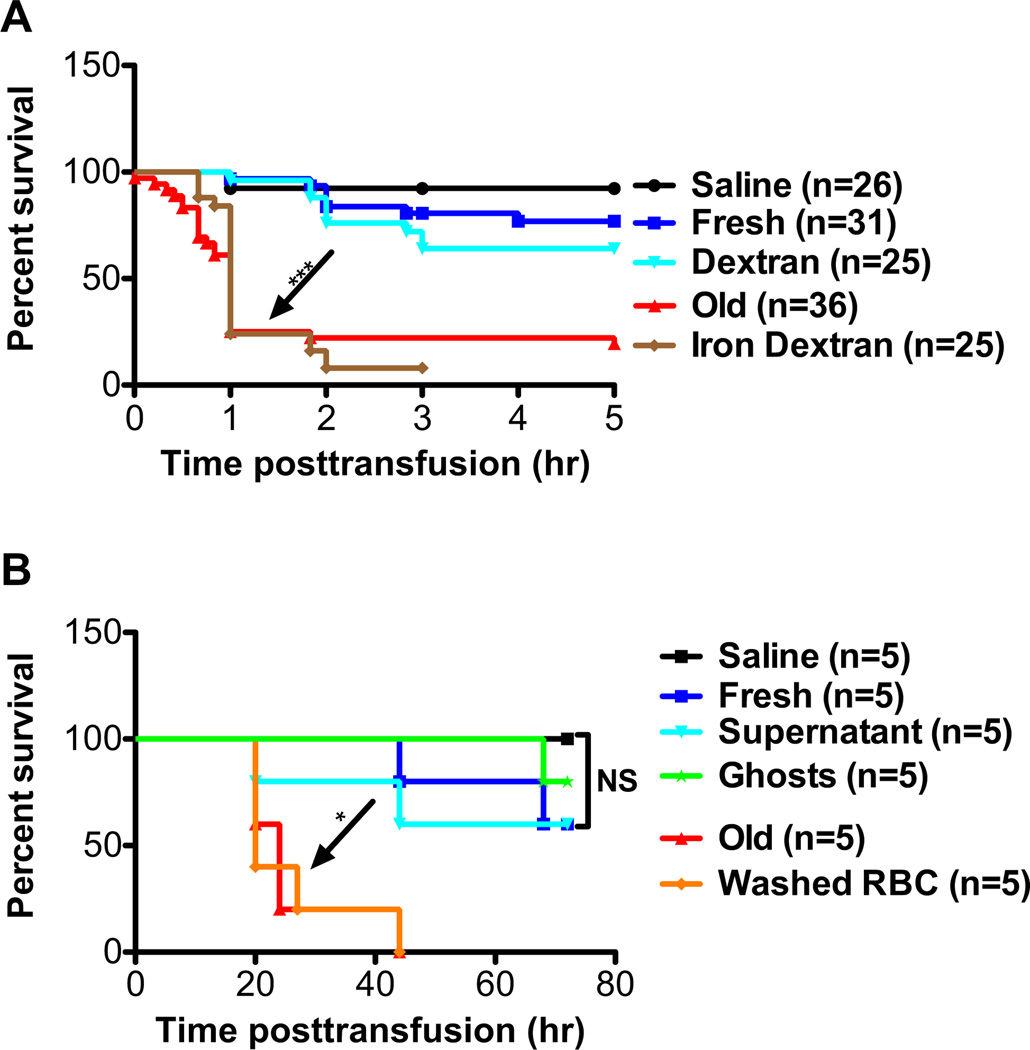
To examine the effect of RBC transfusion and iron delivery to macrophages on infections with extracellular pathogens, CD-1 mice were injected intraperitoneally with E. coli (1 × 108 CFUs) and infused retroorbitally with saline, fresh RBCs, old RBCs, dextran, or iron dextran (at a 0.4-mg equivalent dose of iron). Mice transfused with old RBCs or infused with iron dextran had similar survivals; these were significantly worse than those seen in mice infused with fresh RBCs, saline, or dextran (p < 0.0001; Fig. 3A). To determine whether membrane-encapsulated Hb iron is required, rather than factors accumulating in the supernatant of stored RBCs, E. coli–infected mice received infusions of normalized amounts of 1) fresh RBCs, 2) old RBCs, 3) washed old RBCs, 4) supernatant derived from old RBCs, 5) membrane ghosts prepared from old RBCs, or 6) saline. The survivals of mice transfused with old RBCs or washed old RBCs were similar; these were significantly worse than those seen in mice receiving infusions of fresh RBCs, saline, supernatant, or ghosts (p < 0.05; Fig. 3B).
