Abstract
Context
Sustainable interventions are needed to minimize HIV risk behavior among people living with HIV (PLWH) in South Africa on antiretroviral therapy (ART), a significant proportion of whom do not achieve viral suppression.
Objective
To determine whether a brief lay counselor delivered intervention implemented during routine care can reduce risky sex among PLWH on ART.
Design
Cluster randomized 16 HIV clinical care sites in KwaZulu Natal, South Africa, to intervention or standard-of-care.
Setting
Publicly funded HIV clinical care sites.
Patients
1891 PLWH on ART received the HIV prevention counseling intervention (n = 967) or standard-of-care counseling (n = 924).
Intervention
Lay counselors delivered a brief intervention using motivational interviewing strategies based on the Information—Motivation—Behavioral Skills (IMB) model during routine clinical care.
Main Outcome Measures
Number of sexual events without a condom in the past four weeks with partners of any HIV status, and with partners perceived to be HIV-negative or HIV-status unknown, assessed at baseline, 6, 12, and 18 months.
Results
Intervention participants reported significantly greater reductions in HIV risk behavior on both primary outcomes, compared to standard-of-care participants. Differences in STI incidence between arms were not observed.
Conclusion
Effective behavioral interventions, delivered by lay counselors within the clinical care setting, are consistent with the strategy of linking HIV care and HIV prevention and integrating biomedical and behavioral approaches to stemming the HIV epidemic.
Key words/Phrases: South African HIV Epidemic, Prevention with Positives, HIV Risk Reduction, IMB Model
INTRODUCTION
Since the beginning of the South African epidemic, an estimated 2 million adults have died from HIV/AIDS1,2, 6.1 million South Africans are currently living with HIV3, the prevalence in the 15–49 year age range is 17.9%3, and the incidence is 1.43% per year among those aged 15–494. Over 370,000 HIV infections and 240,000 AIDS-related deaths occur in South Africa each year3.
South Africa’s HIV Testing and Counselling campaign and rollout of antiretroviral therapy (ART) are well established5. More South Africans are learning their HIV status and entering clinical care6, presenting a unique opportunity to link HIV treatment with HIV prevention behavioral interventions for persons living with HIV (PLWH) on ART. PLWH on ART constitute a large and growing population of great significance for impacting South Africa’s epidemic7. Specifically, these individuals, like PLWH everywhere, are variably adherent to ART8 and to safer sex practices9–11, despite clinic-based standard-of-care ART education and counseling and safer sex promotion. Treatment failure with continuing detectable viremia among South African PLWH on ART is not uncommon12,13 and ART resistance has occurred in a sizable proportion of individuals who have been treated and have experienced therapeutic failure14–19. South African PLWH on ART who have experienced treatment failure may serve as relatively healthy but infectious vectors for transmission of drug susceptible and drug resistant virus and may contribute to the maintenance or exacerbation of South Africa’s HIV epidemic. These individuals represent one potential leading edge of the country’s generalized HIV epidemic and merit priority for behavioral safer sex interventions to avert forward HIV transmission. PLWH who engage in unprotected sex also place themselves at risk for other sexually transmitted infections (STI), associated morbidity, accelerated progression of HIV disease20–22, and potential superinfection with drug resistant HIV23–26.
Despite the need for evidence-based safer sex behavioral interventions for PLWH on ART in South Africa and their potential efficacy and efficiency when delivered in the clinical care setting, too few large- scale South African research studies, conducted as rigorous experimental trials in the clinical care context, have been reported27–31. In the United States and other resourced countries, more randomized controlled trials of HIV risk reduction behavioral interventions designed for PLWH and delivered in clinical care settings have been reported32–42 (See 32 for an overview of twenty studies in this area.) We extensively modified the U.S. Options project—a quasi-experimental intervention resulting in significant decreases in HIV risk behavior among PLWH in an HIV clinical care setting34 – for the South African cultural context, HIV risk dynamics, and health care setting. We conducted a successful pilot study of this intervention in South Africa28 and brought it to scale. The current research widely implemented and rigorously evaluated this intervention in the South African HIV clinical care setting to assist PLWH on ART to reduce HIV risk behavior. We hypothesized that over 18-months, PLWH participating in the intervention compared to those receiving standard of care (SOC) would demonstrate significantly greater reductions in HIV sexual risk behavior.
METHODS
Randomization
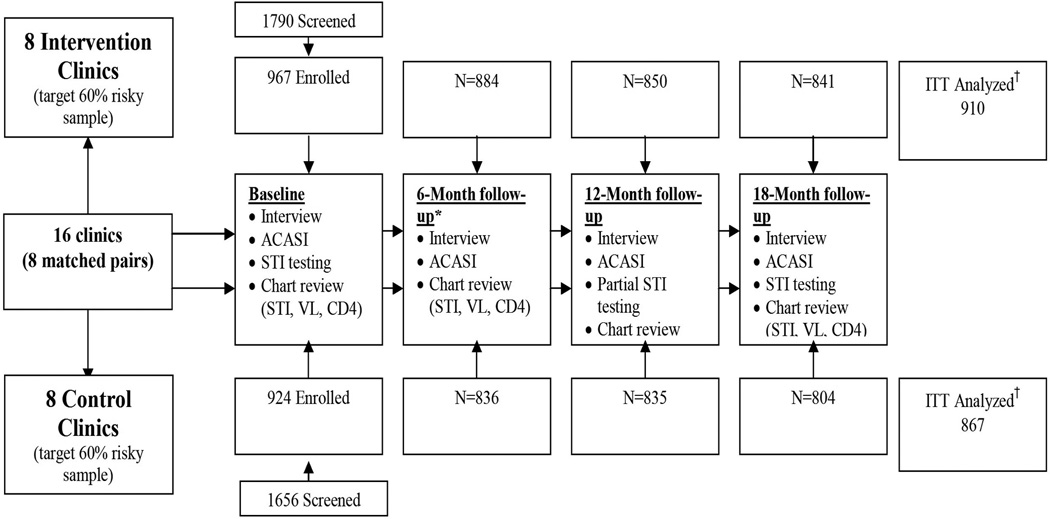
Sixteen urban, peri-urban, and rural Primary Healthcare Clinics and Community Health Centers in the uMgungundlovu and uMkhanyakude health districts of KwaZulu-Natal, South Africa, paired based on geography and other relevant clinic characteristics (e.g., catchment area, patient population, clinic resources), were cluster randomized to intervention (8 clinics) or SOC (8 clinics) arms. These health districts report among the highest rates of HIV in South Africa; antenatal clinic attendee prevalence is 39.8% and 41.1%, respectively4. See Figure 1 for study design.
