Summary
Purpose
CD37 is cell surface tetraspanin present on normal and malignant B cells. Otlertuzumab (TRU-016) is a novel humanized anti-CD37 protein therapeutic that triggers direct caspase independent apoptosis of malignant B cells and induces antibody-dependent cell-mediated cytotoxicity. This study evaluated the safety, pharmacokinetics, and efficacy of otlertuzumab administered in combination with rituximab and bendamustine to patients with relapsed, indolent B-cell non-Hodgkin Lymphoma (NHL).
Methods
Patients with relapsed or refractory NHL received otlertuzumab (10 or 20 mg/kg) intravenously (IV) on days 1 and 15, bendamustine (90 mg/m2) on days 1 and 2, and rituximab (375 mg/m2) on day 1 for up to six 28 day cycles. Responses were determined using standard criteria.
Results
Twelve patients were treated with 6 patients at each dose level; median age was 57 years (range, 51–79), and median number of prior regimens was 3 (range, 1–4). All patients had relapsed after prior rituximab including 7 refractory to their most recent previous treatment. In the 10 and 20 mg/kg dose cohorts, the mean half-life was 8 and 10 days following the first dose, and 12 or 14 days following 12 doses of otlertuzumab, respectively. Overall response rate was 83 % (10/12) with 4 CRs (32 %). The most frequent adverse events were neutropenia, nausea, fatigue, leukopenia, and insomnia; most were grade 1 or 2.
Conclusions
Otlertuzumab in combination with rituximab and bendamustine was well tolerated and induced responses in the majority of patients with relapsed indolent B-NHL.
Keywords: Otlertuzumab, NHL, Rituximab, Bendamustine
Introduction
Rituximab induces cytotoxicity by antibody dependent cell-mediated cytotoxicity (ADCC), complement activation, and apoptosis, and is the most frequently used agent for initial or maintenance therapy of B-cell NHL. Rituximab increases the response rates and progression-free survival for patients with indolent NHL when combined with various chemotherapeutic agents. Unfortunately, most patients relapse so alternative treatments are needed.
The combination of bendamustine and rituximab has been studied in patients with relapsed NHL. In 63 patients with rituximab-naïve mantle cell or low-grade lymphomas in first to third relapse or refractory to previous treatment, the overall response rate was 90 % (95 % CI, 80 % to 96 %) with a complete remission rate (CR) of 60 % (95 % CI, 47 % to 72 %).[1] The median time of progression-free survival was 24 months (range, 5 to 44+ months). In a 67 patient study of BR with relapsed, indolent B-cell or mantle cell lymphoma without documented resistance to prior rituximab overall response rate was 92 % (41 % CR, 14 % unconfirmed CR, and 38 % partial response). Median progression-free survival time was 23 months (95 % CI, 20 to 26 months).[2]
Agents with different mechanism of action are being explored, including ibrutinib [3], a BTK inhibitor; lenalidomide [4, 5], an immunomodulatory agent; everolimus [6, 7] and temsirolimus [8–10], both mTOR inhibitors; and idelalisib, a PI3K-δa inhibitor [11]. There is a strong need for novel treatments in relapsed NHL that overcome resistance to chemotherapy and rituximab [12].
Otlertuzumab is a CD37-specific, single chain, homodimeric therapeutic protein built on the ADAPTIR™ (modular protein technology) platform and has some properties similar to antibodies. ADAPTIR mono-specific molecules are single-chain polypeptides comprised of 3 components: a binding domain (VL and VH), a hinge domain, and an effector domain (huFc). These single-chain polypeptides dimerize within Chinese hamster ovarian (CHO) cells during production. Because of the differentiated structure from monoclonal antibodies, ADAPTIR mono-specific molecules have the capacity to stimulate a unique signaling response [13]. The modular design enables changes in composition of the individual components to tailor the biological activity of the ADAPTIR mono-specific molecule to fit the desired product profile. Like monoclonal antibodies, ADAPTIR mono-specific molecules have the potential to bind cell surface targets as well as neutralize soluble antigens that are implicated in human disease.