Fig. 3.
Iron delivery to macrophages or rapid clearance of transfused RBCs exacerbates E. coli infection. CD-1 mice were used in these experiments. (A) Mice were infused with saline (n = 26; black circles); fresh syngeneic RBCs (<24-hr storage; n = 31; blue squares); older, stored RBCs (2 weeks of storage; n = 36; red triangles); dextran (20 mg; 70,000 MW; n = 25); or iron dextran (0.5 mg of iron; n = 25). Mice were concurrently infected intraperitoneally with E. coli strain Xen 14 (approx. 1 × 108 CFUs). Kaplan-Meier survival estimates are shown. (B) Mice were infused with saline (black circles); syngeneic fresh RBCs (blue squares); older, stored RBCs (red triangles); washed, older, stored RBCs (orange diamonds); supernatant derived from older, stored RBCs (light blue triangles); or ghosts prepared from older, stored RBCs (green stars). Mice were concurrently infected intraperitoneally with E. coli strain Xen 14 (approx. 1 × 108 CFUs). Kaplan-Meier survival estimates are shown. *p < 0.05, ***p < 0.001, as indicated.
Empiric, prophylactic antibiotic therapy ameliorates the increased severity of infection observed with transfusions of old RBCs or infusions of iron dextran
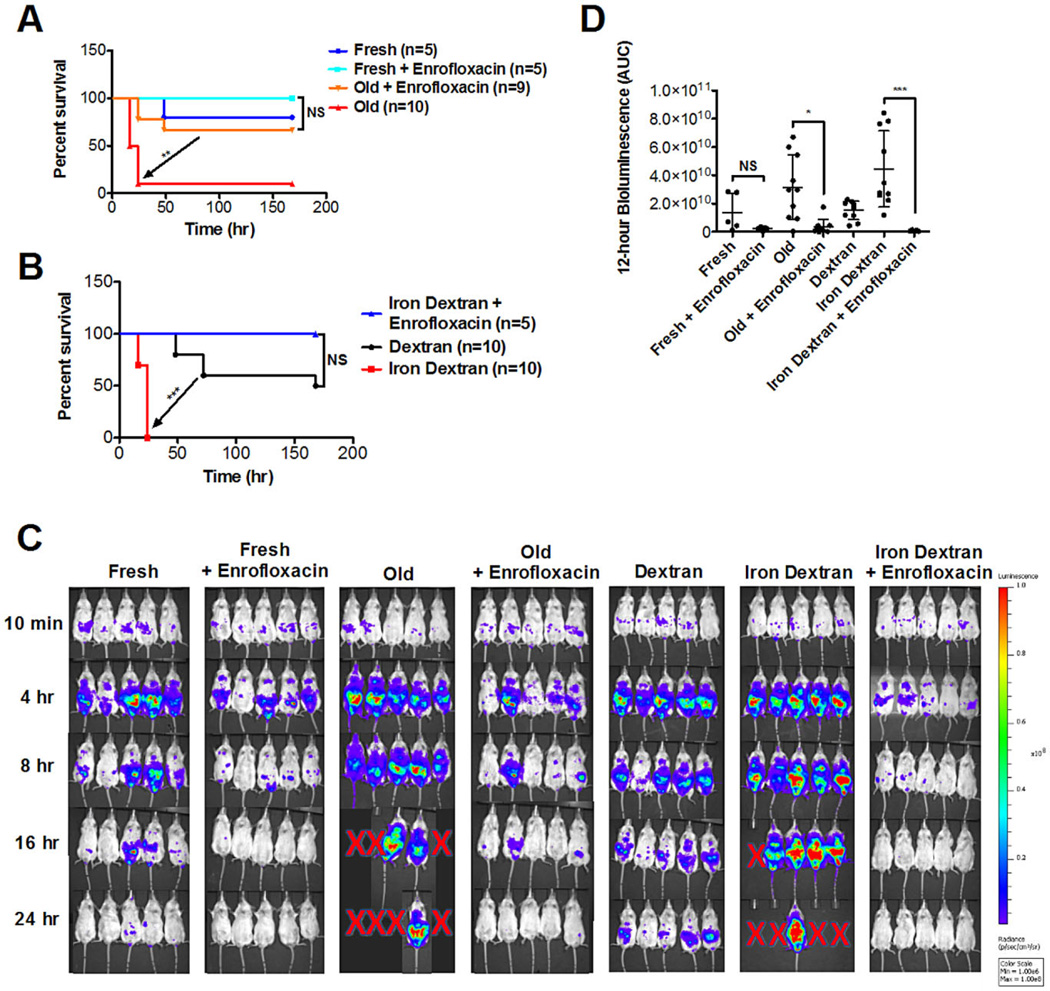
To determine the effect of antibiotic treatment on mice receiving RBC transfusions, E. coli–infected CD-1 mice were transfused with either fresh or old RBCs, with or without treatment with enrofloxacin, a fluoroquinolone antibiotic, before transfusion. Mice treated with antibiotics and transfused with old RBCs had improved survival, compared to mice treated with the vehicle control (p < 0.01; Fig. 4A). Similarly, antibiotic treatment abrogated the decreased survival seen in mice infected with E. coli and infused with iron dextran (p < 0.0001; Fig. 4B). A 24-hour time sequence of bioluminescent flux is shown in Fig. 4C; the area under the curve of bioluminescent flux during the first 12 hours after infection is significantly decreased in mice treated with antibiotics (Fig. 4D).
Fig. 4.