Figure 1. Study design, recruitment and assessment flow Intervention and Standard of Care Arms.
†GEE analyses required two of more assessment periods with a valid score on the primary ACASI collected risk behavior variable to be included in the ITT. 94% of randomized participants met this criterion in the intervention arm and in the control arm.
Participants
HIV-infected participants on ART (N = 1891) were recruited from clinical care sites from June 2008 - May 2010. Inclusion criteria were documented HIV infection, receiving HIV care at a participating clinic, prescribed ART, and minimum age 18 years. To maximize statistical power (≥ 80%) to detect changes in HIV risk behavior, enrollment targets specified a minimum of 16 clinics with a minimum of 125 participants per site and used a sampling strategy oversampling those reporting recent HIV risk behavior. Sampling targeted a 60:40 distribution of those reporting risk behavior during the past 4 weeks on a pre-enrollment screener vs. those not reporting risk. Similar numbers of HIV-infected women and men on ART were recruited.
Procedures
Clinic staff referred eligible PLWH to a research assistant who described the study and screened interested patients for risky sex in the past 4 weeks. Patients meeting criteria were invited to take part in the study and provide informed consent. Participation consisted of (1) completing audio computer-assisted self-interview (ACASI) and interviewer-administered questionnaires (in isiZulu or English) at four time points over 18-months (baseline, 6, 12, and 18 months), (2) providing biological samples assessing sexually transmitted infections (STIs) at three time points (baseline, 12, and 18 months), and (3) consenting to medical chart reviews for CD4 count, HIV viral load, STIs, and health status. As part of routine clinical care, participants in the intervention (n = 967) and SOC (n = 924) arms received counseling from lay counselors concerning issues relevant to PLWH on ART (e.g., adherence education and counseling).
Participants at the eight intervention clinics (n = 967) received brief (10–15-minute), theory and evidence-based, tailored, one-on-one counseling sessions with trained lay counselors concerning sexual risk behavior reduction. Intervention sessions were integrated into PLWHs’ routine clinical care during the 18-month study period. SOC participants received standard of care safer sex promotion messages from counselors, typically involving standard condom promotion messaging. Assessments were carried out by a different individual in a separate research setting at the four specified time points within the 18 month study.
Intervention and SOC participants were compensated for completing measures (R70 ~US $10 per assessment) but not for participation in one-on-one counseling. The study was conducted according to the principles of the Helsinki Declaration and approved by ethics committees at University of Connecticut (USA), University of KwaZulu-Natal (South Africa), Centre for Addiction and Mental Health (Canada), the Research Committee of the KwaZulu-Natal Department of Health, and relevant District Health Offices.
Outcome Measures
The primary outcome measures for intervention evaluation were ACASI-reported number of sexual events without condoms (penile-vaginal or penile-anal) over the past 4 weeks with all partners, regardless of perceived partner serostatus, and number of sexual events without condoms over the past 4 weeks with partners perceived to be HIV-negative or -status unknown. Additional outcome measures included interviewer assessment of participants’ number of unprotected sexual acts during the past 4 weeks, inconsistent condom use over the past 4 weeks and over the past 3 months (1 = “never” to 5 = “always”), and condom nonuse over the past 6 months (“When did you last have sex without a condom?”).These partially overlapping interviewer-delivered measures were included to provide multiple, potentially convergent endpoints assessed via alternative methodologies (ACASI and interviewer) over varying time periods. Data were cleaned prior to analyses; values entered on ACASI surveys judged to be due to touch screen over-sensitivity (e.g., the same number in duplicate [i.e., 88] or triplicate [i.e., 888]) were set to missing (affecting <1.7% of the data, unrelated to study arm).
Self-collected biological samples (vaginal tampons for women, urine samples for men) at baseline, 12, and 18 months assessed incident STIs including Neisseria gonorrhoeae and Chlamydia trachomatis in men and women and Trichomonas vaginalis in women. (The 12-month STI testing was abandoned midway through collection due to financial constraints). Specimens were transported to the laboratory within 48 hours43.
Intervention
The Izindlela Zokuphila/Options for Health HIV risk reduction counseling intervention for PLWH on ART was delivered by lay counselors on an ongoing basis integrated within routine HIV clinical care visits and based on the Information-Motivation-Behavioral Skills (IMB) model of health behavior change44,45. It consisted of brief, collaborative, patient-centered, face-to-face discussions between a lay counselor and a patient. Motivational Interviewing (MI) techniques46,47 were used to: (a) assess the patient’s sexual risk behavior, (b) identify informational, motivational, and behavioral skills barriers to safer sex, (c) explore strategies the patient could use to address barriers, and (d) negotiate an achievable, individually-tailored behavior change (or maintenance) goal. This intervention demonstrated acceptability, feasibility, and fidelity in South African pilot projects27,28 and was adapted for the current study. At the end of each intervention session, lay counselors completed an “Options Record Form” (ORF) serving as a guide for continuing counseling at subsequent sessions and as a measure of intervention fidelity. The full study protocol is available at www.chip.uconn.edu/southafricaoptions.
Lay Counselor Training and Support
Lay counselors from intervention sites (N = 48) participated in an intensive 5-day training to criterion.27,28. Telephone consultation, direct observation, and booster trainings provided ongoing support to lay counselors, who were already employed as clinic staff at intervention and control sites. One additional study-supported lay counselor was hired at each intervention site to assist with intervention delivery; one was hired at each control site to provide resource parity.
