Abstract
Purpose
We aimed to evaluate the effects of statins on mood, sleep, and physical function.
Methods
We performed a systematic computer-aided search of MEDLINE/PubMed, EMBASE, and the Cochrane Central Register and augmented this search by scrutinizing reference lists, and making inquiries among colleagues and experts in the field. All patient populations and study types were considered. We selected studies of statin therapy compared with no statin or placebo. Outcome measures included mood, sleep, and physical function.
Results
Thirty four studies were included in qualitative synthesis. Seven of 8 (88%) observational studies, 4/6 (66%) randomized trials with mood as a primary endpoint (487 total participants; exposure 4 weeks to 1 year), and 3/3 (100%) randomized trials with mood as a secondary endpoint (2,851 total participants; exposure 1–4 years) were not compatible with a negative mood effect of statins. Comparatively fewer studies examined statin effects on sleep and physical function. Studies reporting negative effects contained potential sources of bias, including multiple testing or lack of adjustment for confounders in observational studies, and failure to pre-specify outcomes or report blinding in trials.
Conclusions
A limited body of available evidence is most compatible with no adverse effect of statins on quality of life measures, namely mood, sleep, and physical function. Studies suggesting such effects suffer from an increased risk of bias. High-quality, prospective, and adequately powered studies are needed, especially in the domains of sleep and physical function, with careful attention to patients who may be most vulnerable to adverse effects.
Keywords: Statin therapy, mood, sleep, physical function, energy
INTRODUCTION
Evidence from multiple large randomized controlled trials supports the clinical strategy of treating at-risk patients with statins to prevent atherosclerotic cardiovascular disease events and related mortality [1]. Despite strong evidence for their use and overall safety, concern has persisted for decades regarding potential harms from statins on quality of life. Recent systematic reviews found no evidence overall to support an interaction between statins and short-term cognitive changes and suggested a long-term benefit on cognition, or dementia [2,3].
Comparatively less is known about whether statins and cholesterol lowering impact other potential quality of life measures, namely mood, sleep, and physical functioning. A hypothetical relationship between statins and depressive symptoms arose as a possible explanation for the observation that low serum cholesterol was tied to a higher frequency of deaths from suicide and violence [4,5]. Systematic reviews have reported mixed findings and were published prior to several recent additions to the field [6,7]. Generating more questions about the neuropsychological safety of statins, some reports have suggested adverse effects on sleep duration or quality [8]. Relatedly, impaired energy and physical function were recently proposed as a side effects of statin use [9,10].
Highlighting the importance of critically appraising the totality of existing evidence for these possible side effects, 260 million statin prescriptions were given in 2011 [11]. Moreover, indications for their use appear to be expanding [1,12,13]. In this context, the purpose of this review was to systematically review literature on the impact of statins on mood, sleep, and physical function.
MATERIALS AND METHODS
Data Sources and Searches
We performed a systematic computer-aided search of MEDLINE/PubMed, EMBASE, and the Cochrane Central Register from inception to October 14th, 2013, and augmented this search by scrutinizing reference lists of relevant articles, and making inquiries among colleagues and experts in the field. Our computer-aided search utilized MEDLINE/PubMed’s clinical queries tool, which has 99% sensitivity and 70% specificity for therapy questions [14]. The search strategy was determined with the aid of an information a list at the Johns Hopkins Welch Library.
On MEDLINE/PubMed, we searched for statin OR lipid-lowering agent OR HMG-CoA reductase inhibitor combined with mood OR depression OR well-being OR sleep OR insomnia OR fatigue OR energy OR physical function. Search terms for EMBASE and the Cochrane Central Register were similar and are provided in the online-only supplemental appendix. Language filters were not placed. Unpublished and ongoing research was identified by searching http://controlled-trials.com and http://clinicaltrials.gov. Corresponding authors of such studies were contacted and invited to contribute information about their unpublished study.
To decrease selection bias, we registered the following eligibility criteria a priori with the Welch Library and Ciccarone Center at Johns Hopkins University:
Patients
All patient populations, including those with or without underlying affective disorders.
Methodology
Due to a relative paucity in data, as well as heterogeneity in design and outcome, case reports, case series, case-control, cross-sectional, cohort, and randomized control trials were included.
Exposure
Statins (including all types) versus either no statins or placebo.
Outcomes
The outcomes were three key elements of quality of life (mood, sleep, and physical function), as defined by the search terms above. All outcome measures were considered, whether a primary or non-primary endpoint.