CD37 is a heavily glycosylated cell surface protein that is expressed constitutively at high levels on human B cells including transformed human B cell leukemia and lymphoma cells [14–17]. CD37 is either absent or expressed very weakly on normal T cells, monocytes, and neutrophils, and is absent on platelets and erythrocytes [14], therefore CD37 is considered to be a lineage-specific marker of human B cells and represents a therapeutic target for B cell-directed therapy in NHL [11].
Otlertuzumab has been shown to have two proposed distinct mechanisms of action: to induce direct caspase-independent apoptosis of malignant B cells and to induce potent Fc-dependent cellular cytotoxicity, also known as antibody-dependent cell-mediated cytotoxicity (ADCC).[18] By use of these mechanisms, otlertuzumab has the capability to deplete CD37 expressing B-NHL cells.
Otlertuzumab has potential beneficial clinical attributes for the treatment of malignant human B-cell tumors. First, because otlertuzumab delivers its signal via interaction with CD37 rather than CD20, otlertuzumab offers the possibility for therapeutic benefit when CD20 is shed, blocked, or removed from the surface of the targeted B cells, a limitation that has been reported for chronic lymphocytic leukemia CLL.[19–21] Second, in preclinical models, treatment with otlertuzumab has resulted in increased anti-tumor activity when combined with other therapeutic drugs used for B-cell malignancies. [22, 23] In vitro studies show positive combinatorial activity of otlertuzumab with rituximab and/or bendamustine. These findings were extended to in vivo xeno-graft models, where otlertuzumab plus bendamustine or rituximab resulted in a greater inhibition of tumor growth as compared to that attained with each individual drug. A three-drug regimen with otlertuzumab, rituximab, and bendamustine resulted in an even more profound inhibition of tumor growth compared to the two-drug combination. In addition, the observed efficacy of otlertuzumab with rituximab could be extended through repeated (maintenance) dosing leading to tumor growth delay and overall survival well beyond the dosing period.
The first in human phase 1 trial of otlertuzumab demonstrated encouraging activity in patients with CLL and NHL [24]. The study indicated that otlertuzumab has single-agent clinical activity and appeared to be well tolerated in an advanced CLL and NHL patient population. A maximum tolerated dose (MTD) was not determined in clinical studies of otlertuzumab in patients with CLL. Based on the pharmaco-kinetic modelling the 20 mg/kg dose was expected to produce trough levels ten-fold higher than the in vivo concentration that induced antibody-dependent cell-mediated cytotoxicity (ADCC) or apoptosis.
Thus, we hypothesized that the addition of otlertuzumab to rituximab and bendamustine could further improve the response in relapsed indolent lymphoma patients. The study reported here evaluates the safety, pharmacokinetics, and efficacy of otlertuzumab, bendamustine, and rituximab in subjects with relapsed indolent lymphoma.
Patients, materials, methods
Eligibility criteria and study design
Previously treated patients≥18 years of age with a histologically confirmed diagnosis of indolent non-Hodgkin's B-cell lymphoma (i.e., follicular lymphoma, small lymphocytic lymphoma, and marginal zone lymphoma) that had relapsed (relapsed was defined as confirmed progressive disease (PD) after receiving the most recent prior therapy, or failure to achieve at least a partial response (PR) while receiving the most recent prior therapy) with bi-dimensionally measurable disease including at least one lesion measuring≥1.5 cm in a single dimension were eligible to enroll. Patients were required to have the following: an Eastern Cooperative Oncology Group (ECOG) performance status≤2; creatinine clearance>40 mL/min as calculated by the Cockcroft-Gault method; total bilirubin, serum glutamic oxaloacetic transaminase (SGOT), and serum glutamate pyruvate transaminase (SGPT)≤2.0 × upper limit of normal (ULN); an absolute neutrophil count (ANC)≥1,000/mm3; and a platelet count≥100,000/mm3. Patients were required to have discontinued all previous anti-cancer or investigational therapy at least 28 days prior to study therapy. Patients were not eligible if they had received otlertuzumab, had previously discontinued treatment with rituximab due to unresolved toxicity; were refractory to bendamustine; received therapeutic corticosteroids at doses equivalent to>10 mg prednisone per day for longer than 5 days within 14 days prior to the first dose of study drug; received filgrastim or equivalent within 14 days prior to screening or pegfilgrastim within 28 days prior to screening; had prior stem cell transplant or prior autologous stem cell transplant within 12 months prior to the first dose of study drug; received blood or platelet infusion within 7 days prior to screening; had previous or concurrent additional malignancy except non-invasive, non melanomatous skin cancer or in situ carcinoma of the cervix, or other solid tumors if the subject had been disease-free for a minimum of 2 years prior to the first dose of study drug; central nervous system or leptomeningeal lymphoma; any significant concurrent medical diseases or conditions; an allergy to mannitol; history of positive serology for human immunodeficiency virus; positive serology for hepatitis B or hepatitis C; were pregnant or breastfeeding; or had other severe, acute, or chronic medical or psychiatric condition, laboratory abnormality, or difficulty complying with protocol requirements that could increase the risk associated with study participation or study drug administration or could interfere with safety.