Analytic Approach
Pretest equivalence and attrition analyses were conducted to identify covariates (any baseline variable that was non-equivalent between randomized groups or significantly associated with attrition or missing assessments). Sites were randomized to intervention or SOC control condition, and individuals within sites were assessed on 4 occasions (baseline, 6, 12 and 18 months) on the primary and additional risk-related outcomes. Intention to treat (ITT) outcome analyses used generalized linear mixed effects modeling with non-normal outcome distributions (negative binomial) and AR(1) covariance structure to account for the correlated nature of longitudinal data48,49, negative binomial distributions of outcome measures49,50, and clustering of over time assessments within participants within research site. Analyses used ‘time’ as a continuous variable, with the interaction between time and condition used to determine effect of study condition on changes in risk behavior over time. We repeated analyses using ‘time’ in the class statement to evaluate effects by assessment interval. We found that negative binomial51 (versus Poisson) distribution on count-based outcomes and AR(1) as opposed to other structures were preferable. Outcomes were evaluated with SAS version 9.352 using PROC GLIMMIX which accounts for repeated observations of the same individual over time, nested within clinical care site, and estimates missing observations via all available pairs. Missing data were infrequent; analyses are expected to be robust and consistent with outcomes that adopt multiple imputation strategies to recover larger gaps in data coverage53,54. All main analyses were repeated to determine robustness of effects controlling for identified covariates and participant sex. The potential impact of baseline rates of risky sex was included in the models, as baseline risk set the intercept for each individual’s slope for change over time, although we also compared study arms for potential baseline differences. Newly diagnosed STIs at 18 months relative to baseline were evaluated for study arm differences using simple chi-square tests and logistic regression.
RESULTS
Patient Characteristics
1891 HIV+ patients on ART (mean age 37.3 years; range 18–78 years) were enrolled. At baseline, approximately two-thirds had been on ART less than two years, 30% had CD4+ counts < 200 cells/uL, and approximately one in four (26.1% of men, 22.2% of women) with chart-based viral load data (N=961 of 1891 [51%]) at baseline had detectable viral load. Table 1 provides additional patient characteristic data. Intervention and SOC participants had equal clinical care visits (x̄ = 11, SD = 4.68, range 1–24) over study participation. About 75% of routine clinical care visits included contact with a lay counselor, and in the intervention arm about 75% of visits with counselor contact included intervention sessions. 903 (93.3%) intervention arm participants were exposed to the intervention, receiving an average of five intervention counseling contacts (range 0 to 15, SD = 2.86, normally distributed).
Table 1.
Characteristics of Study Participants.
| Characteristics | SOC* n (%) (n = 924) |
Intervention n (%) (n = 967) |
P value |
|---|---|---|---|
| Female | 514 (55.6%) | 537 (55.5%) | 0.967 |
| Male | 410 (44.4%) | 430 (44.5%) | 0.967 |
| Age (M, SD) | 37.3 (9.0) | 37.3 (9.0) | 0.828 |
| Race/ethnicity | 0.053 | ||
| Black-Zulu | 882 (95.6%) | 935 (97.3%) | |
| Black-Xhosa | 13 (1.4%) | 13 (1.4%) | |
| Black-Another race | 23 (2.5%) | 10 (1.0%) | |
| Indian | 2 (0.2%) | 0 (0.0%) | |
| Coloured | 1 (0.1%) | 3 (0.3%) | |
| Other | 2 (0.2%) | 0 (0.0%) | |
| Education | 0.646 | ||
| No schooling | 134 (14.5%) | 133 (13.8%) | |
| Class1/GR1 - STD7/GR9 | 405 (43.9%) | 440 (45.8%) | |
| STD8/GR10 - STD10/Matric/N3/GR12 | 373 (40.4%) | 381 (39.6%) | |
| Post-secondary | 11 (1.2%) | 7 (0.7%) | |
| Employment and Income | |||
| Currently unemployed | 657 (71.2%) | 698 (72.2%) | 0.629 |
| Household income <R1500/Month (~US $200) | 394 (70.7%) | 538 (72.6%) | 0.354 |
| Relationship and Family | |||
| Married/living with a partner | 213 (23.1%) | 203 (21.1%) | 0.279 |
| Cohabitating with sex partner | 475 (51.4%) | 449 (46.4%) | 0.031 |
| Have one or more children | 809 (88.4%) | 861 (89.4%) | 0.493 |
| Currently trying to have a baby | 227 (24.6%) | 272 (28.1%) | 0.086 |
| Housing | |||
| Housing location = Rural | 617 (66.9%) | 653 (67.5%) | 0.778 |
| Dwelling Type = Informal | 509 (55.1%) | 532 (55.1%) | 0.974 |
| Lives in City or Township | 149 (16.1%) | 204 (21.1%) | 0.006 |
| HIV Diagnosis and Treatment at Study Baseline (Clinical chart extraction) | |||
| HIV positive for 2 or more years | 415 (57.6%) | 372 (56.8%) | 0.752 |
| On ARTs for 2 or more years | 304 (35.9%) | 277 (33.2%) | 0.241 |
| CD4 Count <200 | 233 (29.9%) | 230 (29.7%) | 0.920 |
| HIV viral load <=50 copies/mL= Undetectable (±90 days from Baseline†) | 367 (78.3%) | 364 (74.0%) | 0.121 |
| HIV viral load Mdn, IQR copies/mL among those with detectable viral load | [975, 175: 8800] | [545, 134: 3050] | 0.087 |
| Meets with a counselor at clinic at least every 3 months | 774 (83.8%) | 770 (79.6%) | 0.020 |
| Self-Reported Physical and Mental Health | |||
| FAHI - Physical Health (possible range=0–40) (M, SD) | 28.2 (8.0) | 26.9 (8.2) | 0.001 |
| Depressed (Modified CESD using 15 as cutoff) | 197 (21.3%) | 239 (24.7%) | 0.080 |
| Reported drinking alcohol weekly or more frequently | 48 (5.2%) | 16 (1.7%) | 0.0001 |
Standard-of-care
Percentages are based on the number of participants who indicated a specific response divided by the number of participants who responded to the item in question.
Significant differences presented as adjusted scores using mean substitution for missing values to allow for use of variable in main analyses (2 missing values for living in city or township; 3 missing values for cohabitation; 3 missing values for seeing counselor at least every 3 months; 16 missing values for drinking weekly; and 1 missing value for depression scores). Missingness was unrelated to condition.
Abbreviations: ART - Antiretroviral Medication Therapy; CESD - Centers of Epidemiological Studies- Depression scale; FAHI - Functional Assessment of HIV Infection; GR - Grade level; IQR - Interquartile Range; M - Mean; Mdn - Median; N - National Qualification Framework; n - Number of participants; R - South African Rand; SD - Standard Deviation; STD - Standard
Baseline Equivalence and Attrition
Baseline levels of primary and additional risk outcomes, CD4, and viral load did not differ by condition. Five demographic variables, identified as potential covariates based on non-equivalence between arms at baseline, were used as covariates in intervention outcome analyses (see Table 1).