Study Selection
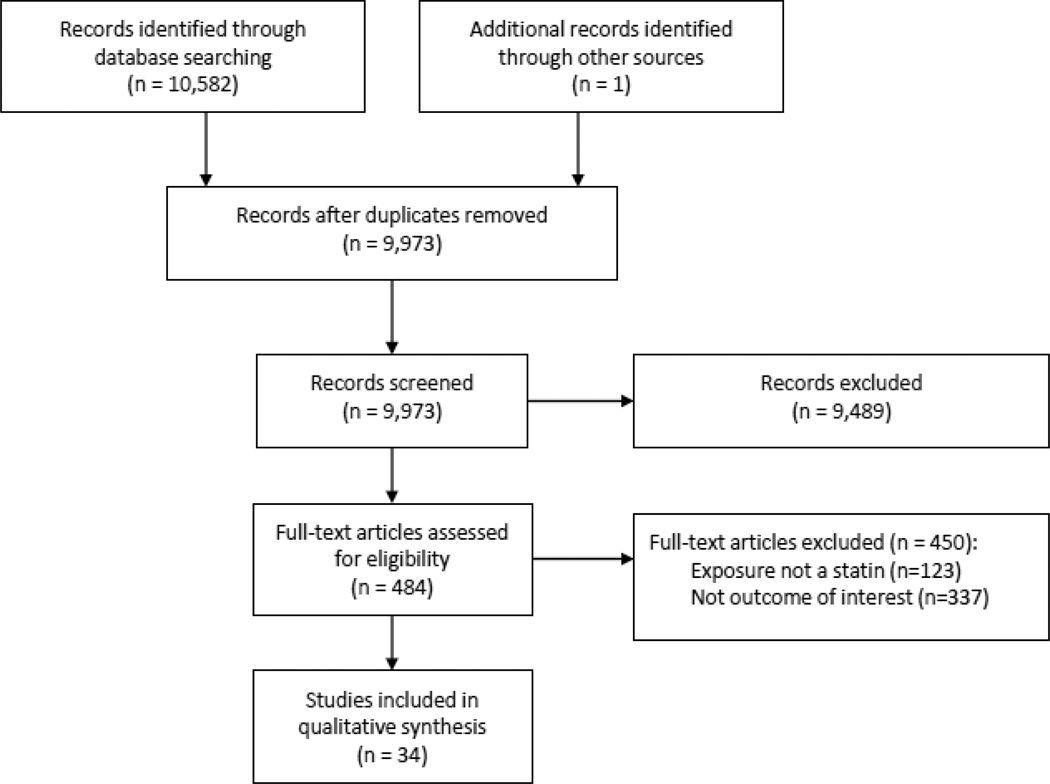
Articles were systematically selected for inclusion (Figure 1). Two independent reviewers (KS and RM) screened all records identified through the initial search strategy. Based on scrutiny of article titles and abstracts, the two reviewers each independently identified potentially eligible articles warranting full-text review. Results were compared and all disagreements were settled by discussion and review of full articles. The kappa coefficient of agreement was 0.73. The full article of all potentially eligible studies was then read by both reviewers and data were extracted to determine whether they met eligibility criteria for our study. Again, any disagreements were settled by discussion between the two independent reviewers. In the case of duplicate publication, we included only the largest, most complete article. Articles were designated for inclusion in the appropriate domain (mood, sleep, physical function).
Figure 1. Flow diagram of study selection process.
Assessing the risk of bias of mood articles
For randomized trials, we utilized the Cochrane Collaboration’s tool for assessing risk of bias [15]. Each article was evaluated for 6 domains including sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessors, completeness of outcome data, selectiveness of outcome reporting, and other sources of bias. A risk of bias assessment (low risk, high risk, or unclear based) was made for each domain from knowledge of each trial’s methods and a judgment about whether those methods are likely to have led to a risk of bias.
For observational studies, we utilized the Newcastle-Ottawa Scale for assessing risk of bias [16]. Each article was evaluated on basis of selection of outcomes assessment, cohort comparability, and quality of population selection. Up to 9 points were allocated with higher scores indicating higher study quality by the Newcastle-Ottawa Scale.
Data Synthesis
Across the studies, there was substantial heterogeneity in the endpoints, measurement scales, and completeness of reporting. Therefore, after careful consideration of a quantitative meta-analysis, we reached a consensus that this would not be appropriate or informative. Therefore, we systematically synthesized the available literature qualitatively. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and accuracy of the data analysis. The Johns Hopkins Institutional Review Board declared that ethics approval was not required for this study.
RESULTS
A PRISMA flow diagram is shown in Figure 1. The search strategy yielded 10,582 records and 34 met eligibility for qualitative synthesis.
Statins and Mood
Observational Studies
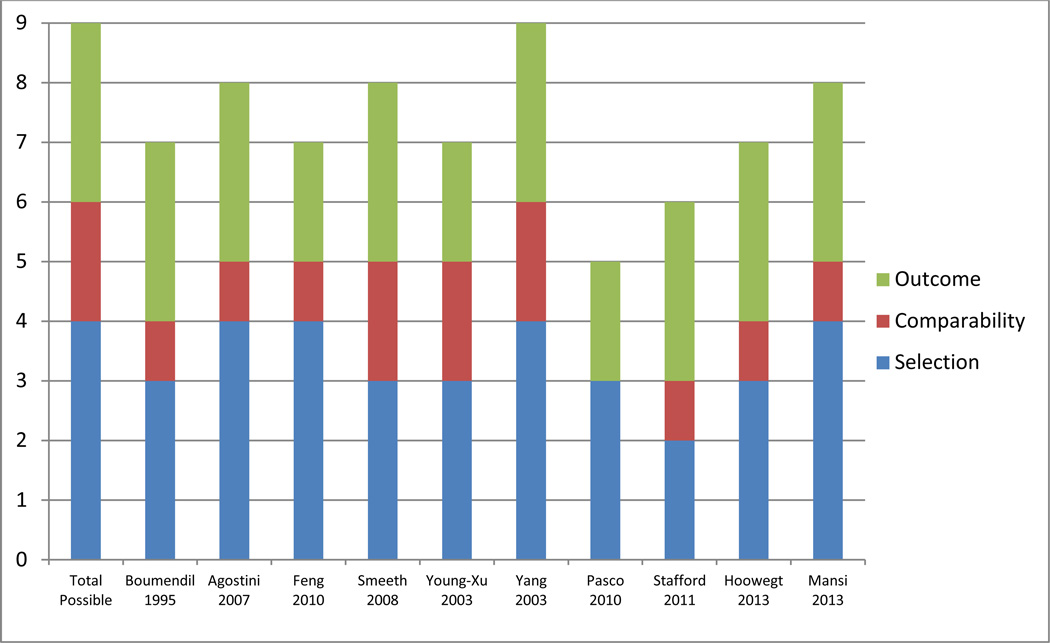
Ten observational studies assessed the association between statin use and mood [17–26]. Study protocols utilized a variety of different outcome measures. Risk of bias assessments are available in Figure 2; of the 10 studies, only one scored the maximum of 9 quality points [24]. Of the 10 observational studies, 1 found an increase in absenteeism from work related to depressive symptoms [17], 5 showed no association [18–21,23], and 4 demonstrated a protective association with statin use and depressive symptoms [22,24–26].
