Abstract
Objectives. We tested whether leukocyte telomere length maintenance, which underlies healthy cellular aging, provides a link between sugar-sweetened beverage (SSB) consumption and the risk of cardiometabolic disease.
Methods. We examined cross-sectional associations between the consumption of SSBs, diet soda, and fruit juice and telomere length in a nationally representative sample of healthy adults. The study population included 5309 US adults, aged 20 to 65 years, with no history of diabetes or cardiovascular disease, from the 1999 to 2002 National Health and Nutrition Examination Surveys. Leukocyte telomere length was assayed from DNA specimens. Diet was assessed using 24-hour dietary recalls. Associations were examined using multivariate linear regression for the outcome of log-transformed telomere length.
Results. After adjustment for sociodemographic and health-related characteristics, sugar-sweetened soda consumption was associated with shorter telomeres (b = –0.010; 95% confidence interval [CI] = −0.020, −0.001; P = .04). Consumption of 100% fruit juice was marginally associated with longer telomeres (b = 0.016; 95% CI = −0.000, 0.033; P = .05). No significant associations were observed between consumption of diet sodas or noncarbonated SSBs and telomere length.
Conclusions. Regular consumption of sugar-sweetened sodas might influence metabolic disease development through accelerated cell aging.
Sugar-sweetened beverages (SSBs), including soft drinks or sodas, fruit-flavored drinks, sports drinks, and energy drinks, are the largest source of added sugar in the US diet.1,2 Between 1999 and 2008, it was estimated that adults aged 20 to 34 years consumed an average of 333 to 421 calories per day, and adults aged 35 years or older consumed an average of 236 to 260 calories per day from SSBs.3 Because of these strikingly high levels of consumption, SSBs have emerged as an important target of public health efforts and policies.4,5
In parallel to trends in SSB intake, the prevalences of obesity and type 2 diabetes have also increased in recent years.6,7 Epidemiological studies have shown that regular consumption of SSBs is associated with increased risks of obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease.8–11 However, the mechanisms for these associations are complex and not yet fully understood. There is evidence to suggest that excess calories (via lowered satiety) and high levels of insulin resistance, oxidative stress, and inflammation, may mediate these associations.9 Because oxidative stress, inflammation, and insulin resistance are also associated with telomere shortening, impaired telomere length maintenance is a potential mechanism that may help to explain the association between SSB consumption and accelerated metabolic disease.12–14
Telomeres are the DNA-protein caps at the end of chromosomes that promote chromosomal stability and protect the genomic DNA from damage. Telomere length naturally shortens with each cell cycle, and if it falls to a critical short length, the cell is no longer able to divide and often malfunctions.15 In addition to biological age, telomere shortness has been linked to lifestyle behaviors and psychological stress.16–22 In turn, shorter telomeres have been associated with increased risks of chronic diseases, including cardiovascular disease, diabetes, and some cancers.17,23–27 In population studies, evidence exists for a causal role of impaired telomere maintenance in raising risks of pulmonary and cardiovascular disease.28 To date, the associations between dietary intake and telomere length have been examined in only a few studies; results for most food groups and nutrients have been mixed.13,29,30
Because of the known effects of SSBs on oxidative stress and insulin resistance, our objective in this study was to examine the associations between SSBs, diet soda, and 100% fruit juice consumption and telomere length in a large, nationally representative sample of healthy adults in the United States. We hypothesized that beverages with high sugar content would be the most detrimental to cellular aging, such that sugar-sweetened sodas and noncarbonated SSBs would show the strongest associations with telomere shortness.
METHODS
The National Health and Nutrition Examination Survey (NHANES) is an ongoing, multistage cross-sectional survey administered by the National Center for Health Statistics. The study population was restricted to 5309 adults, aged 20 to 65 years, who had complete dietary data and leukocyte telomere length (LTL) measured in the 1999 to 2002 NHANES. Adults with a history of diabetes, coronary heart disease, angina, myocardial infarction, stroke or congestive heart failure were excluded.