Thirteen percent (246/1891) of participants discontinued participation before the 18-month assessment. This was evenly distributed between intervention (13.0%) and SOC (13.0%) arms and unrelated to covariates identified in pre-test equivalence analyses or to categorical baseline risk variables. Sex was related to attrition; stratified by study arm, men were lost to follow-up more than women in the SOC condition (17% of men, 10% of women, p = 0.001), with a similar trend in the intervention condition (15% of men, 11% of women, p = 0.06), not an uncommon finding in studies involving HIV care in South Africa55–58. Men and women did not differ in inclusion in the ITT analysis (93% of men and 95% of women had sufficient data for inclusion), but we nonetheless considered sex in the covariate-controlled intervention effects analyses. Missing any risk variable assessment at any point was experienced by 316 (16.7%) participants but was unrelated to condition (X2 = 0.09, p = 0.78) or sex (X2 = 0.20, p= 0.65), and differential measurement attrition by study arm did not occur. Study withdrawals were not related to study arm; there were no adverse events due to intervention exposure.
Analyses of HIV Risk Behavior Outcomes
ITT analyses indicate that, compared to SOC participants, intervention participants showed significantly greater reductions in HIV risk behavior on the primary outcome variables. Over the past four weeks, intervention participants indicated significantly greater reductions in number of sex events (penile-vaginal or penile-anal) without a condom with any partner regardless of serostatus, and in number of sex events without a condom with partners perceived to be HIV-negative or -status unknown (Table 2; Figures 2 and 3).
Table 2.
ITT generalized linear mixed effects modeling with non-normal outcome distributions (negative binomial) and AR(1) covariance structure comparing intervention to control.
| Variable | Estimate | Standard Error | df | t | p |
|---|---|---|---|---|---|
| Number of events without condom with any partner | |||||
| Intercept | 0.6239 | 0.1891 | 6 | 3.30 | 0.0164 |
| Condition (Intervention) | 0.08902 | 0.2646 | 6 | 0.34 | 0.7480 |
| Time effect in Intervention Arm | −0.4462 | 0.05162 | 6628 | −8.64 | <.0001 |
| Time effect in Control Arma | −0.2210 | 0.05098 | 6628 | −4.33 | <.0001 |
| Time by condition (Intervention) | −0.2241 | 0.07351 | 6628 | −3.05 | 0.0023 |
| Number of events without condom with partners perceived to be HIV negative or HIV status unknown partners | |||||
| Intercept | 0.01043 | 0.1353 | 6 | 0.08 | 0.9411 |
| Condition (Intervention) | 0.2293 | 0.1895 | 6 | 1.21 | 0.2717 |
| Time effect in Intervention Arm | −0.7212 | 0.05328 | 6628 | −13.54 | <.0001 |
| Time effect in Control Arma | −0.3124 | 0.04604 | 6628 | −6.79 | <.0001 |
| Time by condition (Intervention) | −0.4088 | 0.07042 | 6628 | −5.81 | <.0001 |
Derived by model where intervention arm served as referent group. Intervention condition=1; Control condition=0.
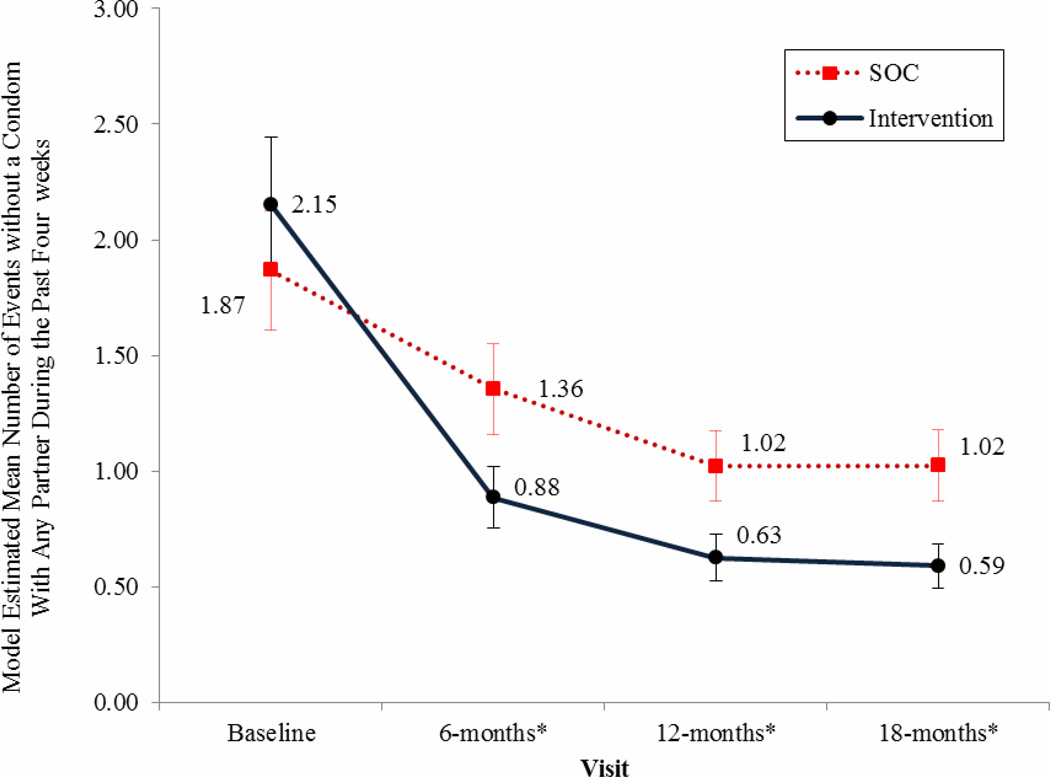
Figure 2. Model Estimated Mean Number of Events without a condom with any Partner During the Past Four weeks.
Results indicate a statistically significant decrease favoring the intervention arm at each assessment point with a 72% total reduction in events without a condom from baseline by 18-months in the intervention group versus a 45% reduction in the control arm.
Error bars represent +/− 1 standard error with non-overlap in errors between group estimates reflecting significant group differences, also designated with * p < 0.05.
