SUMMARY
Accurate chromosome segregation is dependent on the formation and stability of the microtubule spindle apparatus. Meiotic spindle assembly in oocytes differs from the process used during mitosis, and is regulated by unique microtubule organizing centers (MTOCs) that lack centrioles. To gain insight into the molecular composition and function of acentriolar MTOCs in mouse oocytes, we assessed the role of a key MTOC-associated protein, pericentrin (PCNT). In somatic cells, pericentrin functions as a scaffold that binds specific proteins at MTOCs, including γ-tubulin, which is necessary for microtubule nucleation. Pericentrin is expressed in oocytes, but the conservation of its function is not known. Pericentrin localizes specifically to MTOCs during prophase-I arrest in mouse oocytes recovered from pre-ovulatory ovarian follicles, and remains associated with MTOCs at spindle poles during metaphase-I and -II. To test function, specific siRNAs were used to knockdown Pcnt transcripts in mouse oocytes. Efficient protein depletion was confirmed by Western blot as well as immunofluorescence analysis. Notably, meiotic spindle structure and chromosome alignment were disrupted in Pcnt-depleted oocytes. Disorganized spindle structures with reduced microtubule density and misaligned chromosomes were observed in the majority of these oocytes (~70%). In addition, γ-tubulin localization to MTOCs was significantly reduced and microtubule regrowth, following cold treatment, was delayed in Pcnt-depleted oocytes. Thus, pericentrin is a key functional component of the unique acentriolar MTOCs of mouse oocytes, and plays an important role in regulating meiotic spindle assembly and/or stability.
Keywords: Meiosis, γ-Tubulin, Chromosome segregation
INTRODUCTION
Errors in chromosome segregation during meiotic division can disrupt genomic stability in gametes prior to fertilization, leading to aneuploidy – a leading cause of congenital birth defects and pregnancy loss in women. Studies indicate that approximately 7–10% of clinically recognized pregnancies are aneuploid owing to meiotic errors that occur predominantly during the first meiotic division in oocytes. Moreover, the rates of aneuploidy rise significantly with increasing maternal age (Hassold and Hunt, 2001; Nagaoka et al., 2012). Accurate chromosome segregation is critically dependent on the formation and stability of the microtubule spindle apparatus, yet the molecular mechanisms that regulate meiotic spindle assembly and organization in mammalian oocytes are not well defined.
In somatic cells, mitotic spindle assembly is regulated by centrosomes, which function as the primary microtubule organizing centers (MTOCs). Centrosomes are non-membrane bound organelles, typically composed of two centrioles surrounded by a protein matrix of pericentriolar material (PCM). Centrioles reportedly maintain centrosome structure integrity and are also essential for flagella and cilia formation, while the PCM provides microtubule nucleating and anchoring activities (Wiese and Zheng, 2006; Nigg and Raff, 2009). Intriguingly, mammalian oocytes contain unique MTOCs composed of essential pericentriolar matrix proteins, including γ-tubulin for microtubule formation, but lack centrioles (Gueth-Hallonet et al., 1993; Manandhar et al., 2005). Initial electron-microscopy analysis demonstrated that centrioles are present in oogonia and fetal oocytes only up to the pachytene stage (Szollosi et al., 1972), thus centrioles are absent during meiotic division in oocytes. It is only at the time of fertilization that sperm-associated centrioles are reintroduced into the oocyte in most species. In mice, the sperm centriole degrades during fertilization and de novo assembly of centrioles is observed in the early pre-implantation embryo (Gueth-Hallonet et al., 1993; Manandhar et al., 2005).
The acentriolar MTOCs in pre-ovulatory oocytes have been described as aggregates, or foci, of PCM that can nucleate microtubules (Maro et al., 1985; Combelles and Albertini, 2001). A single MTOC is normally detected in pre-ovulatory oocytes arrested at prophase-I. But, upon resumption of meiosis, multiple small MTOCs form in the oocyte cytoplasm and in close proximity to the oocyte nucleus (Can et al., 2003; Schuh and Ellenberg, 2007; Ma et al., 2010; Łuksza et al., 2013). These newly formed perinuclear MTOCs nucleate spindle microtubules and label positively with antibodies that detect key proteins such as γ-tubulin as well as pericentrin. As oocytes progress to prometaphase-I, a dense microtubule array forms and gradually self organizes into a bipolar (barrel-shaped) meiotic spindle (Combelles and Albertini, 2001; Schuh and Ellenberg, 2007; Schatten and Sun, 2011). During metaphase-I and -II, the chromosomes congress to form a metaphase plate with MTOCs localized at both spindle poles in an “O-” or “C-” shaped configuration (Carabatsos et al., 2000; Ma et al., 2010). Currently, the molecular processes that regulate the localization and accumulation of PCM proteins for the formation and function of oocyte MTOCs are not well understood.
To gain insight into the molecular composition and function of acentriolar MTOCs in pre-ovulatory mouse oocytes, we assessed the role of the crucial protein pericentrin. Pericentrin is characterized by a large coil-coiled domain, and is considered an essential scaffolding protein that binds and anchors other proteins at the centrosome in somatic cells. It is an integral centrosome-associated protein that localizes to the PCM, where it interacts with additional proteins, including γ-tubulin (Zimmerman et al., 2004; Lee and Rhee, 2011; Kim and Rhee, 2014). Pericentrin depletion disrupts spindle microtubule organization and reduces γ-tubulin expression at centrosomes during mitosis (Zimmerman et al., 2004). Recent studies also demonstrate that pericentrin plays a key role in centrosome assembly and higher-order organization of the PCM (Lawo et al., 2012; Mennella et al., 2012; Kim and Rhee, 2014). Pericentrin is highly conserved across species; in mouse oocytes, it localizes specifically to MTOCs during meiotic maturation (Carabatsos et al., 2000; Schuh and Ellenberg, 2007; Łuksza et al., 2013). While its function in oocytes is not well-defined, our previous studies indicate that pericentrin likely plays an important role in MTOC composition and function, as it is essential for the accumulation of key proteins, such as NEDD1 (neural precursor cell expressed developmentally down regulated protein 1), at oocyte MTOCs (Ma et al., 2010). Identifying and testing the function of the fundamental components of MTOCs in mammalian oocytes is crucial for an increased understanding of the underlying molecular mechanisms that control meiotic spindle assembly, which is essential for accurate chromosome segregation. In the current study, we test pericentrin function in oocytes by knockdown of pericentin (Pcnt) transcript levels using specific siRNAs and subsequent evaluation of its impact on meiotic spindle assembly and stability.