Figure 2. Risk of bias for observational studies of mood.
Risk of bias assessed by Newcastle-Ottawa Scale; up to 9 points were allocated with higher scores indicating higher study quality.
The only observational study to find a negative association with mood studied retrospective data from the Gazel cohort of 17,424 workers at a large company in France [17]. Greater absenteeism from work due to depressive symptoms (as diagnosed by a company physician) was associated with following a hypolipidemic diet (prevalence ratio, 1.83; 95% CI, 1.30–2.58) and simvastatin use (prevalence ratio, 2.18; 95% CI, 1.18–4.03). The analysis was not adjusted for potential confounders.
Five studies found no association between statin therapy and mood [18–21,23]. After one year of follow-up in 765 veterans taking statins, there was no change in depressive symptom reporting by the Center for Epidemiologic Studies Depression Scale [18]. Similarly, another prospective cohort of 1803 individuals >55 years of age reported no association of statin treatment and depressive symptoms [19]. A large, nested, case-control study of a UK database found no increased use of antidepressants in those taking statins [20]. In 409 patients followed for 12 months after defibrillator implantation, there was no statistically significant association between statin use and anxiety or depression in adjusted models [21]. Finally, in a retrospective cohort study of a military population, there was no statistically significant increase in ICD-9-CM diagnoses for depression, psychosis, bipolar disorder, or schizophrenia in statin users versus nonusers in a propensity score-matched analysis [23].
Four studies noted a protective association of statin use with depressive symptoms and other mood-related disturbances [22,24–26]. In the study with the longest exposure length in this review (up to 7 years), Young-Xu et al reported an odds ratio of 0.63 (95% CI, 0.43–0.93) for depression scores among those taking statins compared with placebo [22]. Similar results were obtained for anxiety (odds ratio, 0.69; 95% CI, 0.47–0.99) and hostility (odds ratio, 0.77; 95% CI, 0.58–0.93). The study was prospective and utilized propensity score matching to control for unknown confounders. Yang et al. noted a similar protective association of statin use with depressive symptoms and suicide in a nested case-control study [24]. Pasco et al, also using a case-control design, found that the rate of diagnosis of major depressive disorder was 1.7 per 1,000 person-years (95% CI, 0.4–6.9) for exposure subjects and 12.2 per 1,000 person-years (95% CI, 7.9–19.0) for those not taking statins [25]. Finally, Stafford et al studied self-reported symptoms via the Hospital Anxiety and Depression Scale at 9 months post-discharge from angioplasty, myocardial infarction, or coronary artery bypass grafting [26]. This study was notable for comprising the only use of statins in secondary prevention in this review and showed a 69% relative reduction in a diagnosis of major depressive disorder, minor depression, and dysthymia.
Randomized Controlled Trials
Seven clinical trials totaling 555 subjects were identified that investigated mood as a primary endpoint, the characteristics of which are provided in Table 1 [27–33]. Exposures included simvastatin (3 trials), pravastatin (2 trials), lovastatin (2 trials), and atorvastatin (1 trial). Participants were generally healthy and free of psychological morbidity, though one study [33] investigated patients with Major Depressive Disorder and found lovastatin augmented standard fluoxetine by improving depression scores. Some investigated subjects >70 years of age, but trials mostly focused on middle-age adults with or without hyperlipidemia. In trials that reported total cholesterol at baseline, average levels ranged from 5.30–7.27 mmol/L (205–281 mg/dL). Cohorts ranged in size from 12 to 209 persons with exposure lengths from 4 weeks to 1 year.
Table 1.
Table of clinical trials featuring mood as an endpoint
| Reference | Exposure/Duration | Patients | Outcome/Assessment | Result |
|---|---|---|---|---|
| Harrison, 1994 [27] | Simvastatin 40 mg/day vs. pravastatin 40 mg/day vs. placebo; 2 week placebo run-in, 4 week treatment phase, 4–6 week washout | 25 healthy volunteers, average age 23.8 years; excluded those with personal or family history psychiatric disease, or evidence of current depression | Hospital Anxiety Depression Scale at conclusion | No effect |
| Muldoon, 2000 [31] | Lovastatin 20 mg/day vs. placebo for 6 months | 209 healthy adults with LDL-C >4.14 mmol/L (160 mg/dL), aged 24–60 years; excluded those with schizophrenia, use of psychotropic medications | Assessments at baseline and conclusion of mood, hostility, hopelessness, anger, and social function using neuropsychological test battery (16 tests), interviews, and daily diary assessments | No effect; in addition, no signal of worsening for those with the greatest LDL-C reduction or those reaching the lowest LDL-C levels. |
| Carlsson, 2002 [28] | Pravastatin 20 mg/day for 6 months then pravastatin + tocopherol (group 1) or tocopherol for 6 months then both medications for an additional 6 months | 41 community-dwelling men and women >70 years old with LDL-C ≥3.62 mmol/L (140 mg/dL); 3 subjects used antidepressants | Health perception and depression (Geriatric Depression Scale) were assessed at baseline, 6 months, and 1 year | No effect at 6 months or 1 year; in addition, no changes by lipid levels. |