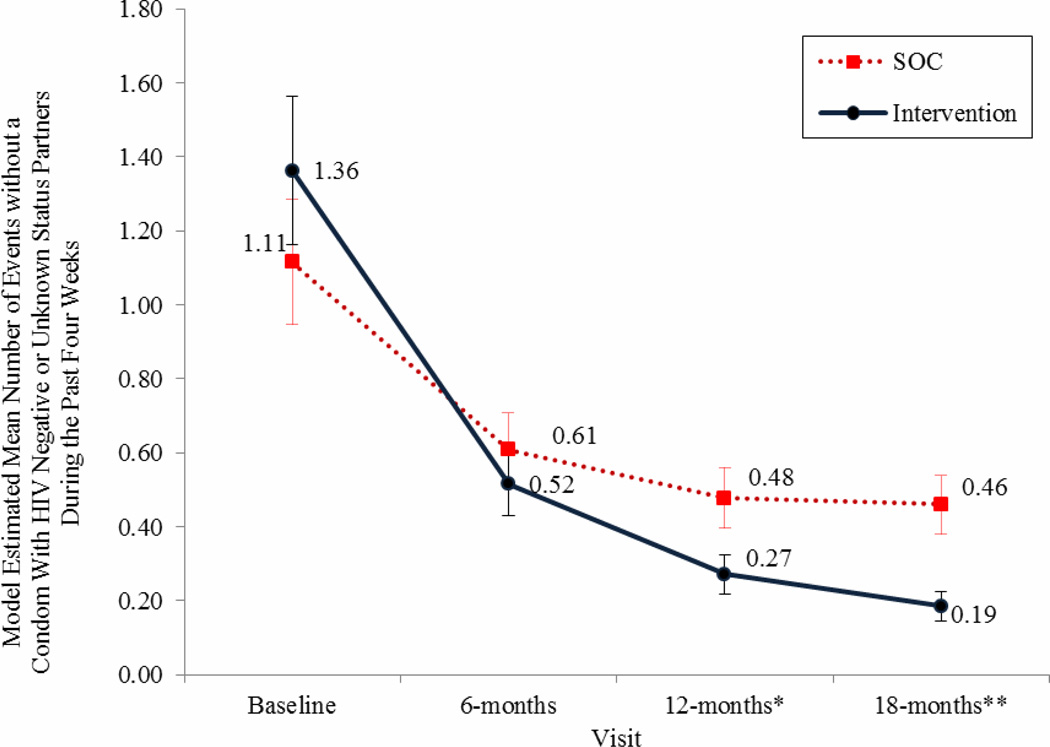
Figure 3. Model Estimated Mean Number of Events without a Condom with Partners Perceived to be HIV-negative or HIV-status unknown During the Past Four weeks.
Results indicate a statistically significant decrease favoring the intervention group with an 86% total reduction in events without a condom from baseline by 18-months in the intervention group versus a 59% reduction in the control arm.
Error bars represent +/− 1 standard error with non-overlap in errors between group estimates reflecting significant group differences, also designated with * p < 0.05 and ** p < 0.01 on over time axis.
Reported number of sex events without a condom with partners regardless of perceived serostatus during the past 4 weeks decreased over time for each group (time effect −0.46, p < .0001 for intervention;- 0.22, p <0.0001 for SOC). However, membership in the intervention condition was associated with greater risk reduction compared to SOC (interaction effect −0.22, p < .002). Study arms significantly differed in favor of the intervention condition at 6, 12 and 18 month assessments for number of events without a condom during the past four weeks with partners regardless of serostatus (see Figure 2). Similar results were found for number of sexual events without a condom during the past four weeks with partners perceived to be HIV-negative or -status unknown. Events without a condom involving these partners decreased over time for intervention and control participants (time effect −0.72, p <0.0001 in the intervention condition; −0.31, p < 0.0001 in the control condition), with the intervention condition associated with greater reduction in sex without a condom (interaction effect −0.41, p < 0.0001). The arms significantly differed in number of sexual events without a condom with HIV-negative and -status unknown partners at 12 and 18-month assessment intervals (see Figure 3).
Repeating analyses controlling for covariates and sex produced similar results. Analysis of the additional outcome data (interviewer administered measures of number of unprotected sexual acts during the past 4 weeks, consistency of condom use during the past 4 weeks and 3 months, and condom nonuse during the past 6 months) produced similar results with intervention participants reporting significantly greater reductions in all additional measures of risk behavior over various time frames, compared to control participants (data not shown).
STI Findings
STI data were available for 1873 (99%) participants at baseline and 1571 (83%) at 18 months. Missing STI data at 18 months was marginally associated with study arm (15.3% of intervention vs. 18.6% of SOC participants were missing STI data at 18 months; X2= 3.82(1,1891), p = 0.06). Excluding 221 participants with a confirmed STI at baseline (111 intervention, 110 control), incident STIs were evaluated for those without STI at baseline who had one at month 18. 53 (7.4% of valid cases) intervention and 44 (6.8% of valid cases) control participants had new STIs at month 18 (X2(N=1366) =.17, ns). Additionally, new STIs did not differ by study arm considered within sex or by specific STI. Results were also unchanged when examining percent of participants with any STI at each time point, regardless of baseline STI status.
DISCUSSION
The findings support the efficacy of our intervention for reduction of HIV risk among HIV-infected South Africans on ART. Intervention compared to SOC participants reported consistent, statistically significant, meaningful reductions in each primary and in each additional risk behavior endpoint. Findings indicated greater intervention than SOC reductions in unprotected sex with all partners and with partners perceived to be HIV-negative or -status unknown. Similar reductions in risk were observed on all additional outcome measures including unprotected sex overall and with HIV-negative or status unknown partners, across assessment intervals from four weeks to six months, as assessed by interviewer and ACASI methodologies. These intervention effects were obtained controlling for site-level differences, which, when significant, were generally small, and few in number, and occurred despite matching sites on key variables, and when controlling for covariates and sex of participant. The intervention was delivered during routine clinical care visits, on an ongoing basis, by trained lay counselors, nearly all already employed at clinical care sites. This approach provides effective and continuing intervention exposure linking HIV treatment with HIV prevention while deploying existing resources effectively.
Results demonstrated a substantial decline in HIV risk behavior and persistence of reduced risk behavior supported by the continuing presence of the intervention. With over-sampling of sexually risky individuals, participants reported roughly two unprotected sexual events with any partner, and approximately one with an HIV- or -status unknown partner during the past four weeks, at study baseline. At 18 months, intervention participants engaged in roughly one-quarter as much risky behavior with any partner, and one-seventh as much risky behavior with partners perceived to be HIV- or -status unknown, compared to their baseline risk, and engaged in significantly less risk than SOC participants at almost every post-baseline assessment. While the current intervention was designed to be an ongoing component of routine clinical care and thus a final post-intervention follow-up period was not part of the study design, results showed sustained reductions in risk behavior in the presence of the intervention, as intended.