RESULTS
Pericentrin Localizes to MTOCs in Oocytes During Meiotic Division
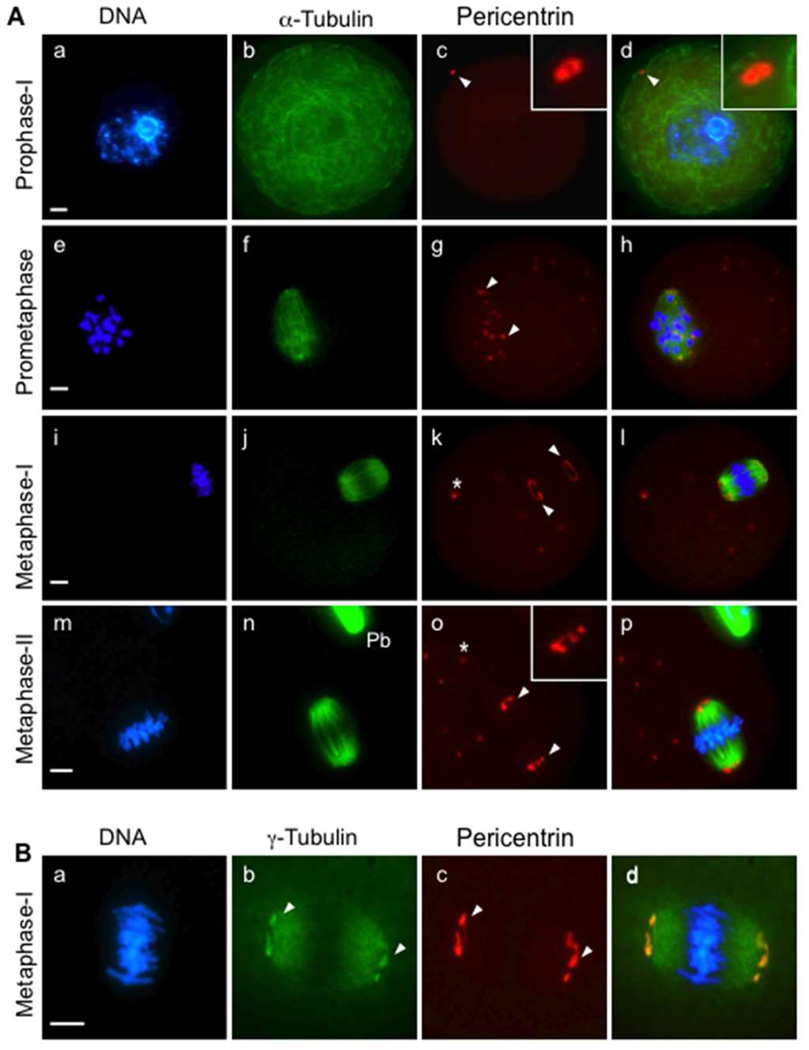
The subcellular distribution of pericentrin was assessed by immunofluorescence during meiotic division, and evaluated in relation to spindle assembly. Pre-ovulatory oocytes arrested at prophase-I (the germinal vesicle stage) exhibited an intact nuclear membrane and a dense cytoplasmic microtubule network (Fig. 1A, a–d). A clear pericentrin signal was detected at a single MTOC (Fig. 1A, c) near the oocyte cortex. Consistent with previous studies, as oocytes resumed meiosis and progressed to prometaphase, the cytoplasmic microtubules depolymerized and multiple discrete foci, labeled with pericentrin (corresponding to MTOCs), were detected around the condensing chromosomes and newly forming spindle microtubule array (Fig. 1A, e–h). During metaphase-I and -II, pericentrin specifically localized to the MTOCs at meiotic spindle poles (Fig, 1A, i–p) as well as at discrete foci distributed in the cytoplasm. Notably, γ-tubulin (Fig. 1B) localized both along the spindle microtubules as well as to MTOCs at the spindle poles. Pericentrin co-localized with γ-tubulin at spindle-pole MTOCs (Fig. 1B, c), yet there was no apparent pericentrin staining along spindle microtubules.
Figure 1.
Pericentrin localizes to MTOCs in oocytes arrested at prophase-I and during meiotic division. (A) Mouse oocytes were collected and fixed for immunofluorescence analysis at specific stages, including prophase-I arrest (a–d, n= 45), prometaphase (e–h, n=51), metaphase-I (i–l, n=65), and metaphase-II (m–p, n=63). The oocytes were double-labeled with specific anti-pericentrin (red, arrows) and anti-acetylated α-tubulin (green) antibodies to detect spindle microtubules. (B) Pericentrin (c, red) co-localizes with γ-tubulin (b, green) specifically at the spindle poles (arrows) in metaphase-I oocytes (n=55). All slides were counterstained with DAPI to detect DNA (blue). Pb: Polar body. *, cytoplasmic MTOCs. Insets show 2× and 4× magnifications of the spindle-pole area. Scale bars, 10 µm.
Efficient Depletion of Pericentrin
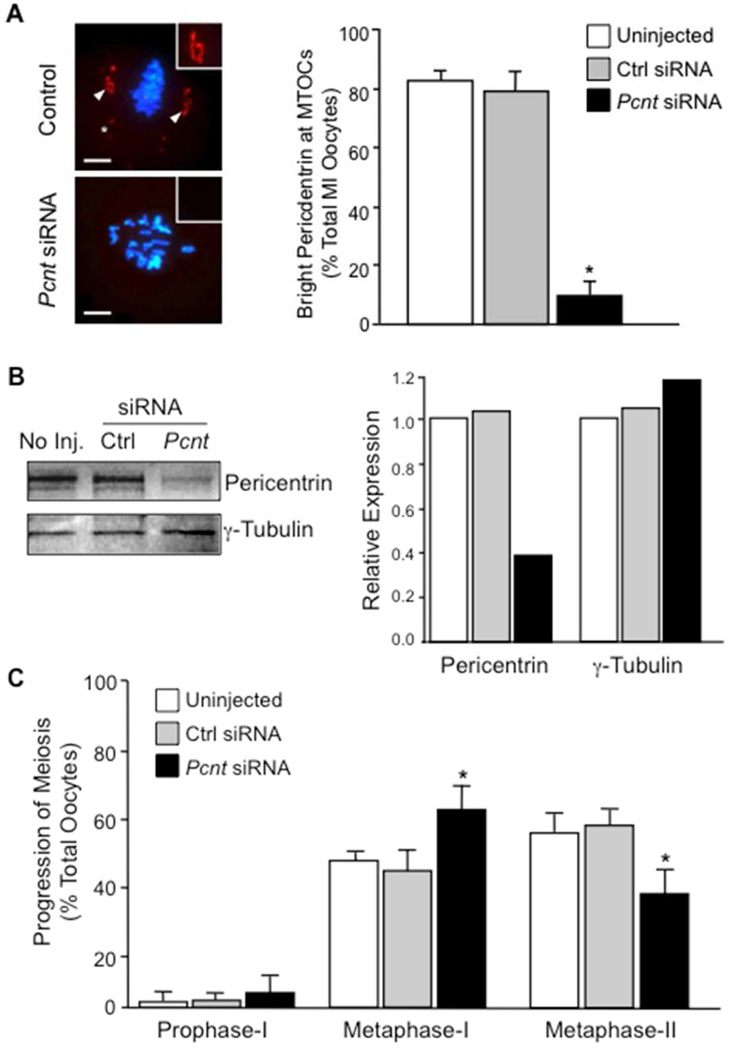
To test function, Pcnt transcripts were knocked down in oocytes using specific siRNAs directed against the mouse coding sequence. Both, immunofluorescence and Western blot analysis revealed efficient protein depletion in the Pcnt siRNA group (Fig. 2). The majority of oocytes injected with Pcnt siRNAs failed to express pericentrin at the meiotic spindle poles (Fig. 2A). Less than 15% of oocytes injected with Pcnt siRNAs contained MTOCs that labeled with anti-pericentrin, compared to over 85% of control oocytes. Moreover, pericentrin levels were significantly reduced in total-protein extracts from oocytes injected with Pcnt-specific siRNAs relative to the uninjected and control siRNA groups (Fig. 2B). Re-probing the same membrane demonstrated equivalent total γ-tubulin levels in all groups, indicating no difference in total-protein levels in each sample. This supports the Pcnt siRNA knockdown specificity and further demonstrates that total levels of another key MTOC protein, γ-tubulin, are unchanged.