| Ormiston, 2003 [29] | Atorvastatin 10–20 mg/day, lovastatin 20–40 mg/day or placebo for 52 weeks (upon unblinding, it was revealed no patients received placebo) | 12 subjects, aged 33–80 years; mean total cholesterol at baseline 7.27 mmol/L (281 mg/dL) | Psychological variables were measured at 4 weeks and 52 weeks using Beck Depression Inventory, Buss-Durkee Hostility Inventory, and the Barrett Impulsivity Scale | Week 4: No effect on depression or hostility; mild increase in impulsivity, not significantly correlated with cholesterol lowering. Week 52: Impulsivity returned to baseline. No change in hostility. Depression mildly but significantly improved compared to baseline. |
| Hyyppa, 2003 [30] | Mediterranean-style diet and/or simvastatin 20 mg/day for 12 weeks | 120 hyperlipidemic but otherwise healthy men aged 35–64 years | Psychological distress assessed by Brief Symptom Inventory that assessed somatization, depression, anxiety, hostility, phobic anxiety and obsessive– compulsive behavior; Aggression was assessed with two questionnaires based on the Strauss Scale of Aggression Questionnaire (completed by subject and partner or cohabitant). | Simvastatin resulted in an increase in depression (p=0.016), and somatization (p=0.034), without changes in the anxiety, hostility scores. Aggression increased by self-report (p=0.049) but not by report from partners |
| Morales, 2006 [32] | Simvastatin, up to 20 mg/day or placebo for 15 weeks after 4–6 week run-in diet phase | 80 participants with an average age of 70 years and high normal/mildly elevated cholesterol | Center for Epidemiological Studies Depression Scale (CES-D), Structured Clinical Interview for DSM, Apathy Evaluation Scale Profile of Mood States, Daily diaries incorporating Lawton Positive Affect and Negative Affect rating scales | No change in CES-D scores Diary data showed time-dependent, cholesterol-related decreases in self-reports of positive affect in the simvastatin group. |
| Ghanizadeh, 2013 [33] | Lovastatin up to 30 mg/day, lovastatin up to 30 mg/day + fluoxetine up to 40 mg/day, or fluoxetine up to 40 mg/day + placebo | 68 participants with diagnosed Major Depression Disorder and without “serious uncontrolled medical conditions” such as hyperthyroidism, hypothyroidism, epilepsy, active substance abuse, diabetes, hypertension, or active suicidal ideation | Hamilton Depression scale via face-to-face interviews at baseline, week 2, and week 6. | Fluoxetine plus lovastatin decreased Major Depressive Disorder symptoms more than fluoxetine plus placebo |
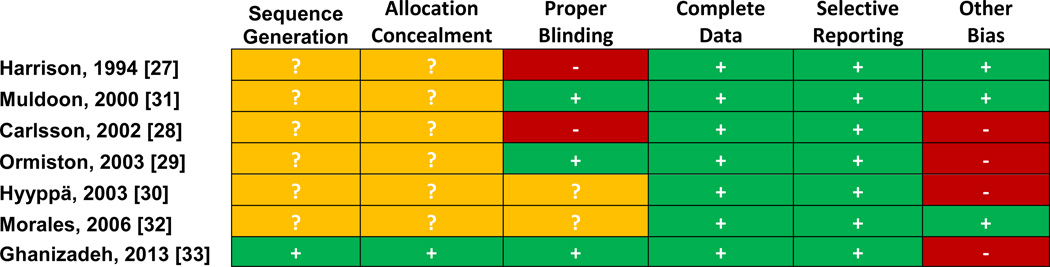
Risk of bias assessments are summarized in Figure 3 and show a pattern of failure to report adequate sequence generation, allocation concealment, and blinding. Trials utilized different assessments of mood including questionnaires, structured interviews, and diaries. Four trials [27–29,31] showed no effect on mood, while 2 [30,32] showed a significant negative effect on some, but not all, mood endpoints. One study, did note an adjuvant effect with co-administration of fluoxetine on in patients with Major Depressive Disorder [33].
Figure 3. Risk of bias for randomized controlled trials with mood as a primary outcome.
Risk of bias assessed by Cochrane tool. ? = unclear, − = high risk, + = low risk
Four large clinical trials studied mood as a secondary endpoint [34–37]. Downs et al analyzed 1100 men and women treated with lovastatin from the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TEXCAPS) with mild to moderate elevations of total cholesterol and LDL-C, and low HDL-C, using an emotional well-being and general health perception scale and found no difference at one year [34]. Wardle et al looked at 621 men and women from the Oxford Cholesterol Study of simvastatin and also found no effect on mood disturbance using a questionnaire [35]. Thirdly, Stewart et al studied 1130 respondents from the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) study of pravastatin over 4 years and found no difference by treatment group in anxiety, depression, anger, impulsiveness, adverse life events, or alcohol intake [36]. An analysis of adverse events from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosvastatin (JUPITER) found no effect on anxiety or anger, and found fewer cases of incident depression among subjects achieving LDL-C levels <1.29 mmol/L (<50 mg/dL) with rosuvastatin relative to those not achieving such levels on rosuvastatin or those taking placebo [37].
Statins and Sleep
Observational data examining statins and sleep were mixed. In a letter to the editor, Schaefer reported that 9 of 51 patients taking lovastatin decreased their sleep (by 1 to 3 hours) compared with 0 of 33 patients taking pravastatin in a randomized trial [38]. In a review of the available data on the short- and long-term effects of lovastatin, Tobert noted that one trial participant withdrew for insomnia and that this was reversible with drug discontinuation [39]. Black et al used a questionnaire in 409 hyperlipidemic patients receiving a diet intervention, lovastatin, simvastatin, pravastatin, or other lipid-lowering agents in a cross-sectional approach [40]. They concluded that sleep in the lovastatin group was no different than in the other groups. In a prospective cohort of 149 patients followed for 6 months, self-reported sleep disturbances were not significantly different between the simvastatin (3.6%) versus lovastatin (4.6%) versus placebo groups (8.7%) [41].