Our intervention is highly compatible with an integrated behavioral and biomedical approach to HIV prevention incorporating ART treatment and adherence to reduce viral load59 and HIV risk reduction to prevent forward transmission from PLWH who are not virally suppressed— 25% of our sample. As such, our intervention represents an important addition to the integrated behavioral and biomedical HIV prevention armamentarium. It has considerable promise for widespread and sustainable dissemination at low cost with existing clinic personnel in low resource settings. The major costs of implementation as a standard approach to integrated behavioral and biomedical HIV risk reduction would involve training lay counselors already on staff in the intervention protocol and training an existing site mentor to provide ongoing intervention fidelity support.
While this research has several strengths, limitations are present. Although the intervention achieved significantly greater risk reduction than the SOC at nearly all assessment points, the SOC also exhibited a significant, although significantly less robust, reduction in risk. Reduction of HIV risk behavior in control conditions of intervention research is commonly observed60–63 and may be a result of research-related attention and monitoring of sexual behavior. A second, important limitation is that we did not observe significant intervention impact on the STI outcome. This would have been a desirable complement to our self-reported outcomes that focus directly on unprotected sexual events, but which are nonetheless subject to potential reporting bias. Complexities in collection and interpretation of STI data as proxies for sexual risk behavior include the inability to account for individuals who acquired symptomatic STIs and were successfully treated between STI assessments, and individuals assessed with an STI and referred to treatment, who did not successfully complete treatment or clear the infection. Failure to observe intervention impact on STI endpoints is present in a number of published HIV prevention behavioral intervention trials63,64. With the current intervention effects replicating on diverse measures of risk behavior (ACASI, interview, asked as count data, as estimates of frequency of condom use, and for varying time intervals), confidence in the integrity and accuracy of the behavioral risk reduction findings may be increased. Finally, although retention was high, among those who left the study early, men were overrepresented across study arms. The percent loss was low in each arm, however, and intervention results were maintained when controlling for sex, suggesting that intervention effects should be generalizable to men and women living with HIV and treated with ART in the South African clinical care setting.
CONCLUSION
Our intervention provides effective, efficient, continuing support for HIV risk reduction among HIV-infected South Africans on ART. It is compatible with an integrated behavioral and biomedical approach to stemming HIV and holds promise for sustainable and widespread dissemination efforts linking treatment and prevention to curtail the South African epidemic. Our intervention, integrated within the clinical care setting and utilizing existing staff, represents an empirically-supported strategy to leverage existing resources and structures to promote HIV risk reduction among HIV-infected individuals on ART in generalized epidemic, resource-limited, sub-Saharan settings.
Acknowledgments
Tremendous thanks go to the KwaZulu-Natal (KZN) Department of Health (DOH) and uMkhanyakude (DC 27) and uMgungundlovu (DC 22) health districts for their collaboration and support. We thank the lay counselors working at the KZN DOH health clinics who delivered the Options counseling intervention to study participants for the duration of the study. We are greatly appreciative of the efforts of the onsite Options for Health research assistants, who assisted patients on a daily basis throughout the project, and the US-based research assistants Lindsay Shepherd, BS, (Jackson State University), Erin Lenz, BA (University of Connecticut), and Colin Barr, BS (University of Connecticut), for administrative, technical, and material support. We would also like to thank the data managers, Ross Greener and Frang Ngomu. Finally, we would like to thank the clinic staff and most particularly the patients who took part in the research at the participating sites. The Options for Health workers and US-based research assistants were compensated, grant-funded positions.
Source of Funding: This study was funded by a US National Institute of Mental Health grant (5R01MH077524-05), Jeffrey D. Fisher, PhD, Principal Investigator.
Footnotes
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Conference presentation: Some of the results in this manuscript were presented at the 19th International AIDS Conference, Washington D.C., July 2012.
Author Contributions
Study concept and design: J Fisher, K Amico, S Christie, D Cornman, W Fisher, G Friedland, U Lalloo, S Pillay and P Shuper.
Acquisition of data: S Christie, S MacDonald, N Ngcobo, S Pillay and P Shuper.
Analysis and interpretation of data: KR Amico, D Cornman, J Fisher, W Fisher, and P Shuper.
Drafting of the manuscript: KR Amico, S Christie, D Cornman, J Fisher, W Fisher, P Shuper.
Critical revision of the manuscript for important intellectual content: J Fisher, D Cornman, P Shuper, S Pillay, S Christie, S MacDonald, N Ngcobo, U Lalloo, G Friedland, W Fisher.
Statistical analysis: KR Amico
Obtained funding: D Cornman, J Fisher, W Fisher, S Pillay, P Shuper, G Friedland
Administrative, technical, or material support: J Fisher, D Cornman, P Shuper, S Pillay, S Christie, S MacDonald, N Ngcobo.
Study supervision: S Christie, D Cornman, J Fisher, W Fisher, G Friedland, U Lalloo, S MacDonald, N Ngcobo, S Pillay, P Shuper.
P. Shuper and KR. Amico had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
SA Options Team
This study would not have been possible without the hard work and dedication of all members of the SA Options Team. For a full list of team members and their respective contributions, please visit the Center for Health, Intervention, and Prevention (CHIP) website (www.chip.uconn.edu).
Contributor Information
Deborah H. Cornman, Email: deborah.cornman@uconn.edu.
Paul A. Shuper, Email: paul_shuper@camh.net.
Sarah Christie, Email: sarahschristie@gmail.com.
Sandy Pillay, Email: sandypillay7@gmail.com.
Susan Macdonald, Email: sue.options@gmail.com.
Ntombenhle Ngcobo, Email: ntombenhle.ngcobo@gmail.com.
K. Rivet Amico, Email: rivetamico@comcast.net.
Umesh Lalloo, Email: lalloo@gmail.com.
Gerald Friedland, Email: gerald.friedland@yale.edu.
William A. Fisher, Email: fisher@uwo.ca.