Figure 2.
Efficient knockdown of pericentrin protein expression in oocytes by specific siRNAs.
(A) Representative images and percent total metaphase-I stage oocytes (mean ± standard error) with bright pericentrin staining at MTOCs (arrows) from the uninjected (n=109) and non-specific siRNA (n=117) controls relative to the Pcnt-siRNA (n=189) group. Pericentrin is shown in red (arrows) and DNA in blue. Insets show a 2× magnification of the spindle pole area. Scale bars, 10 µm.
*, cytoplasmic MTOCs. (B) Western blot analysis of pericentrin and γ-tubulin levels oocyte lysates (50 oocytes/lane) from the control and Pcnt-siRNA groups. Total pericentrin and γ-tubulin values were compared to the uninjected control, which was normalized to 1.0. (C) Progression of meiosis as determined by DAPI-labeled DNA configurations in uninjected (n=244), control siRNA (n=281)-, and Pcnt-siRNA- (n=284) injected oocytes. All oocytes were fixed after a 17-hour culture period. Bars represent the mean value (± standard error). * P<0.05 between control and Pcnt-siRNA groups.
At the end of a 17-hour culture period, DNA configurations were assessed to determine meiotic maturation rates (Fig. 2C). Over 95% of oocytes resumed meiosis in all groups. In the control groups, approximately 60% of oocytes progressed to metaphase-II. In the Pcnt siRNA group, however, an increased percentage (P<0.05) of oocytes remained at metaphase-I, with approximately 38% progressing to metaphase-II.
Disrupted Spindle Structure and Chromosome Alignment in Pcnt-Depleted Oocytes
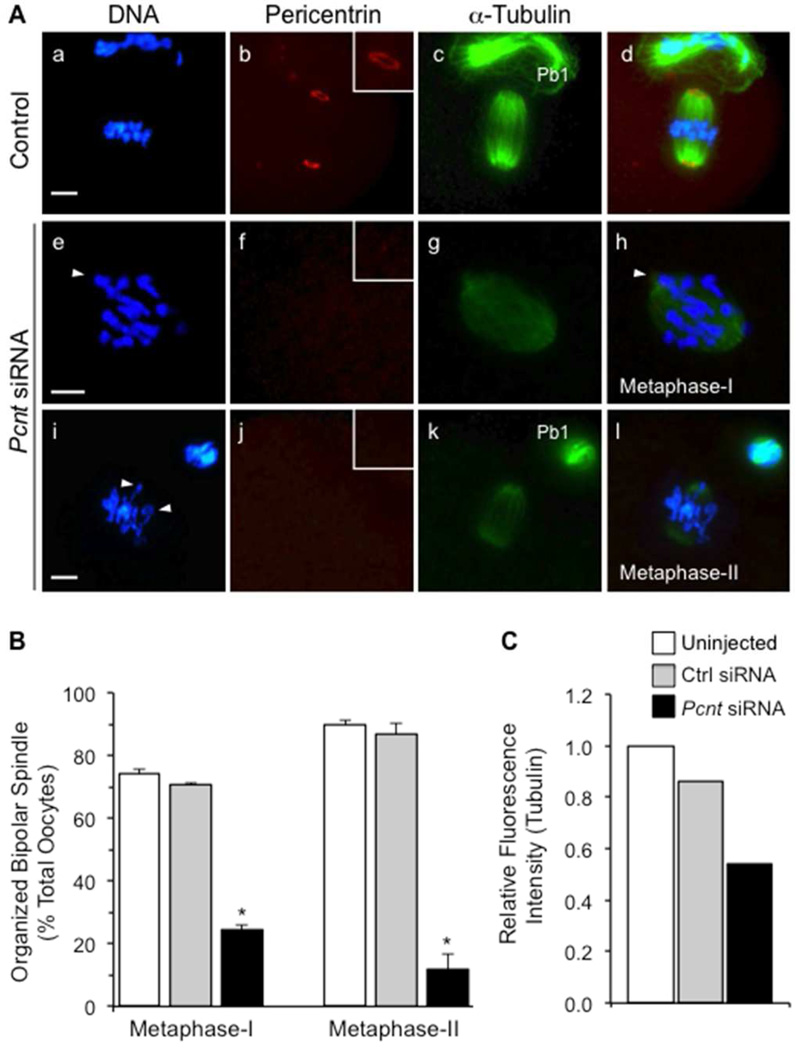
Meiotic spindle organization and chromosome alignment were disrupted in Pcnt-depleted oocytes, as indicated by immunofluorescence labeling of DNA and spindle microtubules (Fig. 3) with DAPI and anti α-tubulin, respectively. After a 17-hour culture period, the majority of oocytes from the uninjected and non-specific siRNA control groups progressed to metaphase-II and contained organized (barrel-shaped) meiotic spindles with aligned chromosomes and clear pericentrin labeling at both spindle poles (Fig. 3A, a–d). In contrast, many oocytes (approximately 62%) from the Pcnt siRNA group remained at metaphase-I and lacked organized spindle structures (Fig. 3B). Disrupted spindle organization was also observed in the Pcnt-depleted oocytes that reached metaphase-II (Fig. 3A, i–l). Broader and shorter spindle structures with significant chromosome misalignment were commonly observed. Moreover, the spindles were characterized by weak labeling with anti α-tubulin antibodies. Quantitative analysis revealed significantly lower relative fluorescence intensity (Fig. 3C), indicating reduced spindle microtubule density in Pcnt-depleted oocytes.