The objective of this study was to evaluate the safety of 10 mg/kg and 20 mg/kg dose levels of otlertuzumab given in combination with rituximab and bendamustine in subjects with relapsed indolent lymphoma. In addition, this study investigated the pharmacokinetics and pharmacodynamics of otlertuzumab.
Dose limiting toxicity included the following toxicities that occurred during Cycle 1: Grade 4 hematological toxicity that had not resolved to≤Grade 2 within 2 weeks of the time that initiation of Cycle 2 was scheduled; ≥Grade 3 nonhematological adverse event, except for Grade 3 fatigue, Grade 3 infection, Grade 3 nausea, and Grade 3 infusion reaction; Grade 3 nausea for more than 5 days despite adequate treatment; Grade 4 febrile neutropenia; Grade 4 acute infusion-related reaction, allergic reaction, anaphylaxis, or cytokine release syndrome; recurrent≥Grade 3 acute infusion-related reaction, allergic reaction, anaphylaxis, or cytokine release syndrome; Grade 5 toxicities.
Dosing
Otlertuzumab was administered by intravenous (IV) infusion over 2 to 3 h at a dose of 10 mg/kg or 20 mg/kg on days 1 and 15 of each 28 day cycle for 6 cycles. The starting dose and schedule were below the maximum tolerated dose (MTD) of 30 mg/kg determined from the previous dose-escalation study using single-agent otlertuzumab in CLL and NHL patients. Bendamustine (90 mg/m2) was administered on days 1 and 2 of each cycle and rituximab (375 mg/m2) was administered on day 2 of each cycle.
Otlertuzumab was supplied by Emergent BioSolutions (Seattle, WA). Bendamustine and rituximab were purchased commercially.
Safety assessments
Toxicity was assessed at each evaluation according to the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
No reduction in otlertuzumab and rituximab dosing was permitted. After Cycle 1, in the event of Grade 4 hematological or≥Grade 3 non-hematological toxicities, the dose of bendamustine was to be reduced to 60 mg/m2. If Grade 4 hematological or≥Grade 3 non-hematological toxicities recurred with 60 mg/m2 bendamustine, the subject had to discontinue study treatment. Use of growth factors was allowed during Cycles 2 through 6, but not during Cycle 1.
Response assessments
Response was assessed by the investigator on the basis of clinical, radiological, and pathological (i.e., bone marrow) criteria, using the Revised Response Criteria for Malignant Lymphoma [25]. CT scans and response assessment was performed between Day 15 and 28 of cycles 2, 4, and 6, and 60 days after end of treatment visit. A bone marrow aspirate and biopsy were performed between Day 15 and Day 28 of an even-numbered cycle if a complete response (CR) was observed and bone marrow was involved by lymphoma prior to initiation of study drug. FDG-PET was not utilized to determine response.