Five clinical trials have evaluated sleep as a primary outcome using both subjective and objective measures (Table 2) [8,27,28,42,43]. Risk of bias assessments are provided in Online Supplemental, Figure 1. The studies were of small size (12 to 41 participants), generally consisted of healthy volunteers, and produced mixed results. The largest and most recent study was compatible with no impact of statin treatment on sleep [28]. Others [8,27,42,43] suggested an effect on sleep quality, but sleep laboratory metrics did not correlate well with patient reports, and the possibility of type I error was noted [8,27,43].
Table 2.
Table of clinical trials featuring sleep as an endpoint
| Reference | Exposure/Duration | Patients | Outcome/Assessment | Result |
|---|---|---|---|---|
| Barth, 1990 [42] | Simvastatin 20 mg/day or colestipol 15g | 30 men with CAD (s/p MI or CABG) initiated on lipid-lowering therapy | Duration and patterns of sleep assessed by questionnaire at baseline, 3 months, 12 months. | Simvastatin group slept less and had greater interruptions |
| Vgontzas, 1991 [8] | Lovastatin 40 mg/day vs. pravastatin 40 mg/day; placebo run-in and withdrawal phases | 12 men and women with normal sleep patterns | Monitored in sleep laboratory over 22 nights along with postsleep questionnaire | Wake time, number of awakenings, and time in stage 1 were increased significantly with lovastatin; no significant correlation with subjective reports. |
| Eckernas, 1993 [43] | Simvastatin 20 mg/day vs. pravastatin 40 mg/day vs. placebo; two-period design with incomplete block design | 24 men with hyperlipidemia | 63 day period split into 3 sections, monitored in sleep laboratory over last 3 days of each section, provided Sleep Problem Questionnaire at conclusion of each section | Inconsistent findings not favoring a particular treatment arm; no difference in subjective reports; not felt to be clinically meaningful |
| Harrison, 1994 [27] | Simvastatin 40 mg/day vs. pravastatin 40 mg/day vs. placebo; 2 week placebo run-in, 4 week treatment phase, 4–6 week washout phase | 25 healthy volunteers, average age 24 years | Leeds Sleep Questionnaire | Simvastatin group with more difficulty getting to sleep vs. pravastatin but not vs. placebo; similar question was not significantly different; felt to be a type I error. |
| Carlsson, 2002 [28] | Pravastatin 20 mg/day for 6 months then pravastatin + tocopherol or tocopherol for 6 months, then both medications for an additional 6 months | 41 community-dwelling men and women aged >70 years with LDL-C ≥ 3.62 mmol/L (140 mg/dL) | Sleep Dysfunction Scale | No effect |
Though not a primary outcome of the trial, JUPITER provides by far the largest randomized comparison of statin therapy and placebo in relation to sleep. Hsia et al found no impact of rosuvastatin use on insomnia among JUPITER subjects [37].
Statins and Physical Function
Most evidence suggested either improved performance [18,44,45] or no effect [28,37,46] of statins on physical functioning. Risk of bias assessments are provided in Online Supplemental, Figure 2. Among JUPITER participants, there was no significant relationship between rosuvastatin use, degree of LDL-C lowering, and fatigue [37]. Additionally, Wardle et al noted the same percentage of individuals (58%) reported fatigue as a symptom in both the simvastatin and placebo group [35]. Many of these studies were observational and conducted in select populations like those with peripheral arterial disease or older individuals [18,44]. Notably, in 5777 participants from the Women’s Health Initiative prospective cohort study, Gray et al found no differences between statin users and non-users in 6-minute walk test performance, repeated chair stands, and grip strength, assessed repeatedly over 6 years [46].
Recently, two studies [9,10] have addressed physical functioning using novel outcomes. Energy and fatigue with exertion were examined in an exploratory analysis of 692 men aged ≥20 years and 324 non-procreative women without diabetes mellitus or cardiovascular disease [9]. Self-reported energy and fatigue with exertion were rated on a scale from 0–10 and ultimately combined to form a composite EnergyFatigEx score. This endpoint was not pre-specified; it was newly created for the purposes of the trial. The investigators found a negative correlation between statin use and the EnergyFatigEx score over 6 months. The authors used imputation for their analysis due to missing data; the extent of missing data was not reported.
Exercise training adaptations were analyzed in thirty-seven participants randomized to exercise training or exercise and simvastatin [10]. Both functional (cardiorespiratory fitness) and biochemical (skeletal muscle mitochondrial content) outcomes were assess and subjects taking statin therapy with exercise were found to have blunted responses to both domains relative to the exercise only group.
DISCUSSION
Based on this systematic review, evidence to date is most compatible with no adverse effect of statins on mood, sleep, and physical function. This conclusion is limited by the paucity of available evidence especially in relation to the large number of studies on statin therapy. The results of this review reflect not only the relative lack of evidence of these side effects, but also the relative weakness of the evidence that does exist, particularly for sleep and physical function. These results are timely and of public health importance given a recent United States Food and Drug Administration statin labeling change brought potential side effects to the forefront.
Mood
The majority of observational and randomized clinical trial data do not indicate that there is an association between mood and statin use though there are outliers in this trend. From observational studies, only one publication noted a negative change in mood, though this was in an outcome measure that served as a proxy for an amalgam of mood states [17]. In terms of trial data, Hyyppä and colleagues noted mood changes after treatment with simvastatin, a finding that contrasts with their earlier work [30]. This was attributed to multiple factors, including differences in data collection and methodology. Changes were found in self-reported measures, however, these were not noted in measures from partners of the participants, suggesting that the differences found may not be clinically evident or significant. Furthermore, subjects were assessed by the Brief Symptom Inventory, an assessment tool rated at a 6th grade reading level, which is an area of concern as upwards of a quarter of the sample population did not possess an elementary school education. Moreover, the Brief Symptom Inventory incorporates information about abdominal pain, dizziness, and body pain. These symptoms are also potential side effects of simvastatin use [47], and therefore this finding may not be evidence of psychological change, but rather other medication side effects.