Figure 3.
Knockdown of pericentrin leads to the disruption of spindle organization and significant chromosome misalignment. (A) Representative images of the meiotic spindle structure in uninjected (n=137) and non-specific siRNA (a–d, n=110) control oocytes, relative to the Pcnt-siRNA-injected group (e–l, n=113). Oocytes were double-labeled with anti-pericentrin (red) as well as anti-acetylated α-tubulin antibodies for the detection of microtubules (green). DNA is shown in blue and arrows denote misaligned chromosomes. Insets show a 2× magnification of the spindle pole area (arrows). Pb1: First polar body. Scale bar, 10 µm. (B) Percentage (mean ± standard error) of metaphase-I and -II oocytes in each group that exhibit an organized bipolar meiotic spindle structure. * P<0.05. (C) Relative fluorescence (pixel) intensity of acetylated α-tubulin (microtubules) in the spindle of metaphase-I oocytes (n=15 per group). The mean fluorescence intensity values from the uninjected control group was standardized to 1.0, and compared to oocytes injected with non-specific control or Pcnt siRNAs.
Reduced γ-Tubulin at MTOCs and Delayed Microtubule Regrowth in Pcnt-Depleted Oocytes
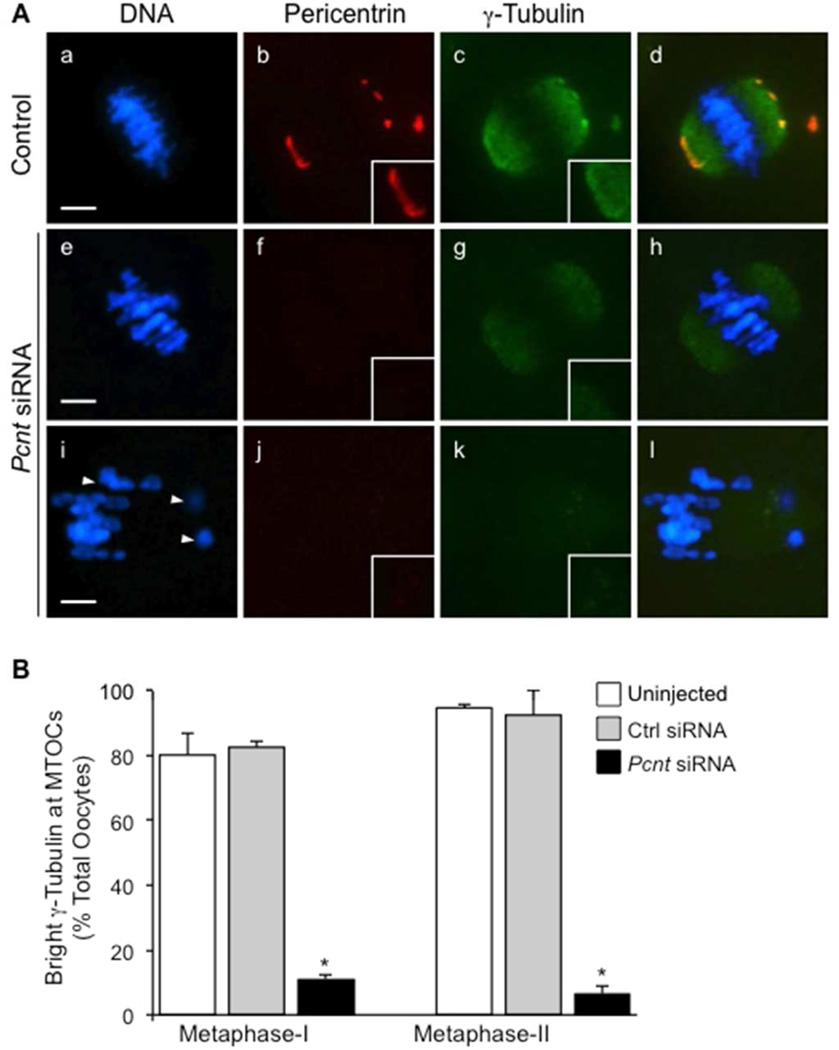
Considering its essential role in microtubule nucleation, we assessed γ-tubulin expression in Pcnt-depleted ooctyes. In the majority of control oocytes (both the uninjected and non-specific siRNA groups), γ-tubulin was consistently detected along the spindle microtubules and co-localized with pericentrin specifically at spindle-pole MTOCs (Fig. 4A, a–d). In contrast, bright labeling of γ-tubulin at MTOCs was only detectable in approximately 10% of oocytes from the Pcnt-siRNA group (Fig. 4A, e–h). Notably, γ-tubulin was markedly reduced at both the spindle pole and cytoplasmic MTOCs. Yet, γ-tubulin labeling was still observed along the spindle microtubules in the majority (~72%) of these oocytes (Fig. 4A, e–h), while no discernable γ-tubulin was detected at the spindle poles or spindle microtubules in a smaller percentage (~28% of oocytes) (Fig. 4A, i–l). There was no difference in total γ-tubulin protein levels between the control and Pcnt-siRNA oocyte groups, as detected by Western blot (Fig. 2A), indicating a specific disruption in the localization of γ-tubulin to oocyte MTOCs.
Figure 4.
Knockdown of pericentrin in oocytes decreased γ-tubulin localization at MTOCs. (A) Representative images of oocytes are shown from the uninjected (n=73) and non-specific siRNA (n=89) controls (a–d) compared to the Pcnt-siRNA group (e–h, n=87). Oocytes were double-labeled with anti-pericentrin (red) and γ-tubulin (green) antibodies. Arrows denote misaligned chromosomes. (B) The percentage (mean ± standard error) of oocytes with bright γ-tubulin detected at MTOCs was lower in the Pcnt-siRNA group relative to the uninjected and non-specific siRNA controls. DNA is shown in blue. * P<0.05. Insets show a 2× magnification of the spindle pole area. Scale bars, 10 µm.