REFERENCES
- 1.Bongaarts J, Francois P, Gerland P. Poverty, Gender, and Youth: Global Treands in AIDS Mortality. In: Rogers RG, Crimmins M, editors. International Handbook of Adult Mortality. 1st ed. Springer; 2009. [Google Scholar]
- 2.Republic of South Africa: Department of Health. The 2010 national antenatal sentinel HIV and syphilis prevalence survey in South Africa. South Africa: National Department of Health; 2011. [Google Scholar]
- 3.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013: Joint United Nations Programme on HIV/AIDS (UNAIDS) 2013 [Google Scholar]
- 4.Republic of South Africa: Department of Health. The 2011 National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa. South Africa: National Department of Health; 2012. [Google Scholar]
- 5.South African National AIDS Council. Statement on the meeting of the South African National AIDS Council. [Accessed 1 November 2011];2011 [Press Release]. http://www.sanac.org.za/files/uploaded/886_Plenary%20Press%20Statement%2012Aug11.pdf. [Google Scholar]
- 6.Republic of South Africa: Department of Health. Country progress report on the declaration of commitment on HIV/AIDS. South Africa: Republic of South Africa; 2010. [Google Scholar]
- 7.South African National AIDS Council, Republic of South Africa: Department of Health. National strategic plan on HIV, STIs and TB: 2012–2016. Pretoria, South Africa: Department of Health, Republic of South Africa; 2011. [Google Scholar]
- 8.Chabikuli NO, Datonye DO, Nachega J, Ansong D. Adherence to antiretroviral therapy, virologic failure and workload at the Rustenburg Provincial Hospital. South Africa Family Practice. 2010;52(4):350–355. [Google Scholar]
- 9.Eisele TP, Mathews C, Chopra M, et al. Changes in risk behavior among HIV-positive patients during their first year of antiretroviral therapy in Cape Town South Africa. AIDS Behav. 2009;13(6):1097–1105. doi: 10.1007/s10461-008-9473-2. [DOI] [PubMed] [Google Scholar]
- 10.Kiene SM, Christie S, Cornman DH, et al. Sexual risk behaviour among HIV-positive individuals in clinical care in urban KwaZulu-Natal, South Africa. AIDS. 2006;20(13):1781–1784. doi: 10.1097/01.aids.0000242827.05120.55. [DOI] [PubMed] [Google Scholar]
- 11.Peltzer K, Ramlagan S. Safer sexual behaviours after 1 year of antiretroviral treatment in KwaZulu-Natal, South Africa: a prospective cohort study. Sexual health. 2010;7(2):135–141. doi: 10.1071/SH09109. [DOI] [PubMed] [Google Scholar]
- 12.El-Khatib Z, Ekstrom AM, Ledwaba J, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS. 2010 Jul 17;24(11):1679–1687. doi: 10.1097/QAD.0b013e32833a097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manasa J, Lessells RJ, Skingsley A, et al. High-levels of acquired drug resistance in adult patients failing first-line antiretroviral therapy in a rural HIV treatment programme in KwaZulu-Natal, South Africa. PloS One. 2013;8(8):e72152. doi: 10.1371/journal.pone.0072152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. The Lancet Infectious Diseases. 2010;10(3):155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 15.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46(10):1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sendagire H, Easterbrook PJ, Nankya I, Arts E, Thomas D, Reynolds SJ. The challenge of HIV-1 antiretroviral resistance in Africa in the era of HAART. AIDS Rev. 2009;11(2):59–70. [PMC free article] [PubMed] [Google Scholar]
- 17.Sigaloff KC, Ramatsebe T, Viana R, de Wit TFR, Wallis CL, Stevens WS. Accumulation of HIV drug resistance mutations in patients failing first-line antiretroviral treatment in South Africa. AIDS research and human retroviruses. 2012;28(2):171–175. doi: 10.1089/aid.2011.0136. [DOI] [PubMed] [Google Scholar]
- 18.Hamers RL, Sigaloff KC, Wensing AM, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clinical infectious diseases. 2012;54(11):1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 19.El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One. 2011;6(3):e17518. doi: 10.1371/journal.pone.0017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anzala AO, Simonsen JN, Kimani J, et al. Acute sexually transmitted infections increase human immunodeficiency virus type 1 plasma viremia, increase plasma type 2 cytokines, and decrease CD4 cell counts. Journal of Infectious Diseases. 2000;182(2):459–466. doi: 10.1086/315733. [DOI] [PubMed] [Google Scholar]
- 21.Holmes K, Sparling P, Stamm W, et al. Sexually Transmitted Diseases. 4th ed. New York: McGraw-Hill; 2007. [Google Scholar]
- 22.Wright PW, Hoesley CJ, Squires KE, Croom-Rivers A, Weiss HL, Gnann JW., Jr A prospective study of genital herpes simplex virus type 2 infection in human immunodeficiency virus type 1 (HIV-1)-seropositive women: correlations with CD4 cell count and plasma HIV-1 RNA level. Clin Infect Dis. 2003;36(2):207–211. doi: 10.1086/345440. [DOI] [PubMed] [Google Scholar]
- 23.Hamers RL, Wallis CL, Kityo C, et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. The Lancet Infectious Diseases. 2011;11(10):750–759. doi: 10.1016/S1473-3099(11)70149-9. [DOI] [PubMed] [Google Scholar]
- 24.Maglione M, Geotz M, Wang Z, et al. Evidence Report/ Technology Assessment No. 156. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Antiretroviral (ARV) Drug Resistance in the Developing World. [PMC free article] [PubMed] [Google Scholar]
- 25.Price MA, Wallis CL, Lakhi S, et al. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses. 2011;27(1):5–12. doi: 10.1089/aid.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters L, Smit E. HIV-1 superinfection. Curr Opin Infect Dis. 2012;25(1):42–50. doi: 10.1097/QCO.0b013e32834ef5af. [DOI] [PubMed] [Google Scholar]
- 27.Cornman DH, Christie S, Shepherd LM, et al. Counsellor-delivered HIV risk reduction intervention addresses safer sex barriers of people living with HIV in KwaZulu-Natal, South Africa. Psychol Health. 2011;26(12):1623–1641. doi: 10.1080/08870446.2011.552180. [DOI] [PubMed] [Google Scholar]
- 28.Cornman DH, Kiene SM, Christie S, et al. Clinic-based intervention reduces unprotected sexual behavior among HIV-infected patients in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2008;48:553–560. doi: 10.1097/QAI.0b013e31817bebd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh-Onoya D, Reddy PS, Ruiter RA, Sifunda S, Wingood G, van den Borne B. Condom use promotion among isiXhosa speaking women living with HIV in the Western Cape Province, South Africa: a pilot study. AIDS care. 2009;21(7):817–825. doi: 10.1080/09540120802537823. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy CE, Medley AM, Sweat MD, O'Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2010;88(8):615–623. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltzer K, Tabane C, Matseke G, Simbayi L. Lay counsellor-based risk reduction intervention with HIV positive diagnosed patients at public HIV counselling and testing sites in Mpumalanga, South Africa. Evaluation and program planning. 2010;33(4):379–385. doi: 10.1016/j.evalprogplan.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Fisher JD, Smith LR, Lenz EM. Secondary prevention of HIV in the United States: past, current, and future perspectives. J Acquir Immune Defic Syndr. 2010;55(2):S106–S115. doi: 10.1097/QAI.0b013e3181fbca2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. HIV/AIDS Prevention Research Synthesis Project. [Accessed April 30, 2012];2012 http://www.cdc.gov/hiv/topics/research/prs/index.htm.