Leukocyte Telomere Length
DNA samples purified from whole blood were collected from NHANES participants aged 20 years and older in the 1999 to 2002 waves to establish a national probability sample of genetic material for future research.31 DNA aliquots were processed by the Division of Laboratory Sciences at the National Center for Environmental Health and provided by the Division of Health and Nutrition Examination Surveys, National Center for Health Statistics, Centers for Disease Control and Prevention. The LTL assay was performed in the laboratory of Elizabeth Blackburn at the University of California, San Francisco, using the quantitative polymerase chain reaction method to measure telomere length relative to standard reference DNA (T/S ratio), as described in detail elsewhere.32,33 The polymerase chain reaction method was preferred over the Southern blot method because of the smaller amount of DNA required for the assay.34,35 Each LTL sample was assayed 3 times on 3 different days. The samples were assayed on duplicate wells, resulting in 6 data points. Sample plates were assayed in groups of 3 plates, and no 2 plates were grouped together more than once. Each assay plate contained 96 control wells with 8 control DNA samples. Assay runs with 8 or more invalid control wells were excluded from further analysis (< 1% of runs). Control DNA values were used to normalize between-run variability. Runs with more than 4 control DNA values falling outside 2.5 SDs from the mean for all assay runs were excluded from further analysis (< 6% of runs). For each sample, any potential outliers were identified and excluded from the calculations (< 2% of samples). The mean and SD of the T/S ratio were then calculated normally. The interassay coefficient of variation was 6.5%. Throughout this article, we refer to the T/S ratio and relative telomere length as telomere length for brevity.
The conversion from the T/S ratio to base pairs was calculated based on comparison of telomeric restriction fragment length from Southern blot analysis and T/S ratios using DNA samples from the human diploid fibroblast cell line IMR90 at different population doublings. The formula used to convert the T/S ratio to base pairs was 3274 + 2413 * (T/S).
Sugar-Sweetened Beverage Intake
One 24-hour dietary recall was administered to NHANES study participants in the Mobile Examination Center. Beverage variables were derived from the NHANES individual food files. Consumption of sugar-sweetened sodas, noncarbonated SSBs (i.e., fruit drinks, sports drinks, energy drinks, sweetened waters), diet sodas, 100% fruit juice, and all SSBs (including sugar-sweetened sodas and noncarbonated SSBs) were identified using data from the US Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies. Serving sizes of 8 ounces (226.8 g) were applied to all beverages.
We used a statistical method developed by the National Cancer Institute to estimate usual dietary intake, because 24-hour dietary recalls might not accurately reflect long-term dietary intake.36 The National Cancer Institute method requires 2 or more days of 24-hour dietary recalls on a subset of participants. Because study participants in the 1999 to 2002 NHANES only contributed one 24-hour dietary recall, we included data from 2003 to 2004 NHANES participants to calibrate the distributions of dietary variables. This method, which uses a 2-part nonlinear mixed model for foods consumed episodically (i.e., SSBs), was applied to participants from 1999 to 2004 NHANES with sociodemographic characteristics and dietary data. We modeled intake distributions for each beverage, correcting for age, gender, race/ethnicity and weekday-to-weekend effects. We then estimated individual beverage intake for all participants in 1999–2004 NHANES, although only the participants in 1999 to 2002 NHANES were retained in the analytical population. In using this method, we assumed that the distributions of SSB intake did not significantly differ between 1999 to 2002 and 2003 to 2004. The National Cancer Institute method’s validity in evaluating associations between usual intake of foods and health outcomes was previously established.37
Study Covariates
Potential confounders included sociodemographic characteristic variables, such as participant’s age (20–24, 25–29, 30–34, 35–39,40–44, 45–49 ,50–54, 55–59, 60–65 years), gender, self-reported race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other race/multiracial), highest educational attainment (< 12 years, high school diploma or equivalent, some college, college graduate), ratio of family income to poverty, using the Department of Health and Human Services annual poverty guidelines (FPL; 0%–100% FPL, 100%–200% FPL, 200%–300% FPL, 300%–400% FPL, and > 400% FPL), and marital status (married or living with partner, never married, separated/widowed/divorced).