Similar findings were found in work by Morales et al, who noted a change toward negative affect measures in the simvastatin treatment group [32]. Of note, these changes were found in diaries kept by participants, not in objective measures of depression. In addition to the subjective nature of this measure, the authors note that while statistically significant, these outcomes are not necessarily clinically significant. Finally, the studies by Morales and Hyyppä may be subject to significant bias as neither explicitly note methods of sequence generation, allocation concealment, and blinding for the respective studies.
One study did note improvement in Major Depressive Disorder with combined fluoxetine and lovastatin treatment [33]. When compared to placebo, co-administration of lovastatin statistically significantly improved scores on the Hamilton Depression Rating scale. The authors note that this may not be a primarily lipid related, but rather a pleotropic effect mediated by anti-inflammatory effect of statin treatment. Additional studies are needed to replicate this preliminary, small study.
Sleep
With regard to sleep, the data from randomized clinical trials and observational studies are limited. Much of the published material is composed of anecdotal evidence rather than rigorous, peer reviewed literature. Those that were published via peer review incorporated small cohorts and assessed different outcomes with varying results; thus, it is difficult to ascertain a clear trend [8,27,28,42,43].
An important consideration is the possibility of Type I error (i.e. rejection of the null hypothesis when it is, in fact, true). If 20 hypothesis tests are run, 1 is expected to reject the null hypothesis based on chance alone. Moreover, epidemiological studies note that both insomnia and depression have significant prevalence in the American adult population. Prevalence of insomnia is as high as 30% in adult populations, while depression affects nearly 10% of persons in certain age groups [48,49]. Therefore, the positive findings in these small studies should be interpreted with caution.
It is possible that some differences between studies could be related to lipophilicity. It has been posited that statins with lipophilic structures would have increased transit across the blood brain barrier [50]. Evidence suggests that the presence of a statin in the cerebrospinal fluid may alter cholesterol metabolism, which could result in the psychological changes associated with statin use [51]. Thus, while overall data are not compatible with adverse neurocognitive effects of statins, including on mood or sleep in this review, further study is needed to evaluate statins with higher lipophilicity could have such undesired effects in certain types of vulnerable patients.
Physical Function
The overall breadth of literature does not support a negative effect of statins on physical functioning [18,37,44–46]. Two recent reports suggest an interaction of statins with energy, fatigue with exertion, and cardiorespiratory fitness [9,10]. In June of 2012, the popular media focused on the question of the effect of statins on energy [52]. The concern stemmed from results of a single exploratory analysis noting a decrease in energy while taking statins [9]. This report had little basis for comparison given the study endpoint was newly created for the analysis. Moreover, the result was published 8 years after trial completion while the primary outcomes of the trial remained unpublished [53]. Publication bias, then, is an important consideration in interpreting these results. Although a recent retrospective analysis of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) study also reported a statistically significant negative effect of statin treatment on energy, the authors noted that findings are of uncertain clinical significance [54]. While energy or fatigue (both ascertained by questionnaire) and physical functioning are not transposable outcomes, physical functioning, as reported by the aforementioned larger body of work, relies at least on objective measures such as the 6 minute walk test and chair stand test. Importantly, overall quality of life appears to be similar in patients who are treated and not treated with statin [34].
The recent trial by Mikus et al. assesses yet another estimate of physical functioning using an objective measure of cardiorespiratory function [10]. Cardiorespiratory fitness was defined as VO2 max, a measurement affected by multiple variables not limited to fitness including muscle diffusion capacity, mitochondrial enzymes, pulmonary diffusion capacity, and demographic factors. As these parameters were not controlled for it is impossible to know whether residual confounding exists. It is also notable that participants and investigators were not blinded to allocation.
Strengths and Limitations
The strengths of this study include a systematic examination of possible sources of bias of each of the included articles. Additionally, while emphasis remained upon randomized controlled trials, the present study included all relevant studies regardless of design. Furthermore, this study provides the first systemic examination of the effect of statin therapy on sleep and physical function.
Publication bias is an important consideration as studies that support the null hypothesis (that statins do not affect mood, sleep, or physical function) may be less likely to be published. Many of the studies analyzed here have small sample sizes (and thus large variance) and are therefore more susceptible to publication bias as larger studies would be less likely to escape publication. We attempted to account for this by thoroughly searching clinicaltrials.gov for ongoing or unpublished studies. It should also be noted that publication bias tends to support positive results (that statins do affect mood, sleep, or physical function) and this review demonstrates a majority of negative studies. We therefore feel that while publication bias may be present it would not change our overall conclusions. Our study was limited to a qualitative synthesis. The disparate methods and outcomes of the reviewed studies did not lend themselves to a quantitative synthesis. Additionally, it is possible that certain mood, sleep, or physical function outcomes may not have been captured in our search strategy. While we focused on statin therapy as a treatment class, statins could have a different adverse effect profile based on their lipophilicity. Finally, randomized trials commonly include run-in periods which can exclude patients more likely to have adverse effects.
Conclusion
Overall, available evidence is limited and most consistent with no adverse effect of statins on mood, sleep, or physical function. Studies suggesting such adverse effects suffer from potential sources of bias, including multiple testing or lack of adjustment for confounders in observational studies, and failure to pre-specify outcomes or report blinding in trials. Future investigations should focus on high-quality, prospective, adequately powered research designs using pre-specified, validated outcome measures. Patients who may be most vulnerable to adverse effects deserve special attention.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Jaime Blanck from the Johns Hopkins Welch library for contributing to the search strategy on this study. SSM is supported by the Pollin Cardiovascular Prevention Fellowship, Marie-Josée and Henry R Kravis endowed fellowship, and a National Institutes of Health training grant (T32HL07024). RSB is supported by the Kenneth Jay Pollin professorship in cardiology.
