Sugita et al. recently reported a Ewing-like sarcoma within the right posterior neck of a 63 year old man that was found to have a novel CIC-FOXO4 gene fusion caused by translocation t(X;19)(q13;q13.3).1 The tumor was composed of sheets of undifferentiated small round blue cells within abundant desmoplastic stroma containing coarse chromatin and conspicuous nucleoli which expressed CD99, CD56, and WT1 but not SMA, desmin, CD34, or EMA. This patient is reportedly alive and disease-free at six months after gross total resection followed by adjuvant radiation and chemotherapy with adriamycin and ifosfamide.1 The prevalence, pathologic characteristics, and clinical behavior of this Ewing-like sarcoma with novel CIC-FOXO4 fusion are undefined.
In our recent genomic analysis of 62 tumors pathologically diagnosed as Ewing sarcoma, we identified seven tumors (11%) which lacked EWSR1 gene fusions and identified one tumor which harbored the same novel CIC-FOXO4 fusion.2 In both cases, a t(X;19)(q13;q13.3) translocation resulted in an in-frame fusion of the N-terminal exons of the CIC gene (breakpoint within exon 19 of the tumor reported by Sugita et al. and within exon 20 of current tumor) with the C-terminal exons of the FOXO4 gene (breakpoint within exon 2 in both tumors). Here we report the clinicopathologic characteristics of this second Ewing-like sarcoma harboring CIC-FOXO4 fusion.
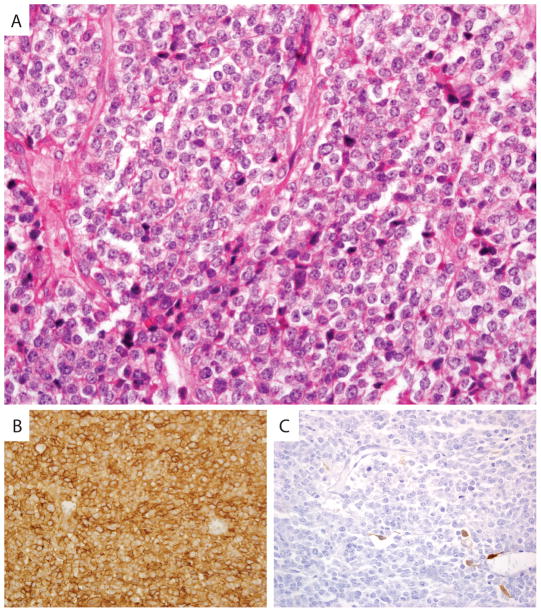
The 13 year old boy initially presented with a peanut-sized, non-tender, non-erythematous mass on his posterior scalp. Resection occurred at four weeks after initial presentation, by which time the mass had grown to be egg-sized. Pathology revealed diffuse sheets of a mitotically active small round blue cell tumor with areas of necrosis and a few interspersed fibrous bands but no well-defined matrix or desmoplastic stroma. The cells have irregular round nuclei with coarse chromatin with conspicuous nucleoli and scant eosinophilic cytoplasm and were positive for CD99 staining and negative for desmin, keratins AE1/AE3, CD56, HMB45, S-100 protein, and nuclear WT1 staining (Fig. 1). Clinical testing was negative for EWSR1-FLI1 fusion. The patient received treatment as per Children’s Oncology Group protocol AEWS0031 (vincristine, doxorubicin, and cyclophosphamide alternating with ifosfamide and etoposide). Five months after diagnosis, the patient had an excision of the surgical scar that was negative for tumor. He relapsed 11 months after his initial diagnosis with three scalp nodules, two of which were resected and showed similar morphology and immunohistochemical profile as his initial tumor with occasional Homer Wright rosettes typical of primitive neuroectodermal tumors. Shortly thereafter, the patient had a restaging chest CT scan that revealed several pulmonary metastases. The patient received salvage chemotherapy with irinotecan and temozolomide and then cabozantanib, the latter on clinical trial. The patient passed away 22 months after his initial presentation.
Figure 1.
Histology and immunoprofile of Ewing-like sarcoma harboring CIC-FOXO4 fusion resected from an occipital scalp mass of a 13 year old male. H&E stain shows a highly cellular and mitotically active tumor composed of small round blue cells with coarse chromatin, prominent nucleoli, and scant eosinophilic cytoplasm (A). Immunohistochemistry shows that the tumor cells are strongly positive for CD99 (B) and negative for S-100 protein (C) with occasional entrapped S-100 positive dendritic cells.
This is the second reported case of an undifferentiated small round cell sarcoma with CIC-FOXO4 gene fusion. Compared to the prior case, this tumor occurred in a teenager on the scalp and had aggressive clinical course with both local recurrence and lung metastasis leading to the patient’s death despite total resection and intensive chemotherapy. While the cellular morphology of the current tumor is similar to the prior case, the current tumor does not have abundant desmoplastic stroma mimicking desmoplastic small round cell tumor (DSRCT) as did the prior case reported by Sugita et al. Additionally, the current tumor is negative for CD56 and nuclear WT1 staining compared to the previous tumor. The morphology and immunoprofile of this tumor has a striking similarity to the group of atypical Ewing sarcomas described by Llombart-Bosch et al. in 2009.3 Further identification and clinical follow-up of Ewing-like sarcomas lacking EWSR1 rearrangement with alternative fusions including CIC-FOXO4, CIC-DUX4, and BCOR-CCNB3 will allow refined prognostic subclassification of this emerging group of tumors.
Acknowledgments
Funding disclosures: J.K. and M.M. are supported by the Intramural Research Program of the National Cancer Institue, National Institutes of Health.
References
- 1.Sugita S, Arai Y, Tonooka A, et al. A novel CIC-FOXO4 gene fusion in undifferentiated small round cell sarcoma: a genetically distinct variant of Ewing-like sarcoma. Am J Surg Pathol. doi: 10.1097/PAS.0000000000000286. Epub 2014 Jul 8. [DOI] [PubMed] [Google Scholar]
- 2.Brohl AS, Solomon DA, Chang W, et al. The genomic landscape of the Ewing sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet. 2014;10:e1004475. doi: 10.1371/journal.pgen.1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llombart-Bosch A, Machado I, Navarro S, et al. Histological heterogeneity of Ewing’s sarcoma/PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch. 2009;455:397–411. doi: 10.1007/s00428-009-0842-7. [DOI] [PubMed] [Google Scholar]