Pharmacokinetic analyses
Serum samples for PK analysis were analyzed by a qualified and sensitive ELISA assay specifically developed for otlertuzumab using a monoclonal antibody specific for the CD37 binding domain of otlertuzumab, which is used to capture and detect otlertuzumab in serum with a standard bridging ELISA format. Actual times after otlertuzumab dose administration for individual subjects were used in all pharmacokinetic calculations; however, the proscribed times were used for graphing. Patients not receiving a full dose of otlertuzumab were excluded from pharmacokinetic parameter calculations such as mean Cmax and total AUC. Values for Cmax and time to reach Cmax (Tmax) were obtained by direct inspection of data. Area under the concentration-time curve (AUCt) was determined by the log-linear trapezoidal rule from time 0 to the last observed concentration (Ct) at time t using GraphPad Prism® Version 6.01 (GraphPad Software, San Diego CA). Otlertuzumab pharmacokinetic parameters were estimated using validated WinNonlin Professional Version 6.3 software (Pharsight Corporation, Mountain View, CA) with non-compartmental methods when a patient had sufficient late time points available for PK analysis. Individual concentration-time profiles were plotted and the terminal disposition rate constant (λz) was determined by the log-linear regression of at least 3 points judged to be in the terminal phase. Descriptive statistics, such as means, standard deviations, and precision (% CV) were calculated for variables using Microsoft® Excel® 2010 (Microsoft Corporation, Redmond, WA).
Statistical methods
Data analyses were based on descriptive statistics. For continuous variables, these statistics included the following: mean, median, standard deviation, minimum, and maximum. Final study analyses were conducted after the last patient stopped study treatment and response was assessed using intent-to-treat analysis. Time-to-event variables were described using Kaplan-Meier estimates, as well as mean and median time with 2-sided 80 % confidence intervals of the mean and median [26].
Results
Patient characteristics and treatment
Twelve adult patients, 6 in each dose group, were enrolled at 4 sites between May and October 2011. Demographic characteristics are summarized in Table 1 and Table 2. The median age of patients was 57 years (range, 51 to 79 years). Nine patients had follicular lymphoma and 3 had small lymphocytic lymphoma. Four patients had bulky disease (≥5 cm). Seven of 11 patients were Ann Arbor stage III or IV and Follicular Lymphoma International Prognostic Index (FLIPI) scores at study entry were: 2 high, 4 intermediate, and 6 low. All patients had relapsed after prior rituximab including 7 refractory to their most recent previous treatment. Prior therapies included CHOP-R (cyclophosphamide, doxorubicin vincristine, and prednisone) in 8 patients, RICE (rituximab, ifosfamide, carboplatin, etoposide) in 5 patients, single agent rituximab in 5 patients, and autologous transplant in 2 patients. No patients had received prior bendamustine. The median number of prior regimens was 3 with a range of 1 to 4. Time since last treatment was more than 1 year in 7 patients. The median numbers of otlertuzumab infusions was 12 (range, 4–12). Seven patients completed all 6 cycles. Two patients were responding but discontinued therapy to receive a transplant.
Table 1.
Summary of Demographics
| 10 mg/kg (N=6) | 20 mg/kg (N=6) | Total (N=12) | |
|---|---|---|---|
| Age (year) | |||
| Median | 56.5 | 62.0 | 57.0 |
| (Min, Max) | (51, 65) | (54, 79) | (51, 79) |
| Sex | |||
| Male, n (%) | 5 (83.3 %) | 3 (50.0 %) | 8 (66.7 %) |
| Female, n (%) | 1 (16.7 %) | 3 (50.0 %) | 4 (33.3 %) |
| Race | |||
| White, n (%) | 5 (83.3 %) | 6 (100.0 %) | 11 (91.7%) |
| Black, n (%) | 1 (16.7 %) | 0 | 1 (8.3 %) |
| β2-microglobulin (mg/L) | |||
| Median | 2.6 | 2.1 | 2.3 |
| (Min, Max) | (0.3, 4.3) | (1.8, 6.3) | (0.3, 6.3) |
| β2-microglobulin (mg/L) group | |||
| n | 6 | 5 | 11 |
| 0–3.0, n (%) | 5 (83.3 %) | 4 (66.7 %) | 9 (75.0 %) |
| 3.1–4.0, n (%) | 0 | 0 | 0 |
| 4.1–5.0, n (%) | 1 (16.7 %) | 0 | 1 (8.3 %) |
| 5.1–10.0, n (%) | 0 | 1 (16.7 %) | 1 (8.3 %) |
| >10.0, n (%) | 0 | 0 | 0 |
| Direct Anti-globulin Test (DAT) | |||
| n | 6 | 5 | 11 |
| Positive, n (%) | 0 | 0 | 0 |
| Negative, n (%) | 6 (100.0 %) | 5 (83.3 %) | 11 (91.7%) |
| Sum of product diameters (SPD), cm2 | |||
| Median | 39.5 | 22.7 | 27.9 |
| (Min, Max) | (9.6, 77.6) | (1.9, 80.4) | (1.9, 80.4) |
Mm, Minimum; Max, Maximum; STD, Standard deviation
Table 2.