Health-related variables included smoking status (never, former, current), pack-years of smoking (0, < 30, 30–60, and > 60), physical activity assessed from questionnaire (some activity, no activity), total energy intake, alcohol intake, and Healthy Eating Index 2005 (HEI 2005) score, which is a dietary pattern developed by the USDA to measure compliance with national dietary guidelines.38 The HEI 2005 is scored out of 100 points and comprises 12 components: total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, total grains, whole grains, milk, meat and beans, oils, saturated fat, sodium, and calories from solid fats, alcoholic beverages, and added sugars. We collapsed HEI 2005 scores into gender-specific quartiles: for men, the cutpoints were 42.1, 45.9, and 50.5; for women, the cutpoints were 44.4, 48.6, and 53.5. We defined alcohol intake as low (0–0.5 drinks/day for men and women), moderate (0.5–2.0 drinks/day for men; 0.5–1.5 drinks/day for women), and heavy ( > 2 drinks/day for men, > 1.5 drinks/day for women).
Adiposity measures included body mass index (BMI) and waist circumference. We calculated BMI from self-reported height (in meters) and weight (in kilograms squared), measured by trained personnel using a stadiometer and Toledo weight scale (Toledo Scale, Honolulu, HI).39 We defined BMI categories as underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5–24.9 kg/m2), overweight (BMI = 25.0–29.9 kg/m2), and obese (BMI ≥ 30 kg/m2). Waist circumference (centimeters) was measured at the upper lateral border of the right ilium. We defined elevated waist circumference as 102 centimeters or greater for men and 88 centimeters or greater for women.
Missing indicators were used to account for missing education level (n = 6, 0.16% missing), marital status (n = 258, 5.5% missing), smoking status (n = 8, 0.15% missing), pack-years of smoking (n = 523, 9.1% missing), household income (n = 416, 6.9% missing), BMI (n = 89, 1.6% missing), and waist circumference (n = 126, 2.0% missing).
Statistical Analysis
To make nationally representative estimates, analyses accounted for the complex NHANES sampling design by incorporating sampling weights for the genetic subsample and strata and primary sampling unit indicators. The sampling weights accounted for different sampling probabilities and the potential nonresponse bias of the participants in the NHANES subsample who consented to the use of DNA specimens for future genetic research. First, we examined bivariate associations between LTL and individual-level characteristics. Because of the skewness of LTL, LTL was log-transformed before fitting to the regression models. Linear regression models were then fit for log-transformed LTL to estimate the difference in LTL for a 1-serving increase in beverage intake. The first model adjusted for age categories, gender, and total energy intake. The second model adjusted for all sociodemographic characteristics and health-related variables. We also examined heterogeneity in the associations between beverage intake and LTL by gender and race/ethnicity by introducing product terms between beverages and the individual modifiers in the fully adjusted models. Statistical significance of the product terms was determined with the Wald test.
All statistical tests were 2-sided, and statistical significance was considered at P < .05. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and conducted using ANDRE (Centers for Disease Control and Prevention, Atlanta, GA), the Centers for Disease Control and Prevention remote access system for restricted data analysis.
RESULTS
As expected, age was linearly associated with shorter telomeres (P < .001) (Table 1). Mean telomere length was longest in Blacks and Hispanics, in never smokers, and in normal weight adults, as observed in previous studies.18,31,40–43
TABLE 1—
Mean Leukocyte Telomere Length by Sociodemographic Characteristics and Lifestyle Behaviors of 5309 Adults Aged 20–65 Years: National Health and Nutrition Examination Surveys, United States, 1999–2002
| Characteristics | No. (Weighted %a) or Mean ±SE | LTL, Mean (SE) | Pb |