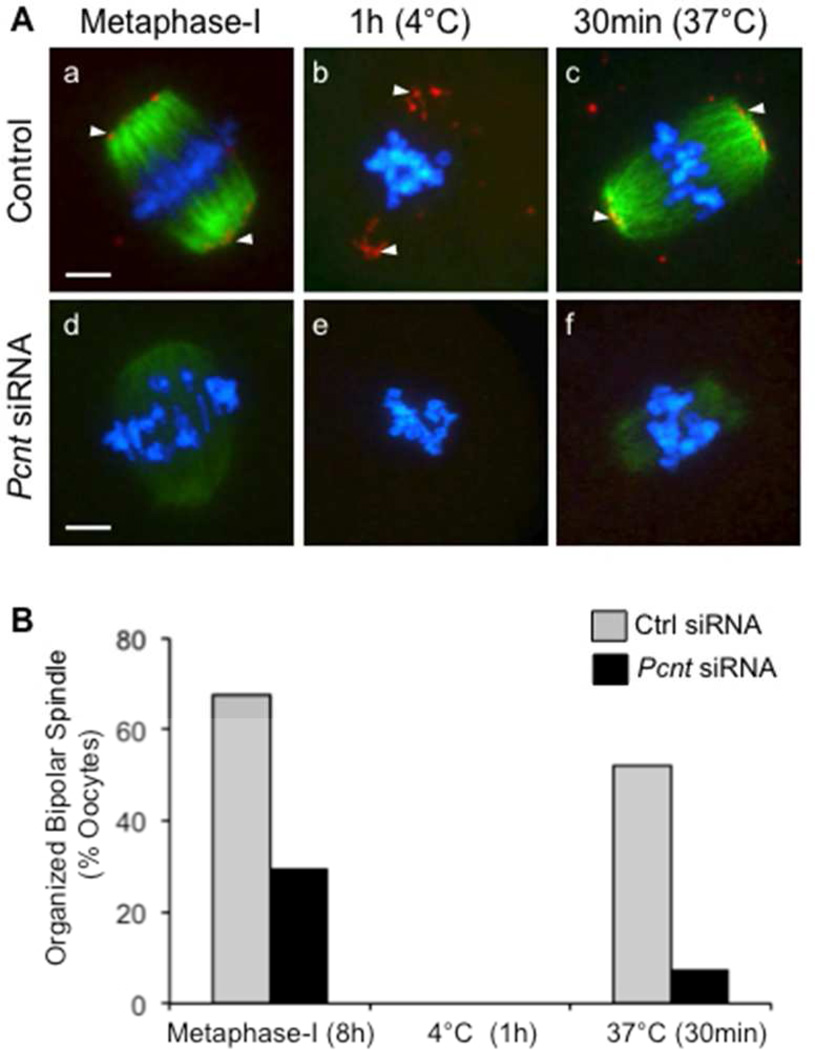
We also assessed spindle microtubule regrowth, following depolymerization by cold treatment at 4°C, in control and Pcnt-depleted metaphase-I oocytes (Fig. 5). The majority of control oocytes (Fig. 5A, a–c) analyzed after an 8-hour culture period had progressed to metaphase-I, and contained a bipolar spindle with aligned chromosomes and clear pericentrin labeling at MTOCs. Oocyte incubation at 4°C for 1 hour depolymerized all detectable spindle microtubules, whereas pericentrin foci were still detected at the poles. After rewarming at 37°C for 30 minutes, the microtubules reassembled and organized into a bipolar spindle in the majority of control oocytes (Fig. 5A, a–c). Analysis of the Pcnt-siRNA group showed that most Pcnt-depleted, metaphase-I stage oocytes (Fig. 5A, d–f) contained disrupted spindle structures with misaligned chromosomes after an 8-hour culture period, which is consistent with our observations after a 17-hour culture period. Cold treatment depolymerized all detectable microtubules. In contrast to the control group, the majority of Pcnt-depleted oocytes exhibited only faint labeling with anti α-tubulin after rewarming for 30 minutes, indicating limited or delayed microtubule regrowth.
Figure 5.
Delayed microtubule re-growth in Pcnt-depleted oocytes. (A) Following an 8-hour culture, metaphase-I stage oocytes were cold treated for 1 hour at 4°C to depolymerize microtubules, then transferred to warm media and incubated for 30 minutes at 37°C to assess microtubule regrowth. Representative images of oocytes from the control (a–c, n=62) as well as Pcnt-siRNA (e–f, n=65) groups fixed at each stage. (B) Percentage (mean ± standard error) of oocytes in each group that exhibit an organized bipolar meiotic spindle structure at each time point. * P<0.05. Oocytes were double-labeled for immunofluorescence analysis with anti-pericentrin (red, arrows) and anti-acetylated α-tubulin (green) to detect spindle microtubules. DNA was counterstained with DAPI (blue). Scale bars, 10 µm.
DISCUSSION
Pericentrin expression at oocyte MTOCs has been previously reported, however its functional role had not been tested. In this study we demonstrate that depletion of pericentrin by siRNAs in vitro significantly disrupts meiotic spindle organization and chromosome alignment in mouse oocytes. Pcnt-depleted oocytes contained disorganized spindle structures with reduced microtubule density and highly misaligned chromosomes. Meiotic spindle disruption was associated with reduced γ-tubulin localization at MTOCs and a diminished capacity of spindle microtubules to regrow after depolymerization. Thus, pericentrin is a key functional component of the unique acentriolar MTOCs in mouse oocytes, playing an important role in regulating γ-tubulin concentration at MTOCs that is essential for microtubule nucleation and meiotic-spindle formation and/or stability.
Pericentrin is a Key Component of Oocyte MTOCs
Consistent with previous studies (Carabatsos et al., 2000; Combelles and Albertini, 2001; Can et al., 2003; Schuh and Ellenberg, 2007), we show that pericentrin is expressed at MTOCs in oocytes arrested at prophase-I as well as during meiotic division. In metaphase-I and -II-stage oocytes, pericentrin co-localized with γ-tubulin at meiotic spindle poles as well as at discrete foci in the cytoplasm. Cytoplasmic MTOCs were previously identified in mouse oocytes, and do not appear to contribute to meiotic-spindle formation (Maro et al., 1985; Schuh and Ellenberg, 2007). γ-tubulin was detected both at MTOCs as well as along the length of meiotic spindle microtubules, yet its co-localization with pericentrin was only observed at MTOCs; therefore, the interaction of pericentrin and γ-tubulin in oocytes is likely specific to MTOCs.