The effect of old RBC transfusions on exacerbating infection is ameliorated by antibiotic treatment. Experiments were performed using CD-1 mice, treated with or without a fluoroquinolone antibiotic (i.e., enrofloxacin). (A) Mice were transfused with syngeneic fresh RBCs (<24-hr storage) or old RBCs (2 weeks of storage) and concurrently infected intraperitoneally with E. coli strain Xen 14 (approx. 1 × 108 CFUs). Kaplan-Meier survival estimates are shown. (B) Mice were infused with dextran (20 mg; 70,000 MW) or iron dextran (0.5 mg iron) and concurrently infected intraperitoneally with E. coli strain Xen 14 (approx. 1 × 108 CFUs). Kaplan-Meier survival estimates are shown. (C) Representative bioluminescence images of mice at defined time points after infection, as described above. Red X’s represent animal death. (D) Bioluminescence was quantified in a defined region over the abdomen of each mouse and the area under the curve (AUC) was calculated for the first 12 hours (i.e., before animal dropout due to death or euthanasia for severe morbidity). *p < 0.05, **p < 0.01, ***p < 0.001, as indicated.
DISCUSSION
Taken together, these results suggest that iron delivery due to the rapid clearance of transfused, older, stored RBCs; from infusions of iron dextran; or from concomitant malaria infection can each exacerbate intracellular or extracellular bacterial infections. Interestingly, the effects of old RBC transfusions or coinfection with malaria were very similar in enhancing the morbidity and mortality of a S. typhimurium infection. Nonetheless, these effects were more pronounced than those seen using a similar dose of iron in the form of iron dextran; this suggests that additional mechanisms, other than simply nutritional iron delivery, are involved in the exacerbation of intracellular infection with Salmonella by old RBC transfusions or malaria.26 In contrast, with E. coli, an extracellular pathogen, the effects of old RBC transfusions were very similar to those seen when a similar dose of iron was administered as iron dextran. This suggests that the method of iron delivery can have differing effects on bacterial infections caused by different microbial pathogens.