Footnotes
Conflict of Interests: Dr. Martin is listed as a co-inventor on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation. The other authors have no conflicts of interest to disclose.
Contributions of Authors Statement: KJS and RJM systematically searched the literature, selected studies, and performed risk of bias assessments. All authors synthesized the data. KJS drafted the manuscript and all other authors edited it for intellectual content.
REFERENCES
- 1.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25) Suppl 2:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213–1221. doi: 10.1016/j.mayocp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688–697. doi: 10.7326/0003-4819-159-10-201311190-00007. [DOI] [PubMed] [Google Scholar]
- 4.Muldoon MF, Rossouw JE, Manuck SB, Glueck CJ, Kaplan JR, Kaufmann PG. Low or lowered cholesterol and risk of death from suicide and trauma. Metabolism. 1993;42:45–56. doi: 10.1016/0026-0495(93)90259-q. [DOI] [PubMed] [Google Scholar]
- 5.You H, Lu W, Zhao S, Hu Z, Zhang J. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14:1467–1476. doi: 10.1517/14656566.2013.803067. [DOI] [PubMed] [Google Scholar]
- 6.O'Neil A, Sanna L, Redlich C, Sanderson K, Jacka F, Williams LJ, Pasco JA, Berk M. The impact of statins on psychological wellbeing: a systematic review and meta-analysis. BMC Med. 2012;10:154. doi: 10.1186/1741-7015-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.While A, Keen L. The effects of statins on mood: a review of the literature. Eur J Cardiovasc Nurs. 2012;11:85–96. doi: 10.1016/j.ejcnurse.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Kales A, Bixler EO, Manfredi RL, Tyson KL. Effects of lovastatin and pravastatin on sleep efficiency and sleep stages. Clin Pharmacol Ther. 1991;50:730–737. doi: 10.1038/clpt.1991.213. [DOI] [PubMed] [Google Scholar]
- 9.Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172:1180–1182. doi: 10.1001/archinternmed.2012.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP. Simvastatin impairs exercise training adaptation. J Am Coll Cardiol. 2013;62:709–714. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2011. [Accessed 14 July 2014];2012 http://www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/IHII_Medicines_in_U.S_Report_2011.pdf. [Google Scholar]
- 12.Martin SS, Metkus TS, Horne A, Blaha MJ, Hasan R, Campbell CY, Yousuf O, Joshi P, Kaul S, Miller M, Michos ED, Jones SR, Gluckman TJ, Cannon CP, Sperling LS, Blumenthal RS. Waiting for the National Cholesterol Education Program Adult Treatment Panel IV Guidelines, and in the meantime, some challenges and recommendations. Am J Cardiol. 2012;110:307–313. doi: 10.1016/j.amjcard.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR Hedges Team. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ. 2005;330:1179. doi: 10.1136/bmj.38446.498542.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J. Chapter 8: Assessing risk of bias in included studies: Table 8.5 a. [Accessed 14 July 2014];Cochrane Handbook for Systematic Reviews. 2008 http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Ch08_Bias.pdf. [Google Scholar]
- 16.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [Accessed 14 July 2014];2014 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 17.Boumendil E, Tubert-Bitter P. Depression-induced absenteeism in relation to antihyperlipidemic treatment: a study using GAZEL cohort data. Epidemiology. 1995;6:322–325. doi: 10.1097/00001648-199505000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Agostini JV, Tinetti ME, Han L, McAvay G, Foody JM, Concato J. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55:420–425. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 19.Feng L, Yap KB, Kua EH, Ng TP. Statin use and depressive symptoms in a prospective study of community-living older persons. Pharmacoepidemiol Drug Saf. 2010;19:942–948. doi: 10.1002/pds.1993. [DOI] [PubMed] [Google Scholar]
- 20.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoogwegt MT, Theuns DA, Kupper N, Jordaens L, Pedersen SS. Relation of statin therapy to psychological functioning in patients with an implantable cardioverter defibrillator. Am J Cardiol. 2013;111:1169–1174. doi: 10.1016/j.amjcard.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 22.Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol. 2003;42:690–697. doi: 10.1016/S0735-1097(03)00785-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansi I, Frei CR, Pugh MJ, Mortensen EM. Psychologic disorders and statin use: a propensity score-matched analysis. Pharmacotherapy. 2013;33:615–626. doi: 10.1002/phar.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C-C, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163:1926–1932. doi: 10.1001/archinte.163.16.1926. [DOI] [PubMed] [Google Scholar]
- 25.Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, Berk M. Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. 2010;79:323–325. doi: 10.1159/000319530. [DOI] [PubMed] [Google Scholar]
- 26.Stafford L, Berk M. The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression: proof of concept for the inflammatory and oxidative hypotheses of depression? J Clin Psychiatry. 2011;72:1229–1235. doi: 10.4088/JCP.09m05825blu. [DOI] [PubMed] [Google Scholar]
- 27.Harrison RW, Ashton CH. Do cholesterol-lowering agents affect brain activity? A comparison of simvastatin, pravastatin, and placebo in healthy volunteers. Br J Clin Pharmacol. 1994;37:231–236. doi: 10.1111/j.1365-2125.1994.tb04268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlsson CM, Papcke-Benson K, Carnes M, McBride PE, Stein JH. Health-related quality of life and long-term therapy with pravastatin and tocopherol (vitamin E) in older adults. Drugs Aging. 2002;19:793–805. doi: 10.2165/00002512-200219100-00008. [DOI] [PubMed] [Google Scholar]