| Age, y | 39.7 ±0.3 | 1.10 (0.01) | < .001 |
| Gender | .27 | ||
| Men | 2473 (48.2) | 1.09 (0.01) | |
| Women | 2836 (51.8) | 1.10 (0.02) | |
| Race | .009 | ||
| Non-Hispanic White | 2510 (69.2) | 1.09 (0.02) | |
| Non-Hispanic Black | 934 (11.0) | 1.15 (0.02) | |
| Hispanic/Latino | 1687 (15.2) | 1.11 (0.02) | |
| Other race/Multirace | 178 (4.6) | 1.09 (0.02) | |
| Education level | .25 | ||
| < 12 y | 1554 (18.7) | 1.08 (0.02) | |
| High school diploma | 1227 (25.4) | 1.09 (0.02) | |
| Some college | 1429 (30.0) | 1.11 (0.02) | |
| College graduate | 1093 (26.0) | 1.11 (0.02) | |
| Marital status | < .001 | ||
| Married or living with partner | 3310 (65.6) | 1.07 (0.02) | |
| Never married | 1021 (20.5) | 1.19 (0.02) | |
| Separated, widowed or divorced | 720 (13.9) | 1.04 (0.02) | |
| Federal poverty level,c % | .09 | ||
| 0–100 | 887 (14.3) | 1.15 (0.03) | |
| 100–200 | 1111 (18.3) | 1.09 (0.02) | |
| 200–300 | 757 (15.1) | 1.09 (0.02) | |
| 300–400 | 619 (13.9) | 1.10 (0.02) | |
| > 400 | 1519 (38.4) | 1.08 (0.02) | |
| Pack-years of smoking | < .001 | ||
| 0 | 2872 (57.4) | 1.11 (0.02) | |
| < 30 | 1533 (34.0) | 1.09 (0.02) | |
| 30–60 | 278 (6.3) | 1.00 (0.02) | |
| > 60 | 103 (2.3) | 0.96 (0.02) | |
| Physical activity | .03 | ||
| Some activity | 1974 (30.7) | 1.08 (0.02) | |
| No activity | 3332 (69.3) | 1.11 (0.01) | |
| BMI (kg/m2) | .004 | ||
| Underweight (< 18.5) | 78 (1.8) | 1.13 (0.03) | |
| Normal weight (18.5–24.9) | 1673 (35.0) | 1.13 (0.02) | |
| Overweight (25.0–29.9) | 1849 (34.4) | 1.08 (0.02) | |
| Obese (≥ 30 kg/m2) | 1620 (28.9) | 1.07 (0.02) | |
| Waist circumference (cm) | .003 | ||
| Normal (< 102 for men; < 88 for women) | 2693 (55.1) | 1.12 (0.02) | |
| Elevated (≥ 102 for men; ≥ 88 for women) | 2490 (42.9) | 1.07 (0.02) |
Note. BMI = body mass index; LTL = leukocyte telomere length.
Weighted percentages are representative of the United States civilian, noninstitutionalized population.
From χ2 test and univariate linear regression.
Ratio of family income to poverty, using the Department of Health and Human Services annual poverty guidelines.
Pearson’s correlation coefficients for associations among self-reported intakes of different beverages are shown in Table 2. Overall, correlations between beverages were modest. The intakes of sugar-sweetened sodas, noncarbonated SSBs, and 100% fruit juice were positively correlated with each other. Diet soda was negatively correlated with SSBs and 100% fruit juice intakes.
TABLE 2—
Pearson’s Correlations Coefficients for Sugar-Sweetened Beverages: National Health and Nutrition Examination Surveys, United States, 1999–2002
| Beverage | Sugar-Sweetened Soda | Noncarbonated SSB | Diet Soda | 100% Fruit Juice |
| Sugar-sweetened soda | 1.00 | |||
| Noncarbonated SSB | 0.20 | 1.00 | ||
| Diet soda | −0.23 | −0.10 | 1.00 | |
| 100% fruit juice | 0.04 | 0.13 | −0.07 | 1.00 |
Note. SSB = sugar-sweetened beverage.
Average sugar-sweetened soda consumption was 1.5 servings (12 ounces) per day. Average consumption of diet sodas, noncarbonated SSBs, and 100% fruit juice was lower, ranging from 0.3 to 0.5 servings per day. Consumption of all SSBs (including sugar-sweetened soda and noncarbonated SSBs) was averaged at 2.1 servings (16.8 ounces) per day. Associations between SSB intake and telomere length are shown in Table 3. After adjustment for sociodemographic characteristics and health-related variables and adiposity, sugar-sweetened soda consumption was inversely associated with telomere length (b = −0.010; 95% CI = −0.020, −0.001). Holding other covariates constant, this difference corresponded to a deficit of 14 base pairs. Because of the model-based estimate in this sample of the age-associated rate of telomere shortening of 13.6 base pairs per year, this was equivalent to 1.9 additional years of aging for an 8-ounce serving of sugar-sweetened sodas. For a daily consumption of the current standard 20-ounce serving size for sugar-sweetened sodas, this corresponds to 4.6 additional years of aging. Approximately 21% of adults in the study population reported daily consumption of 20 ounces or more of sugar-sweetened soda (data not shown).