In humans, iron dextran administration first delivers iron to cells of the mononuclear phagocyte system, from where it is then released into plasma, primarily attached to transferrin, to be used during erythropoiesis.19 However, when humans are exposed to sufficiently high doses of iron dextran, circulating non–transferrin-bound iron levels can increase, subsequently producing a proinflammatory cytokine response and generating intracellular reactive oxygen species in cells of the mononuclear phagocyte system.40 Iron dextran injections in mice, up to doses of 50 mg, predominantly deliver iron to cells of the reticuloendothelial system.41 Similarly, we previously showed in mice that transfusions of old RBCs resulted in iron delivery to the mononuclear phagocyte system, followed by a proinflammatory cytokine response; in addition, the subsequent release of iron into plasma, at a rate that overwhelmed the binding ability of transferrin, produced circulating non–transferrin-bound iron.16 Although RBC transfusions to healthy adult volunteers were not followed by an inflammatory response,18 RBC transfusions in neonates were associated with a proinflammatory cytokine response42 and transfusions of old, but not fresh, RBCs into neonates43 or healthy human volunteers18 induced increased levels of circulating non– transferrin-bound iron for 4 to 24 hours posttransfusion. Finally, iron dextran administration to human newborns increased the rate of bacterial sepsis by approximately sixfold, with a particular predilection for E. coli sepsis.2 Therefore, we hypothesized that, in mice, old RBC transfusions would be similar to iron dextran administration with regard to E. coli infection; indeed, our current findings suggest this to be true.
Human tissues are iron restricted, and there is fierce competition between host and pathogen for this essential nutrient.6,44 Thus, bacteria have multiple mechanisms for acquiring iron; for example, by secreting iron-chelating compounds (i.e., siderophores), which are then imported using specific membrane receptors.45 When iron is abundant, bacterial growth is stimulated. This effect varies among different organisms depending on their relative ferrophilia;36 for example, hemolysis (leading to iron release) increases the morbidity of infections with S. typhimurium, E. coli, and Staphylococcus aureus, but not with Streptococcus pneumoniae.36 Furthermore, free heme may provide another source of iron after RBC transfusion, and increasing circulating heme levels correlate with increasing mortality in human sepsis.46 In a recent study using a massive transfusion canine model, the mortality from S. aureus pneumonia increased in dogs transfused with older RBCs.47 In this case, older, stored RBCs had a propensity to hemolyze for several days after transfusion, releasing cell-free Hb, and, thereby, increasing pulmonary hypertension and ischemic damage.47 The possibility of ongoing hemolysis after transfusion was not tested in our study; however, given that iron dextran alone produced effects similar to old RBC transfusions, it is unlikely that cell-free Hb was the only cause of the observed exacerbations of infection.
By challenging mice with only 1000 CFUs of S. typhimurium, the median survival in control animals was approximately 4 weeks. However, a single transfusion of only 2 units of old, but not fresh, RBCs, decreased the median survival to less than 1 week. Thus, these results suggest that a single transfusion event can rapidly convert a clinically well-controlled and imperceptible infection into fulminant and fatal sepsis. However, depending on the microbial pathogen and the initial inoculum, the enhancing effects of an old RBC transfusion may not be perceived until several days later, at which point most physicians would not associate the clinically evident infection with the prior transfusion. However, when using a much larger dose of E. coli (i.e., 1 × 108 CFUs per mouse), the effects of the old RBC transfusion were noticeable within 24 hours; in contrast, lower doses of E. coli did not result in the death of any mice (data not shown). Nonetheless, even in this 24-hour period, the old RBC transfusions converted an infection that would have otherwise been cleared into overwhelming sepsis (Fig. 4C).