Pericentrin has been identified at MTOCs in both growing (Łuksza et al., 2013) and fully-grown, pre-ovulatory, mouse oocytes (Carabatsos et al., 2000; Combelles and Albertini, 2001; Can et al., 2003; Schuh and Ellenberg, 2007). Both, fixed- and live-cell immunofluorescence analysis demonstrate that pericentrin progressively accumulates at forming MTOCs upon the resumption of meiosis, although the cellular mechanisms that regulate pericentrin concentration at MTOCs in oocytes are not known. In somatic cells, a process of ‘centrosome maturation’ occurs upon mitotic entry, whereby the expression of key factors essential for microtubule formation increases significantly at centrosomes. During this process, pericentrin levels increase considerably at the centrosome and is dependent on two other key proteins, CEP192 (Gomez-Ferreria et al., 2007; Zhu et al., 2008) and CEP215 (Kim and Rhee, 2014). In turn, pericentrin,, CEP192, and CEP215 together regulate the binding and accumulation of additional proteins that are necessary for increased microtubule nucleating activity, including γ-tubulin, at the centrosomes (Gomez-Ferreria et al., 2007; Zhu et al., 2008; Kim and Rhee, 2014). Whether or not similar mechanisms regulating acentriolar MTOC protein composition and function operate in the mammalian oocyte upon the resumption of meiosis remains to be determined.
Depletion of Pcnt Reduces γ-Tubulin Localization to MTOCs and Disrupts Meiotic Spindle Organization
We tested pericentrin function by microinjecting a specific Pcnt siRNA, which efficiently reduced total pericentrin protein levels in oocytes. Pcnt-depleted oocytes exhibited defects in meiotic spindle organization and chromosome alignment, which correlated with reduced γ-tubulin at MTOCs whereas faint γ-tubulin labeling was still evident along the spindle microtubules of most Pcnt-depleted oocytes. Since total γ-tubulin protein levels were not altered, depletion of pericentrin specifically disrupts the localization of γ-tubulin to oocyte MTOCs. These findings are consistent with observations in somatic cells (Zimmerman et al., 2004; Kim and Rhee, 2014).
We previously demonstrated an important interaction between pericentrin and another key MTOC-associated protein, NEDD1, in oocytes (Ma et al., 2010). NEDD1 (also referred to as GC-WD40) is a unique γ-tubulin ring complex (γTuRC)-associated protein that plays a critical role in recruiting γ-tubulin to MTOCs in somatic cells (Haren et al., 2006; Luders et al., 2006), and requires pericentrin for its proper localization to mouse oocyte MTOCs (Ma et al., 2010). Knockdown of NEDD1 had no effect on pericentrin targeting to oocyte MTOCs, however. Thus, pericentrin function is conserved –at least in part– as an important scaffolding protein in acentriolar MTOCs in mouse oocytes. In addition, pericentrin likely anchors γ-tubulin at oocyte MTOCs through potential interactions with key γTuRC-associated proteins such as NEDD1.
γ-Tubulin is essential for microtubule nucleation at MTOCs. This member of the tubulin superfamily is highly conserved across species, and functions to catalyze α- and β-tubulin dimer assembly into microtubules (Raynaud-Messina and Merdes, 2007). Depleting total γ-tubulin in oocytes by siRNA has been shown to reduce meiotic spindle size in mouse oocytes (Barrett and Albertini, 2007). Here, we show that γ-tubulin localization to MTOCs is dependent on pericentrin, albeit γ-tubulin localization to the spindle microtubules was not fully disrupted after Pcnt knockdown, suggesting that other regulatory mechanisms may be at work. Importantly, reduced γ-tubulin at MTOCs in Pcnt-depleted oocytes associated with disrupted meiotic spindle structure, especially disorganized spindles with significant chromosome misalignment, as characterized by scattered and misaligned chromosomes. Quantitative analysis of the relative fluorescence intensity also revealed weak labeling with anti-α-tubulin antibodies, suggesting that spindle microtubule density is reduced in Pcnt-depleted oocytes.
Microtubules are highly dynamic and have a short half-life, thus spindle stability requires continuous formation of new microtubules (Raynaud-Messina and Merdes, 2007). Microtubule regrowth (following cold treatment) was delayed and/or diminished in Pcnt-depleted oocytes, further supporting that pericentrin function at MTOCs is important for microtubule nucleation during meiotic spindle assembly. Disruption of meiotic spindle formation, organization, or stability can perturb chromosome-microtubule interactions, and potentially accounts for the chromosome alignment errors observed in Pcnt-depleted oocytes. Fewer of these Pcnt-depleted oocytes progress to metaphase-II, suggesting that some chromosomes may remain unattached, which could activate the spindle assembly checkpoint and thus block or delay the first meiotic division (Homer et al., 2005; McGuinness et al., 2009). In earlier studies, we noted that the majority of control oocytes under similar culture conditions progress to metaphase-II after 10–11 hours of culture (Ma et al., 2010), so failure to extrude the first polar body by 17 hours indicates a considerable delay – but further studies are warranted to assess the timing of polar body extrus are warranted to assess the timing of polar ion, and whether or not the oocytes remain arrested with extended culture.
Interestingly, approximately 38% of Pcnt-depleted oocytes did progress to metaphase-II, despite significant disruption of the meiotic spindle. Subtle differences in the efficiency of siRNA-mediated knockdown of pericentrin may occur among individual oocytes, such that oocytes with low levels of residual pericentrin may have less-severe spindle defects that enabled progression to metaphase-II. Alternatively, it is possible that the metaphase-II oocytes might have escaped the spindle checkpoint, despite their meiotic spindle defects. In previous studies, we observed that disruption of the meiotic spindle in oocytes can lead to spindle-assembly checkpoint activation, based on persistent MAD2 at kinetochores and delayed polar-body emission. Even with checkpoint activation, however, some oocytes progressed to metaphase-II; of these oocytes, a high percentage of the resultant metaphase-II eggs were aneuploidy, so spindle-assembly checkpoint activation did not permanently arrest all oocytes or preclude chromosome segregation errors (Ma et al., 2010).
MTOC Function Promotes Meiotic Spindle Stability in Mammalian Oocytes
Disruptions of the meiotic spindle in Pcnt-depleted oocytes implicates an important role for pericentrin in regulating MTOC-mediated microtubule nucleation. While depletion of Pcnt lead to significant defects in spindle organization and chromosome attachment, it did not completely block meiotic spindle assembly in oocytes since spindle microtubules did form in Pcnt-depleted oocytes, albeit with disruptions. It is possible that low endogenous levels of pericentrin persist in siRNA-injected oocytes, or that additional MTOC-associated proteins may partly compensate for the depleted pericentrin. Yet, non-MTOC-mediated microtubule nucleation can also contribute to meiotic spindle assembly. For example, Ran GTPase activity promotes microtubule nucleation around the chromosomes in mouse oocytes (Hinkle et al., 2002; Dumont et al., 2007), as has been described in mitotic somatic cells (Khodjakov et al., 2000; Caudron et al., 2005; Kalab et al., 2006). Live-cell image analyses of oocytes further demonstrated that microtubules nucleate from MTOCs, and also increase around the condensing chromosomes in a Ran-dependent manner (Dumont et al., 2007; Schuh and Ellenberg, 2007). Disruption of Ran GTPase activity does not completely inhibit meiotic spindle formation, though (Dumont et al., 2007).