- 34.Fisher JD, Cornman DH, Osborn CY, Amico KR, Fisher WA, Friedland GA. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: Formative Research, Acceptability, and Fidelity of the Options Project. J Acquir Immune Defic Syndr. 2004 Oct 1;37(Suppl 2):S78–S87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 35.NIMH Multisite HIV/STD Prevention Trial for African American Couples Group. Eban HIV/STD risk reduction intervention: Conceptual basis and procedures. Journal of acquired immune deficiency syndromes. 2008;49(Suppl 1):S15–S27. doi: 10.1097/QAI.0b013e318184255d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healthy Living Project Team. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the healthy living project randomized controlled study. Journal of Acquired Immune Deficiency Syndromes. 2007;44(2):213–221. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- 37.El-Bassel N, Jemmott JB, Landis JR, et al. National Institute of Mental Health multisite Eban HIV/STD prevention intervention for African American HIV serodiscordant couples: a cluster randomized trial. Archives of Internal Medicine. 2010;170(17):1594–1601. doi: 10.1001/archinternmed.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golin CE, Davis RA, Przybyla SM, et al. SafeTalk, a multicomponent, motivational interviewing-based, safer sex counseling program for people living with HIV/AIDS: a qualitative assessment of patients' views. AIDS patient care and STDs. 2010;24(4):237–245. doi: 10.1089/apc.2009.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golin CE, Earp JA, Grodensky CA, et al. Longitudinal effects of SafeTalk, a motivational interviewing-based program to improve safer sex practices among people living with HIV/AIDS. AIDS and Behavior. 2012;16(5):1182–1191. doi: 10.1007/s10461-011-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingood GM, DiClemente RJ, Mikhail I, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: The WiLLOW Program. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004;37:S58–S67. doi: 10.1097/01.qai.0000140603.57478.a9. [DOI] [PubMed] [Google Scholar]
- 41.Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. American journal of public health. 2011;101(3):531–538. doi: 10.2105/AJPH.2010.197608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotheram-Borus MJ, Swendeman D, Comulada WS, Weiss RE, Lee M, Lightfoot M. Prevention for substance-using HIV-positive young people: telephone and in-person delivery. Journal of acquired immune deficiency syndromes (1999) 2004;37(Suppl 2):S68–S77. doi: 10.1097/01.qai.0000140604.57478.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardick J, Yang S, Lin S, Duncan D, Gaydos C. Use of the Roche LightCycler instrument in a real-time PCR for Trichomonas vaginalis in urine samples from females and males. Journal of clinical microbiology. 2003;41(12):5619–5622. doi: 10.1128/JCM.41.12.5619-5622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychological Bulletin. 1992;111(3):455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 45.Fisher JD, Fisher WA, Shuper PA. The Information Motivation Behavioral Skills Model of HIV preventive behavior. In: DiClemente R, Crosby R, Kegler M, editors. Emerging theories in health promotion practice and research model of HIV preventive behavior. 2nd ed. San Francisco, CA: Jossey Bass Publishers; 2009. pp. 22–63. [Google Scholar]
- 46.Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- 47.Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. 1st ed. Churchill Livinstone: 1999. [Google Scholar]
- 48.Diggle PJ, Liang KY, Zeger SL. The analysis of Longitudinal Data. Oxford, England: Oxford University Press; 1994. [Google Scholar]
- 49.Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatric Pulmonology. 2000;30(4):330–344. doi: 10.1002/1099-0496(200010)30:4<330::aid-ppul10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 50.Agresti A. Categorical data analysis. 1st ed. New York: Wiley; 1990. [Google Scholar]
- 51.Long JS. Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, CA: Sage Publications; 1997. [Google Scholar]
- 52.SAS [computer program] Version 9.02. Cary, NC: The SAS Institute; 2008. [Google Scholar]
- 53.Chen Q, Ibrahim JG, Chen MH, Senchaudhuri P. Theory and inference for regression models with missing responses and covariates. Journal of multivariate analysis. 2008 Jul;99(6):1302–1331. doi: 10.1016/j.jmva.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibrahim JG, Chu H, Chen MH. Missing data in clinical studies: Issues and methods. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Sep 10;30(26):3297–3303. doi: 10.1200/JCO.2011.38.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lessells RJ, Mutevedzi PC, Cooke GS, Newell M-L. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. Journal of acquired immune deficiency syndromes (1999) 2011;56(3):e79. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ekouevi DK, Balestre E, Ba-Gomis FO, et al. Low retention of HIV-infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Tropical Medicine & International Health. 2010;15(s1):34–42. doi: 10.1111/j.1365-3156.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charurat M, Oyegunle M, Benjamin R, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One. 2010;5(5):e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdool Karim KQ, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science (New York, N.Y.) 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baeten J, Celum C. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: the Partners PrEP study. Paper presented at: 6th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; Rome, Italy. 2011. [Google Scholar]
- 63.The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science (New York, N.Y.) 1998;280(5371):1889–1894. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- 64.Metcalf CA, Malotte CK, Douglas JMJ, et al. Efficacy of a booster counseling session 6 months after HIV testing and counseling: a randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005;32(2):123–129. doi: 10.1097/01.olq.0000151420.92624.c0. [DOI] [PubMed] [Google Scholar]