We also show that empiric antibiotic use can ameliorate the adverse effects of old RBC transfusions with regard to infectious complications in mice. In the study of iron dextran and human newborns,2 which documented a sixfold increase in Gram-negative sepsis, the newborns were not initially treated with antibiotics. Thus, these results highlight important implications for epidemiologic studies of the adverse effects of older, stored RBC transfusions on infectious risk; for example, the predicted effects are expected to be less prominent when study subjects receive prophylactic antibiotic therapy. Furthermore, adverse effects of RBC transfusions on infectious risk are more likely to be seen with highly ferrophilic pathogens. Finally, the clinically apparent infectious complications may occur days or weeks after the transfusion event.
One limitation of this study is that mice were not anemic nor iron deficient before transfusion, as is the case in most transfused patients. Furthermore, CD-1 RBCs store significantly less well than human RBCs. This may affect the generalizability of these findings from mice to human patients. Finally, it should be noted that mononuclear phagocyte metabolism, the precise kinetics of the appearance of circulating iron, and the types of iron present all differ in the three models used in this study (i.e., transfusion, iron dextran, and malaria). Thus, these data do not definitively demonstrate that iron delivery to the mononuclear phagocytic system alone is directly responsible for the increased mortality seen after older, stored RBC transfusions, and other indirect mechanisms are potentially involved. Nonetheless, neither transfusions of fresh RBCs nor infusions of RBC ghosts or supernatants from stored RBCs exacerbated sepsis with E. coli. Taken together, our current and prior16 results suggest that delivery of the contents of storage-damaged RBCs to the mononuclear phagocyte system are responsible for at least some of the infectious complications observed after transfusion, thereby implicating RBC transfusion after prolonged storage as a risk factor for adverse events.
In conclusion, the murine transfusion and monobacterial infection models described herein provide evidence that transfusions of older, stored RBCs can exacerbate infections with certain bacterial pathogens, at least in the absence of antibiotic prophylaxis. In addition, the effects of old RBC transfusions with E. coli infection are similar to those seen with iron dextran. Moreover, the virulence-enhancing effects of a single transfusion episode on an underlying S. typhimurium infection can be equivalent to those seen when the host is simultaneously infected with malaria. Interestingly, in Africa, clinically apparent S. typhimurium infections exhibit a seasonality that coincides with the malaria season; in addition, Salmonella infections are associated with sickle cell anemia, a hemolytic disorder.30,31 Finally, we previously showed that transfusions of older, stored RBCs produce extravascular hemolysis in mice,16 dogs,17 and humans.18 Although observations from mouse models may not always be directly applicable to humans,48 the abundant human data implicating hemolysis and iron delivery as risk factors for infections1,2,49 suggest that iron delivery to macrophages may be an underappreciated mechanism mediating at least some of the adverse effects of RBC transfusions.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grants K08-HL103756 (to EAH) and U01-HD064827 (to SLS) and a Louis V. Gerstner Scholars Award (to EAH).
Footnotes
AUTHORSHIP CONTRIBUTIONS
KP, SLS, GMB, DAF, and EAH designed the study; KP, SB, ROF, and EAH acquired the data; KP, ROF, KF, and EAH controlled and analyzed the data; KP and EAH wrote the paper; and all authors edited drafts and reviewed the final version of the manuscript.
CONFLICT OF INTEREST
KF is an employee of PerkinElmer. The other authors have disclosed no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s Web site:
Fig. S1. Storage properties of CD-1 RBCs. Fresh blood from 20 CD-1 mice was collected in 14% CPDA-1. A sample was obtained pre- and post-leukoreduction and the number of white blood cells enumerated by flow cytometry using the BD Leucocount Residual White Blood Cell Enumeration Kit (BD Biosciences) by following the manufacturer’s instructions. (A) Flow plots obtained pre- and post-leukoreduction counting 20,000 events in gate P2 (depicted), which represents leucocount beads, shows that leukoreduction leads to a greater than 3-log10 decrease in the number of white blood cells enumerated in R3 (white blood cell gate, depicted), from 2810 white blood cells/μL to 0.15 white blood cells/μL. (B) After two weeks of storage, 12 recipient CD-1 mice were transfused with stored RBCs and the recovery of transfused RBCs was calculated by dual-label flow cytometric tracking at 10 min, 30 min, 1-hr, 2-hr, and 24-hr after transfusion. The results are from one representative experiment and are presented as mean ± s.d. The post-transfusion 24-hr recovery of fresh RBCs is ~100% (not shown).