Baseline Characteristics and Subject Response
| Subject | Age | Gender | Histology | FLIPI | Bulky Disease (≥5 cm) | Ann Arbor Staging at Diagnosis | Prior NHL Lines of Therapy | Number of Study Drug Cycles | Responsea |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle 2 Day 15–28 | Cycle 4 Day 15–28 | Cycle 6 Day 15–28 | Follow up Day 60 | |||||||||
| Dose Cohort 1:10 mg/kg Otlertuzumab | ||||||||||||
| 1301 | 56 | Male | SLL | Int | No | IV | 1 | 6 | CR | CR | CR | CR |
| 1302 | 55 | Female | FL | Int | No | IV | 3 | 6 | CR | CR | CR | CR |
| 1901 | 60 | Male | FL | Low | Yes | II | 1 | 6 | PR | PR | PR | CR |
| 1103 | 51 | Male | FL | Low | No | I | 1 | 6 | SD | SD | SD | SD |
| 1303 | 57 | Male | SLL | Int | Yes | ND | 3 | 2 | PD | Progression of disease | ||
| 1104 | 65 | Male | FL | Low | No | III | 4 | 6 | PR | PR | PR | PR |
| Dose Cohort 2: 20 mg/kg Otlertuzumab | ||||||||||||
| 1106 | 55 | Female | FL | Low | No | III | 4 | 5 | PR | PR | Discontinued-transplant | |
| 1105 | 57 | Male | FL | Low | No | IV | 3 | 3 | PR | Discontinued-AEb | ||
| 1304 | 54 | Female | FL | High | No | III | 4 | 2 | CR | Discontinued-transplant | ||
| 1107 | 74 | Female | FL | Low | Yes | IV | 4 | 6 | PR | PR | PR | PR |
| 1402 | 67 | Male | FL | Int | No | II | 1 | 3 | CR | Discontinued-ANC did not recoverc, started other treatment | ||
| 1902 | 79 | Male | SLL | High | Yes | IV | 2 | 6 | PR | PR | PR | PR |
Abbreviations: AE, Adverse event; CR, Complete response; FL, Follicular lymphoma; FLIPI, follicular lymphoma international prognostic index; Int, Intermediate; NHL, Non-Hodgkin's lymphoma; ND, Not done; PD, Progressive disease; PR, Partial response; SD, Stable disease; SLL, Small lymphocytic lymphoma
Response assessed using CT scan. Last assessment at Day 60 follow up
Subject prematurely discontinued the study drugs due to an AE of Grade 2 thrombocytopenia. The subject later experienced an SAE of Grade 3 myelodysplastic syndrome and subsequently discontinued from the study
Per protocol, subject was discontinued because ANC did not recover within 2 weeks
As shown in Table 2, in the 10 mg cohort, 5 patients completed treatment and 1 patient discontinued after Cycle 1 due to disease progression. In the 20 mg cohort, 2 patients completed treatment, 2 discontinued study drug early (after Cycle 2 and Cycle 4) to receive a transplant, and 2 discontinued due to adverse events (one for thrombocytopenia and myelodysplastic syndrome and 1 for neutropenia as required by the protocol).