TABLE 3—
Associations Between Beverage Intake and Log-Transformed Leukocyte Telomere Length (T/S Ratio): National Health and Nutrition Examination Surveys, United States, 1999–2002
| Mean LTL by Quartile of Intake |
Model 1 |
Model 2 |
||||
| Beverage Typea | Q1 | Q2 | Q3 | Q4 | b (95% CI) | b (95% CI) |
| All sugar-sweetened beveragesb | 1.05 | 1.10 | 1.11 | 1.13 | −0.010 (−0.021, 0.001) | −0.008 (−0.020, 0.004) |
| Sugar-sweetened soda | 1.04 | 1.13 | 1.09 | 1.12 | −0.013* (−0.023, −0.003) | −0.010* (−0.020, −0.001) |
| Noncarbonated sugar-sweetened beverages | 1.03 | 1.10 | 1.12 | 1.13 | 0.000 (−0.029, 0.029) | −0.001 (−0.030, 0.028) |
| Diet soda | 1.10 | 1.08 | 1.09 | 1.10 | −0.003 (−0.021, 0.016) | −0.000 (−0.019, 0.018) |
| 100% fruit juice | 1.10 | 1.08 | 1.08 | 1.11 | 0.022* (0.003, 0.041) | 0.016 (−0.000, 0.033) |
Note. CI = confidence interval; LTL = leukocyte telomere length. Model 1 included age, gender, and total energy. Model 2 included age, gender, race/ethnicity, education level, marital status, smoking status, pack-years of smoking, physical activity, poverty level, total energy, alcohol intake, Healthy Eating Index 2005 scores, body mass index categories, and waist circumference categories.
Expressed in servings (8 oz).
Includes sugar-sweetened soda and noncarbonated sugar-sweetened beverages.
*P < .05.
No associations were observed between diet soda or noncarbonated SSBs and telomere length. Although a positive association was observed between 100% fruit juice and telomere length in the first model after adjusting for age, gender, and total energy, this association was attenuated after the inclusion of other potential confounders (P = .05).
Stratified associations by gender and race/ethnicity are available as a supplement to the online version of this article at http://www.ajph.org. There was no evidence of heterogeneity in the associations by gender or racial/ethnic groups.
DISCUSSION
In this nationally representative sample of healthy adults, the average daily consumption of sugar-sweetened soda was 12 ounces (1.5 servings), a level in excess of the American Heart Association recommended limit for added sugar.5 Consistent with our hypothesis, we found that each daily 8-ounce serving of sugar-sweetened sodas was linearly associated with shorter telomeres, roughly equivalent to 1.9 additional years of aging, independent of sociodemographic characteristics and health-related variables. For a daily 20-ounce serving, the current standard serving size, this translates into approximately 4.6 additional years of aging. More than 20% of adults in the study population reported at least 20 ounces of sugar-sweetened soda consumption per day. Although these were modest associations, the magnitude of the association for consuming 20 ounces of sugar-sweetened soda was comparable to observed associations between telomere length and moderate or vigorous levels of physical activity (4.4 years, in the opposite direction) and smoking (4.6 years).18,20 To our knowledge, ours was the first study to link sugar-sweetened soda consumption with telomere length in a large, nationally representative sample of healthy adults.
Results of telomere length associations with other various dietary aspects were inconsistent.13,29,30 The Multi-Ethnic Study of Atherosclerosis (MESA) previously examined sugar-sweetened soda consumption in relation to telomere length among adults.30 Their results showed no association after adjustment for sociodemographic characteristics, lifestyle factors, and BMI. The fact that MESA had a smaller sample size and an older population, on average than NHANES, might account for why an association was found in our study but not in MESA.