REFERENCES
- 1.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 2.Barry DM, Reeve AW. Increased incidence of gram-negative neonatal sepsis with intramuscula iron administration. Pediatrics. 1977;60:908–912. [PubMed] [Google Scholar]
- 3.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- 4.Raiten DJ, Namaste S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden—executive summary. Int J Vitam Nutr Res. 2011;81:57–71. doi: 10.1024/0300-9831/a000051. [DOI] [PubMed] [Google Scholar]
- 5.Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock V, Ferrieres L, Klemm P. The ferric yersiniabactin uptake receptor FyuA is required for efficient biofilm formation by urinary tract infectious Escherichia coli in human urine. Microbiology. 2008;154:167–175. doi: 10.1099/mic.0.2007/011981-0. [DOI] [PubMed] [Google Scholar]
- 7.Carless PA, Henry DA, Carson JL, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2010;(10):CD002042. doi: 10.1002/14651858.CD002042.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, et al. Influence of erythrocyte concentrate storage time on post-surgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98:815–822. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 10.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 11.Juffermans NP, Prins DJ, Vlaar AP, et al. Transfusion-related risk of secondary bacterial infections in sepsis patients: a retrospective cohort study. Shock. 2011;35:355–359. doi: 10.1097/SHK.0b013e3182086094. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg JA, McGwin G, Jr, Marques MB, et al. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 13.Vandromme MJ, McGwin G, Jr, Marques MB, et al. Transfusion and pneumonia in the trauma intensive care unit: an examination of the temporal relationship. J Trauma. 2009;67:97–101. doi: 10.1097/TA.0b013e3181a5a8f9. [DOI] [PubMed] [Google Scholar]
- 14.Hassan M, Pham TN, Cuschieri J, et al. The association between the transfusion of older blood and outcomes after trauma. Shock. 2011;35:3–8. doi: 10.1097/SHK.0b013e3181e76274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachoin JS, Daher R, Schorr C, et al. Microbiology, time course and clinical characteristics of infection in critically ill patients receiving packed red blood cell transfusion. Vox Sang. 2009;97:294–302. doi: 10.1111/j.1423-0410.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 16.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callan MB, Patel RT, Rux AH, et al. Transfusion of 28-day-old leucoreduced or non-leucoreduced stored red blood cells induces an inflammatory response in healthy dogs. Vox Sang. 2013;105:319–327. doi: 10.1111/vox.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimes AJ, Hutt MS. Metabolism of 59Fe-dextran complex in human subjects. BMJ. 1957;2:1074–1077. doi: 10.1136/bmj.2.5053.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noyes WD, Bothwell TH, Finch CA. The role of the reticulo-endothelial cell in iron metabolism. Br J Haematol. 1960;6:43–55. doi: 10.1111/j.1365-2141.1960.tb06216.x. [DOI] [PubMed] [Google Scholar]
- 21.Menendez C, Fleming A, Alonso P. Malaria-related anaemia. Parasitol Today. 2000;16:469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- 22.Berkley J, Mwarumba S, Bramham K, et al. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–286. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 23.Ukaga CN, Orji CN, Orogwu S, et al. Concomitant bacteria in the blood of malaria patients in Owerri, southeastern Nigeria. Tanzan Health Res Bull. 2006;8:186–188. doi: 10.4314/thrb.v8i3.45119. [DOI] [PubMed] [Google Scholar]
- 24.Uneke CJ. Concurrent malaria and typhoid fever in the tropics: the diagnostic challenges and public health implications. J Vector Borne Dis. 2008;45:133–142. [PubMed] [Google Scholar]