In the current study we also note that, although diminished, spindle microtubules do form and γ-tubulin localization along the spindle microtubules persists in the majority of Pcnt-depleted oocytes. Together these findings indicate that MTOC-mediated, as well as Ran GTPase-dependent, microtubule nucleation are important activities in oocytes. Moreover, Pcnt-depleted oocytes provide a valuable model to help determine the relative contribution of these two mechanisms to meiotic spindle assembly and stability in oocytes.
Conclusion
We demonstrated that pericentrin is a key conserved and functional component of the unique acentriolar MTOCs in oocytes. Notably, depletion of pericentrin specifically disrupts γ-tubulin localization to MTOCs, which is associated with the disruption of meiotic spindle organization and chromosome alignment defects. Our findings support that MTOC-medicated microtubule formation is regulated, at least in part, by pericentrin in oocytes and plays a critical role in spindle formation and/or stability during the progression of meiosis.
MATERIALS AND METHODS
Oocyte Collection and Culture
The subcellular distribution of pericentrin was assessed during various stages of meiotic division in oocytes obtained from B6D2F1 mice (C57BL/6J females×DBA/2J males). For recovery of pre-ovulatory stage oocytes, 21- to 23-day-old females were injected with 5 IU pregnant mare serum gonadotropin (EMD Biosciences) to stimulate follicle development, and the ovaries were collected 44 to 48 hours later. Cumulus cell-oocyte complexes (COCs) were quickly released from ovarian follicles in media supplemented with 1 µg/ml of the phosphodiesterase inhibitor milrinone (Sigma) to maintain prophase-I arrest. The surrounding cumulus cells were removed by gentle pipetting, and the denuded oocytes were cultured in milrinone-free media to undergo meiotic maturation. The oocytes were fixed at specific stages for analysis. Oocytes undergoing nuclear-envelope breakdown upon the resumption of meiosis, as well as those at prometaphase and the metaphase-I stages, were collected following a 2-, 4-, and 8-hour culture period, respectively. Mature metaphase-II eggs were collected following a 17-hour culture period, or from the oviducts of superovulated females approximately 16 hours after treatment with 5 IU human chorionic gonadotropin (EMD Biosciences). All oocytes were cultured in Minimal Essential Medium (MEM) supplemented with 3 mg/ml crystallized bovine serum albumin (BSA) (Sigma) and 10% fetal calf serum (Hyclone). Cultures were maintained at 37°C with 5% CO2, 5% O2 and 90% N2.
Immunofluorescence Analysis
Oocytes were fixed and immunostained as previously described (Ma et al., 2008). In brief, the oocytes were fixed with 4% paraformaldehyde in PEM Buffer (100 mM Pipes [pH 6.9], 1 mM MgCl2, 1 mM EGTA) with 0.5% Triton-X for 1 hour, then rinsed and blocked in phosphate-buffered saline (PBS) with 5% serum for immunostaining. Antibodies were used at a 1:1000 dilution to detect pericentrin (BD Biosciences), γ-tubulin (Sigma), and spindle microtubules with acetylated α-tubulin (Sigma). DNA was counterstained with DAPI contained in the mounting medium (Vector Laboratories). Alexa Fluor-conjugated 488 and 555 secondary antibodies were purchased from Molecular Probes (Invitrogen), and fluorescence was assessed using an upright fluorescent microscope with imaging software (Leica Microsystems).
Western Blot Analysis
Oocytes devoid of surrounding granulosa cells were collected and frozen in Laemmli buffer with protease inhibitors. Prior to analysis, the samples were thawed then heated to 100°C for 5 minutes. The proteins were separated in 10% acrylamide gels containing 0.1% sodium dodecyl sulfate (SDS), and transferred onto hydrophobic polyvinylidene fluoride (PVDF) membranes (Amersham). Membranes were blocked (PBS supplemented with 2% BSA and 0.1% Tween-20) overnight at 4°C, then incubated with anti-pericentrin (BD Biosciences) diluted 1:2000, for 2 hours at room temperature, followed by three (20-minute) washes in PBST (PBS with 0.1% Tween-20). A peroxidase-conjugated secondary antibody (Jackson Immuno Research) was added for 1 hour prior to processing using an ECL-plus detection system (Amersham). The membrane was then re-probed with anti-γ-tubulin (Sigma) using similar conditions. Individual band intensity was quantified using Image J software, and the relative total protein values in each group were compared to the uninjected control, which was normalized to 1.0.
Pericentrin (Pcnt) Transcript Knockdown Using Specific siRNAs
Short interfering RNAs (siRNA) were used to knockdown and test pericentrin function in oocytes. A mix of four specific, pre-designed and validated siRNAs directed against the mouse Pcnt gene sequence (Qiagen) were used. Control groups included (i) non-injected oocytes that were subject to the same culture conditions as well as (ii) oocytes injected with control non-specific siRNAs (Qiagen). Approximately, 10 pl of a 1 µM solution of the siRNAs were microinjected directly into the cytoplasm of denuded oocytes arrested at prophase-I in M2 medium (composition?) supplemented with 1 µg/ml milrinone (Ma et al., 2010). Injection volumes were standardized using Femtotip capillaries and a Femptojet microinjector (Eppendorf). The oocytes were subsequently maintained in prophase-I arrest for 24 hours in milrinone-supplemented MEM media to permit sufficient transcript knockdown, then washed and transferred into milrinone-free MEM for an additional 17-hour culture period for meiotic maturation.
Initial control experiments were undertaken to determine the efficacy of the Pcnt-specific siRNAs. At the end of culture, a total of 50 oocytes from each group were processed for Western-blot analysis to assess pericentrin levels in total-protein extracts. This was a representative sample of the oocytes at the end of culture, and contained both a mixture of metaphase-I and –II-stage oocytes; the few prophase-I arrested oocytes observed were excluded. In additional experiments, all the oocytes in each group were fixed for immunodetection of pericentrin at MTOCs, and to assess the progression of meiosis as well as chromosome and meiotic spindle configurations by immunofluorescence. Spindle microtubules were detected using an anti-acetylated α-tubulin antibody, and the chromosomes were stained with DAPI. Immunofluoresecence analysis of γ-tubulin at MTOCs was also undertaken in control and Pcnt-depleted oocytes.