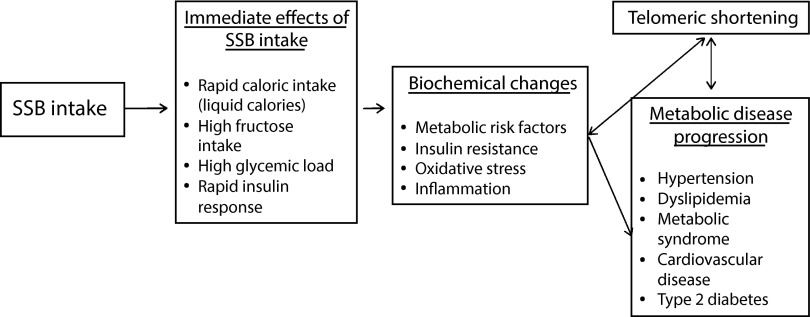
Our hypothesis that consumption of SSBs were related to shorter telomeres was derived from the known effects of SSB consumption on impaired fasting glucose and insulin resistance.8–11 SSBs have been known to increase oxidative stress and systemic inflammation, which are both processes that can influence telomere attrition.13,14 Telomere shortening in response to, and perhaps contributing to, these disease processes was reported, reflecting the overall burden of cardiometabolic disease.27,44,45 Our results suggested that another link between sugar-sweetened soda consumption and metabolic disease might be through shortened telomere length, a biomarker and mechanism of cellular aging (Figure 1).
FIGURE 1—
Conceptual model of the effects of sugar-sweetened beverage (SSB) intake on telomeric shortening and metabolic disease progression: National Health and Nutrition Examination Surveys, United States, 1999–2002.
Note. High SSB intake leads to rapid caloric and fructose intake, and high glycemic load. This results in an increased risk of metabolic risk factors and a biochemical environment of high insulin resistance, oxidative stress, and inflammation. In turn, this can affect telomeric shortening and influence metabolic disease progression, including metabolic syndrome, type 2 diabetes, and cardiovascular disease.
We observed no significant associations between consumption of noncarbonated SSBs and telomere length. The lack of association might be attributed to the large degree of heterogeneity in sugar content across beverages.46 In the study population, the average consumption of noncarbonated SSBs (0.3 servings/day) was substantially lower than the average consumption of sugar-sweetened sodas (1.5 servings/day); it might be that sugar consumption in beverages affected telomere length only at higher intake levels. Consumption of noncarbonated SSBs has increased in recent years, whereas overall intakes of sugar-sweetened sodas have decreased, and an association between consumption of noncarbonated SSBs and telomere length might emerge in future studies.3 Even lacking a significant current association with telomere length, decreasing consumption of SSBs to reduce risks of obesity-related chronic disease seems prudent.8,10
A marginally positive association was shown in our study between 100% fruit juice consumption and telomere length. Previous studies that examined fruit juice and health outcomes yielded mixed findings. Fruit juice was associated with an increased risk of type 2 diabetes in some,47–49 but not all studies.10,50–52 Consumption of 100% fruit juice was not shown to have the same effect on cardiometabolic risk factors53 or markers of insulin resistance, oxidative stress, or inflammation as SSB consumption.54–56 Fruit juice consumption might result in different metabolic effects compared with SSBs, with potentially beneficial effects of phytochemicals and micronutrients balancing out the harmful effect of liquid sugars. Consumption levels of 100% fruit juice were also generally lower than levels of sugar-sweetened soda consumption, as was shown in our study. Because fruit juice consumption was not associated with long-term health benefits in epidemiological studies, limiting its consumption in preference of whole fruit might be advisable.
Our study was strengthened by the use of a large, nationally representative sample of adults. In addition, we used a validated method to estimate usual beverage intake from the extensive NHANES dietary data. Furthermore, the NHANES response rates from 1999 to 2002 ranged from 76% to 80%; these were considerably higher than other national health surveys, and helped to improve the generalizability of our findings.57 We also took steps to avoid spurious findings, including examining a small number of dietary components for which there were substantially strong a priori hypotheses for associations with oxidative stress and biomarkers of aging.
Study Limitations
Our study had limitations. First, the cross-sectional nature of the data made it difficult to infer causation. Longitudinal studies of dietary intake and telomere length are needed to understand how dietary intake can influence telomere length over time, and whether the associations are explained by the mechanisms proposed in Figure 1. Collection of biochemical data, such as insulin resistance, oxidative stress, and inflammation, would also help to inform the understanding of the mechanisms of the association between SSB intake and telomeric shortening. LTL was measured from a single DNA specimen, which did not provide information on rates of telomere shortening. Similarly, beverage intake was estimated from a 24-hour dietary recall conducted at the time of the survey, which might not reflect diet or beverage patterns over the life course.