- 25.Leung KY, Finlay BB. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roux CM, Butler BP, Chau JY, et al. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to nontyphoidal Salmonella enterica serovar typhimurium infection in mice. Infect Immun. 2010;78:1520–1527. doi: 10.1128/IAI.00887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 28.Ammah A, Nkuo-Akenji T, Ndip R, et al. An update on concurrent malaria and typhoid fever in Cameroon. Trans R Soc Trop Med Hyg. 1999;93:127–129. doi: 10.1016/s0035-9203(99)90282-1. [DOI] [PubMed] [Google Scholar]
- 29.Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–1321. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 30.Okuonghae HO, Nwankwo MU, Offor EC. Pattern of bacteraemia in febrile children with sickle cell anaemia. Ann Trop Paediatr. 1993;13:55–64. doi: 10.1080/02724936.1993.11747625. [DOI] [PubMed] [Google Scholar]
- 31.Onwubalili JK. Sickle cell disease and infection. J Infect. 1983;7:2–20. doi: 10.1016/s0163-4453(83)90863-0. [DOI] [PubMed] [Google Scholar]
- 32.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 33.Shukla G, Singh D, Sharma L, et al. Effect of Plasmodium and Salmonella co-infection in a murine model. Cent Eur J Med. 2009;4:340–347. [Google Scholar]
- 34.Akinyemi KO, Bamiro BS, Coker AO. Salmonellosis in Lagos, Nigeria: incidence of Plasmodium falciparum-associated co-infection, patterns of antimicrobial resistance, and emergence of reduced susceptibility to fluoroquinolones. J Health Popul Nutr. 2007;25:351–358. [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 36.Kaye D, Hook EW. The influence of hemolysis or blood loss on susceptibility to infection. J Immunol. 1963;91:65–75. [PubMed] [Google Scholar]
- 37.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129:275–278. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 39.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transf. Med Rev. 2002;16:46–60. doi: 10.1053/tmrv.2002.29404. [DOI] [PubMed] [Google Scholar]
- 40.Pai AB, Conner T, McQuade CR, et al. Non-transferrin bound iron, cytokine activation and intracellular reactive oxygen species generation in hemodialysis patients receiving intravenous iron dextran or iron sucrose. Biometals. 2011;24:603–613. doi: 10.1007/s10534-011-9409-6. [DOI] [PubMed] [Google Scholar]
- 41.Richter GW. The cellular transformation of injected colloidal iron complexes into ferritin and hemosiderin in experimental animals; a study with the aid of electron microscopy. J Exp Med. 1959;109:197–216. doi: 10.1084/jem.109.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keir AK, McPhee AJ, Andersen CC, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. 2013;73:75–79. doi: 10.1038/pr.2012.144. [DOI] [PubMed] [Google Scholar]
- 43.Stark MJ, Keir AK, Andersen CC. Does non-transferrin bound iron contribute to transfusion related immune-modulation in preterms? Arch Dis Child Fetal Neonatal Ed. 2013;98:F424–F429. doi: 10.1136/archdischild-2012-303353. [DOI] [PubMed] [Google Scholar]
- 44.Nairz M, Schroll A, Sonnweber T, et al. The struggle for iron—a metal at the host-pathogen interface. Cell Microbiol. 2010;12:1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 45.Martinez JL, Delgado-Iribarren A, Baquero F. Mechanisms of iron acquisition and bacterial virulence. FEMS Microbiol Rev. 1990;6:45–56. doi: 10.1111/j.1574-6968.1990.tb04085.x. [DOI] [PubMed] [Google Scholar]
- 46.Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 47.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimring JC, Spitalnik SL. On the appropriate use and interpretation of animal models in transfusion medicine research. Transfusion. 2013;53:2334–2339. doi: 10.1111/trf.12131. [DOI] [PubMed] [Google Scholar]
- 49.Bullen J, Rogers H, Spalding P, et al. Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol. 2005;43:325–230. doi: 10.1016/j.femsim.2004.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.