- 29.Ormiston T, Wolkowitz OM, Reus VI, Manfredi F. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44:412–414. doi: 10.1176/appi.psy.44.5.412. [DOI] [PubMed] [Google Scholar]
- 30.Hyyppä MT, Kronholm E, Virtanen A, Leino A, Jula A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology. 2003;28:181–194. doi: 10.1016/s0306-4530(02)00014-8. [DOI] [PubMed] [Google Scholar]
- 31.Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108:538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 32.Morales K, Wittink M, Datto C, DiFilippo S, Cary M, TenHave T, Katz IR. Simvastatin causes changes in affective processes in elderly volunteers. J Am Geriatr Soc. 2006;54:70–76. doi: 10.1111/j.1532-5415.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 33.Ghanizadeh A, Hedayati A. Augmentation of fluoxetine with lovastatin for treating major depressive disorder, a randomized double-blind placebo controlled-clinical trial. Depress Anxiety. 2013;30:1084–1088. doi: 10.1002/da.22195. [DOI] [PubMed] [Google Scholar]
- 34.Downs JR, Oster G, Santanello NC. HMG CoA reductase inhibitors and quality of life. JAMA. 1993;269:3107–3108. doi: 10.1001/jama.269.24.3107. [DOI] [PubMed] [Google Scholar]
- 35.Wardle J, Armitage J, Collins R, Wallendszus K, Keech A, Lawson A. Randomised placebo controlled trial of effect on mood of lowering cholesterol concentration. Oxford Cholesterol Study Group. BMJ. 1996;313:75–78. doi: 10.1136/bmj.313.7049.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart R, Sharples K. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. Arch Int Med. 2000;160:3144–3152. doi: 10.1001/archinte.160.20.3144. [DOI] [PubMed] [Google Scholar]
- 37.Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) J Am Coll Cardiol. 2011;57:1666–1675. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer E. HMG-CoA reductase inhibitors for hypercholesterolemia. N Engl J Med. 1988;319:1222–1223. doi: 10.1056/NEJM198811033191811. [DOI] [PubMed] [Google Scholar]
- 39.Tobert JA. Efficacy and long-term adverse effect pattern of lovastatin. Am J Cardiol. 1988;62:28J–34J. doi: 10.1016/0002-9149(88)90004-5. [DOI] [PubMed] [Google Scholar]
- 40.Black DM, Lamkin G, Olivera EH, Laskarzewski PM, Stein EA. Sleep disturbance and HMG CoA reductase inhibitors. JAMA. 1990;264:1105. doi: 10.1001/jama.1990.03450090041020. [DOI] [PubMed] [Google Scholar]
- 41.Ditschuneit HH, Kuhn K, Ditschuneit H. Comparison of different HMG-CoA reductase inhibitors. Eur J Clin Pharmacol. 1991;40(Suppl 1):S27–S32. [PubMed] [Google Scholar]
- 42.Barth JD, Kruisbrink OA, Van Dijk AL. Inhibitors of hydroxymethylglutaryl coenzyme A reductase for treating hypercholesterolaemia. BMJ. 1990;301:669. doi: 10.1136/bmj.301.6753.669-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckernäs SA, Roos BE, Kvidal P, Eriksson LO, Block GA, Neafus RP, Haigh JR. The effects of simvastatin and pravastatin on objective and subjective measures of nocturnal sleep: a comparison of two structurally different HMG CoA reductase inhibitors in patients with primary moderate hypercholesterolaemia. Br J Clin Pharmacol. 1993;35:284–289. [PMC free article] [PubMed] [Google Scholar]
- 44.Giri J, McDermott MM, Greenland P, Guralnik JM, Criqui MH, Liu K, Ferrucci L, Green D, Schneider JR, Tian L. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47:998–1004. doi: 10.1016/j.jacc.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 45.Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, Pastore M, Borrello F, Belcastro M, Picchi A, Nami R. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–364. doi: 10.1016/s0002-9343(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 46.Gray SL, Aragaki AK, LaMonte MJ, Cochrane BB, Kooperberg C, Robinson JG, Woods NF, LaCroix AZ. Statins, angiotensin-converting enzyme inhibitors, and physical performance in older women. J Am Geriatr Soc. 2012;60:2206–2214. doi: 10.1111/jgs.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MedlinePlus. Simvastatin. [Accessed 14 July 2014];2014 http://www.nlm.nih.gov/medlineplus/druginfo/meds/a692030.html.
- 48.National Institute of Mental Health. Major Depressive Disorder Among Adults. [Accessed 14 July 2014];2014 http://www.nimh.nih.gov/statistics/1MDD_ADULT.shtml.
- 49.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 50.Botti RE, Triscari J, Pan HY, Zayat J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin Neuropharmacol. 1991;14:256–261. doi: 10.1097/00002826-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Locatelli S, Lütjohann D, Schmidt HH, Otto C, Beisiegel U, von Bergmann K. Reduction of plasma 24S-hydroxycholesterol (cerebrosterol) levels using high-dosage simvastatin in patients with hypercholesterolemia: evidence that simvastatin affects cholesterol metabolism in the human brain. Arch Neurol. 2002;59:213–216. doi: 10.1001/archneur.59.2.213. [DOI] [PubMed] [Google Scholar]
- 52.Reuters. Do statins drain your energy? [Accessed 14 July 2014];2012 http://www.foxnews.com/health/2012/06/12/do-statins-drain-your-energy/. [Google Scholar]
- 53.Golomb BA, Criqui MH, White H, Dimsdale JE. Conceptual foundations of the UCSD Statin Study: a randomized controlled trial assessing the impact of statins on cognition, behavior, and biochemistry. Arch Intern Med. 2004;164:153–162. doi: 10.1001/archinte.164.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez AC, Jhund P, Preiss D, Kjekshus J, McMurray JJ. Effect of rosuvastatin on fatigue in patients with heart failure. J Am Coll Cardiol. 2013;61:1121–1122. doi: 10.1016/j.jacc.2012.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.