Telomere research in clinical studies is a relatively new field, and researchers are still identifying important individual and lifestyle determinants of telomere length. Thus, there is always the possibility of unmeasured confounding. For example, genetic differences may contribute to telomeric shortening; however, the degree of this confounding could be small because it is unlikely that any potential single nucleotide polymorphisms predictive of telomere length are strongly associated with beverage consumption. Psychosocial stress is another important determinant of telomeric shortening; unfortunately, this construct was not captured within the NHANES questionnaires. Our analyses included all potential sociodemographic characteristics and health variables known to be related to telomere length and dietary intake, some of which might act as proxies for psychological stress; the inclusion of these variables did not substantially change the model estimates. Because we examined healthy adults without a history of diabetes or cardiovascular disease, the associations should reflect sugar-sweetened soda consumption independent of cardiometabolic disease.
Conclusions
Understanding the role that nutrition plays in telomere length maintenance is critical in understanding how to improve dietary intake. Independent of adiposity and other individual characteristics, our study results suggested that regular consumption of sugar-sweetened sodas was associated with significantly shorter telomeres. Further epidemiological studies are needed to confirm this association in longitudinal settings, and experimental research can examine the pathway from soda to cell to better understand the mechanism of this relationship. Still, there is sufficient evidence to limit our consumption of all SSBs to improve cardiometabolic risk factors, reduce chronic disease risk, and improve overall health. Our study supported a new link, shortened immune cell telomere length, which is a biological risk factor for aging, between sugar-sweetened soda consumption and metabolic disease.
Acknowledgments
This research was funded by grant R01AG033592-01A1 from the National Institute on Aging (E. S. Epel, principal investigator). C. W. Leung was supported by a grant from the University of California Multicampus Research Program and Initiatives proposal #142691.
J. Lin is a shareholder of Telomere Diagnostics, Inc., a telomere measurement company.
This article was presented as an abstract at the Epidemiology and Prevention/Nutrition, Physical Activity and Metabolism 2014 Scientific Sessions of the American Heart Association; March 18–21; San Francisco, CA.
The authors would like to thank RDC Analyst Carolyn Neal, PhD, for her assistance. Carolyn Neal did not receive any personal compensation for her work associated with this article.
Human Participant Protection
Institutional review board approval was not needed because secondary data from National Health and Nutrition Examination Survey (NHANES) data were used to conduct this study. NHANES is approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. Our proposal to analyze the genetic data was approved by the NCHS Research Data Center.
References
- 1.Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988-1994 to 1999-2004. Am J Clin Nutr. 2009;89(1):372–381. doi: 10.3945/ajcn.2008.26883. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988-2004. Pediatrics. 2008;121(6):e1604–1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- 3.Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the United States. J Acad Nutr Diet. 2013;113(1):43–53. doi: 10.1016/j.jand.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomeranz JL. Advanced policy options to regulate sugar-sweetened beverages to support public health. J Public Health Policy. 2012;33(1):75–88. doi: 10.1057/jphp.2011.46. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RK, Appel LJ, Brands M et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 6.Crude and Age-Adjusted Percentage of Civilian, Noninstitutionalized Adults With Diagnosed Diabetes. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; 2013. pp. 1980–2011. [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, 2009-2010. Atlanta, GA: National Center for Health Statistics, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 8.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulze MB, Manson JE, Ludwig DS et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 12.Demissie S, Levy D, Benjamin EJ et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 13.Shiels PG, McGlynn LM, MacIntyre A et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS ONE. 2011;6(7):e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 15.Puterman E, Epel E. An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2012;6(11):807–825. doi: 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler N, Pantell MS, O’Donovan A et al. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun. 2013;27(1):15–21. doi: 10.1016/j.bbi.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 18.Valdes AM, Andrew T, Gardner JP et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 19.Cherkas LF, Hunkin JL, Kato BS et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 20.Du M, Prescott J, Kraft P et al. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol. 2012;175(5):414–422. doi: 10.1093/aje/kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008;40(10):1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epel ES, Blackburn EH, Lin J et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzpatrick AL, Kronmal RA, Gardner JP et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 24.Murillo-Ortiz B, Albarran-Tamayo F, Arenas-Aranda D et al. Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male. 2012;15(1):54–58. doi: 10.3109/13685538.2011.593658. [DOI] [PubMed] [Google Scholar]