Assessment of Microtubule Regrowth
Spindle microtubule regrowth was assessed in control and Pcnt-depleted oocytes, following microtubule depolymerization by cold treatment (Haren et al., 2006). Microinjection was conducted as described, and the oocytes from the control (non-specific siRNA) and Pcnt siRNAs groups were subsequently placed in culture for 8 hours for assembly of the first meiotic spindle. At the end of the culture, sample oocytes (n=15) from each group were fixed for immunofluorescence analysis. The remaining oocytes were placed in cold M2 media at 4°C for 1 hour to depolymerize the spindle microtubules. After cold-treatment, oocytes from the control and Pcnt-siRNA groups were transferred to fresh, pre-warmed MEM media at 37°C to initiate microtubule regrowth, then fixed for immunofluorescence analysis at 0 and 30 minutes post-warming.
Statistical Analysis
All data are presented as mean percentages (± standard error) from a minimum of 3 independent experimental replicates. For evaluation of the differences between groups, all percentages were subjected to arcsine transformation. The transformed data were analyzed by ANOVA, and comparison of all pairs by Tukey-Kramer HSD using JMP Start Statistics (SAS Institute Inc.). Differences were considered significant when P<0.05.
ACKNOWLEDGEMENTS
The authors thank Dr. Rabindranath De La Fuente and Dr. Claudia Baumann for critical review of the manuscript. This work was supported by National Institutes of Health funding from NICHD grant HD071330 to MMV
Abbreviations
- MTOC
microtubule organizing center
- NEDD1
neural precursor cell expressed developmentally down regulated protein 1
- PCM
pericentriolar material
- Pcnt
pericentrin.
REFERENCES
- Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- Can A, Semiz O, Cinar O. Centrosome and microtubule dynamics during early stages of meiosis in mouse oocytes. Mol Hum Reprod. 2003;9:749–756. doi: 10.1093/molehr/gag093. [DOI] [PubMed] [Google Scholar]
- Carabatsos M, Combelles CMH, Messinger SM, Albertini DF. Sorting and reorganization of centrosomes during oocyte maturation in the mouse. Microsc Res Techn. 2000;49:435–444. doi: 10.1002/(SICI)1097-0029(20000601)49:5<435::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Caudron M, Bunt G, Bastiaens P, Karsenti E. Spatial coordination of spindle assembly by chromosome-mediated signaling gradients. Science. 2005;309:1373–1376. doi: 10.1126/science.1115964. [DOI] [PubMed] [Google Scholar]
- Combelles CMH, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of γ-tubulin. Dev Biol. 2001;239:281–294. doi: 10.1006/dbio.2001.0444. [DOI] [PubMed] [Google Scholar]
- Dumont J, Petri S, Pellegrin F, Terret M-E, Bohnsack MT, Rassinier P, Georget V, Kalab P, Gruss OJ, Verlhac M-H. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ferreria MA, Rath U, Buster DW, Chanda SK, Caldwell JS, Rines DR, Sharp DJ. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr Biol. 2007;17:1960–1966. doi: 10.1016/j.cub.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Gueth-Hallonet C, Antony C, Aghion J, Santa-Maria A, Lajoie-Mazenc I, Wright M, Maro B. gamma-Tubulin is present in acentriolar MTOCs during early mouse development. J Cell Sci. 1993;105:157–166. doi: 10.1242/jcs.105.1.157. [DOI] [PubMed] [Google Scholar]
- Haren L, Remy M-H, Bazin I, Callebaut I, Wright M, Merdes A. NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J Cell Biol. 2006;172:505–515. doi: 10.1083/jcb.200510028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold TJ, Hunt PA. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hinkle B, Slepchenko B, Rolls MM, Walther TC, Stein PA, Mehlmann LM, Ellenberg J, Terasaki M. Chromosomal association of Ran during meiotic and mitotic divisions. J Cell Sci. 2002;115:4685–4693. doi: 10.1242/jcs.00136. [DOI] [PubMed] [Google Scholar]
- Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005;19:202–207. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P, Pralle A, Isacoff EY, Heald R, Weis K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature. 2006;440:697–701. doi: 10.1038/nature04589. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Kim S, Rhee K. Importance of the CEP215-pericentrin interaction for centrosome maturation during mitosis. PLoS ONE. 2014;9:e87016. doi: 10.1371/journal.pone.0087016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat Cell Biol. 2012;14:1149–1158. doi: 10.1038/ncb2591. [DOI] [PubMed] [Google Scholar]
- Lee K, Rhee K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J Cell Biol. 2011;195:1093–1101. doi: 10.1083/jcb.201106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J, Patel UK, Stearns T. GCP-WD is a [gamma]-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat Cell Biol. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- Łuksza M, Queguigner I, Verlhac M-H, Brunet S. Rebuilding MTOCs upon centriole loss during mouse oogenesis. Dev Biol. 2013;382:48–56. doi: 10.1016/j.ydbio.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Ma W, Baumann C, Viveiros MM. NEDD1 is crucial for meiotic spindle stability and accurate chromosome segregation in mammalian oocytes. Dev Biol. 2010;339:439–450. doi: 10.1016/j.ydbio.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Ma W, Koch JA, Viveiros MM. Protein kinase c delta (PKCδ) interacts with microtubule organizing center (mtoc)-associated proteins and participates in meiotic spindle organization. Dev Biol. 2008;320:414–425. doi: 10.1016/j.ydbio.2008.05.550. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Schatten H, Sutovsky P. centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- Maro B, Howlett SK, Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol. 1985;101:1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness BE, Anger M, Kouznetsova A, Gil-Bernab AM, Helmhart W, Kudo NR, Wuensche A, Taylor S, Hoog C, Novak B, Nasmyth K. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol. 2009;19:369–380. doi: 10.1016/j.cub.2009.01.064. [DOI] [PubMed] [Google Scholar]
- Mennella V, Keszthelyi B, McDonald KL, Chhun B, Kan F, Rogers GC, Huang B, Agard DA. Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nat Cell Biol. 2012;14:1159–1168. doi: 10.1038/ncb2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina B, Merdes A. [gamma]-Tubulin complexes and microtubule organization. Curr Opin Cell Biol. 2007;19:24–30. doi: 10.1016/j.ceb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Schatten H, Sun Q-Y. Centrosome dynamics during mammalian oocyte maturation with a focus on meiotic spindle formation. Mol Reprod Dev. 2011;78:757–768. doi: 10.1002/mrd.21380. [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Szollosi D, Calarco P, Donahue RP. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–4153. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- Zhu F, Lawo S, Bird A, Pinchev D, Ralph A, Richter C, Muller-Reichert T, Kittler R, Hyman AA, Pelletier L. The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr Biol. 2008;18:136–141. doi: 10.1016/j.cub.2007.12.055. [DOI] [PubMed] [Google Scholar]
- Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell. 2004;15:3642–3647. doi: 10.1091/mbc.E03-11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]