- 25.Willeit P, Willeit J, Mayr A et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 26.Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22(10):895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Zhu Y, Lin J et al. Short leukocyte telomere length predicts risk of diabetes in American Indians: the Strong Heart Family study. Diabetes. 2014;63(1):354–362. doi: 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. doi: 10.1038/ng.2528. 427e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassidy A, De Vivo I, Liu Y et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. 2010;91(5):1273–1280. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR., Jr Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Needham BL, Adler N, Gregorich S et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Soc Sci Med. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Epel E, Cheon J et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1-2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura M, Stone RC, Hunt SC et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5(9):1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 35.Rehkopf DH, Dow WH, Rosero-Bixby L, Lin J, Epel ES, Blackburn EH. Longer leukocyte telomere length in Costa Rica’s Nicoya Peninsula: a population-based study. Exp Gerontol. 2013;48(11):1266–1273. doi: 10.1016/j.exger.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usual Dietary Intakes. The NCI Method. Bethesda, MD: Division of Cancer Control and Population Sciences, National Cancer Institute; 2011. [Google Scholar]
- 37.Kipnis V, Midthune D, Buckman DW et al. Modeling data with excess zeros and measurement error: application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics. 2009;65(4):1003–1010. doi: 10.1111/j.1541-0420.2009.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ervin RB. Healthy Eating Index-2005 Total and Component Scores for Adults Aged 20 and Over: National Health and Nutrition Examination Survey, 2003-2004, National Health Statistics Reports: No 44. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 39.National Center for Health Statistics. Anthropometry Procedures Manual. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 40.Morlá M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27(3):525–528. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Wang X, Gutin B et al. Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J Pediatr. 2011;158(2):215–220. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Parks CG, DeRoo LA et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18(3):816–820. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt SC, Chen W, Gardner JP et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huzen J, Wong LS, van Veldhuisen DJ et al. Telomere length loss due to smoking and metabolic traits. J Intern Med. 2014;275(2):155–163. doi: 10.1111/joim.12149. [DOI] [PubMed] [Google Scholar]
- 45.Dei Cas A, Spigoni V, Franzini L et al. Lower endothelial progenitor cell number, family history of cardiovascular disease and reduced HDL-cholesterol levels are associated with shorter leukocyte telomere length in healthy young adults. Nutr Metab Cardiovasc Dis. 2013;23(3):272–278. doi: 10.1016/j.numecd.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Harris JL, Schwartz MB, Brownell KD . Sugary Drink FACTS: Evaluating Sugary Drink Nutrition and Marketing to Youth. New Haven, CT: Yale Rudd Center for Food Policy and Obesity; 2011. [Google Scholar]
- 47.Bazzano LA, He J, Ogden LG et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76(1):93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 48.Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol. 2010;171(6):701–708. doi: 10.1093/aje/kwp452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muraki I, Imamura F, Manson JE et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–1327. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eshak ES, Iso H, Mizoue T, Inoue M, Noda M, Tsugane S. Soft drink, 100% fruit juice, and vegetable juice intakes and risk of diabetes mellitus. Clin Nutr. 2013;32(2):300–308. doi: 10.1016/j.clnu.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Xi B, Li S, Liu Z et al. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2014;9(3):e93471. doi: 10.1371/journal.pone.0093471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K, Xing A, Chen K et al. Effect of fruit juice on cholesterol and blood pressure in adults: a meta-analysis of 19 randomized controlled trials. PLoS ONE. 2013;8(4):e61420. doi: 10.1371/journal.pone.0061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Sia CL, Dandona P. Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care. 2007;30(6):1406–1411. doi: 10.2337/dc06-1458. [DOI] [PubMed] [Google Scholar]
- 55.Gao X, Qi L, Qiao N et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50(2):306–312. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida M, McKeown NM, Rogers G et al. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr. 2007;137(9):2121–2127. doi: 10.1093/jn/137.9.2121. [DOI] [PubMed] [Google Scholar]
- 57.National Center for Health Statistics. NHANES Response Rates and Population Totals. Atlanta, GA: Centers for Disease Control and Prevention; 2013. [Google Scholar]