Toxicities
Adverse event data are summarized in Table 3. No DLTs were observed. The most frequently reported (>25 %) adverse events were neutropenia, nausea, fatigue, decreased white blood cells, and insomnia. Grade 1 and 2 infections were reported for 3 patients in the 20 mg/kg cohort, one grade 1 infection was considered related to otlertuzumab. Severe (grade 3/4) adverse events reported in more than 1 patient were neutropenia (5 patients), decreased neutro-phil count (3 patients), decreased white blood cell count, hypophosphatemia, lymphopenia, pulmonary thrombosis (2 patients each), and nausea, abdominal pain, lymphocyte count decreased, and hyperglycemia (1 patient each). Eight serious adverse events were reported in 5 patients. Three patients had serious adverse events considered related to otlertuzumab; one patient had neutropenia, one had pulmonary artery thrombosis, and one had pulmonary artery thrombosis, deep vein thrombosis, and retinal vein occlusion.
Table 3.
Summary of All Treatment-Emergent Adverse Events by Preferred Term in≥2 Subjects Overall
| Preferred Term | 10 mg/kg (N=6) | 20 mg/kg (N=6) | Total (N=12) |
|
|---|---|---|---|---|
| All Events | Grade 3/4 | |||
| Subjects who reported at Adverse Event (%) | 6 (100.0 %) | 6 (100.0 %) | 12 (100.0%) | 10 (83.3 %) |
| Neutropenia | 5 (83.3 %) | 2 (33.3 %) | 7 (58.3 %) | 5 (41.6 %) |
| Nausea | 3 (50.0 %) | 4 (66.7 %) | 7 (58.3 %) | 1 (8.3 %) |
| Fatigue | 3 (50.0 %) | 4 (66.7 %) | 7 (58.3 %) | 0 |
| White blood cell count decreased | 3 (50.0 %) | 1 (16.7 %) | 4 (33.3 %) | 2 (16.7 %) |
| Insomnia | 2 (33.3 %) | 2 (33.3 %) | 4 (33.3 %) | 0 |
| Anaemia | 2 (33.3 %) | 1 (16.7 %) | 3 (25.0 %) | 0 |
| Thrombocytopenia | 0 | 3 (50.0 %) | 3 (25.0 %) | 0 |
| Abdominal pain | 0 | 3 (50.0 %) | 3 (25.0 %) | 1 (8.3 %) |
| Diarrhoea | 1 (16.7 %) | 2 (33.3 %) | 3 (25.0 %) | 0 |
| Vomiting | 1 (16.7 %) | 2 (33.3 %) | 3 (25.0 %) | 0 |
| Infections and infestations | 0 | 3 (50.0 %) | 3 (25.0 %) | 0 |
| Neutrophil count decreased | 3 (50.0 %) | 0 | 3 (25.0 %) | 3 (25 %) |
| Hypophosphatasemia | 3 (50.0 %) | 0 | 3 (25.0 %) | 2 (16.7 %) |
| Headache | 2 (33.3 %) | 1 (16.7 %) | 3 (25.0 %) | 0 |
| Productive cough | 0 | 3 (50.0 %) | 3 (25.0 %) | 0 |
| Leukopenia | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 0 |
| Lymphopenia | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 2 (16.7 %) |
| Constipation | 0 | 2 (33.3 %) | 2 (16.7 %) | 0 |
| Chills | 0 | 2 (33.3 %) | 2 (16.7 %) | 0 |
| Pyrexia | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 0 |
| Alanine aminotransferase increased | 2 (33.3 %) | 0 | 2 (16.7 %) | 0 |
| Lymphocyte count decreased | 2 (33.3 %) | 0 | 2 (16.7 %) | 1 (8.3 %) |
| Hyperglycemia | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 1 (8.3 %) |
| Arthralgia | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 0 |
| Back pain | 0 | 2 (33.3 %) | 2 (16.7 %) | 0 |
| Cough | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 0 |
| Dyspnea exertional | 0 | 2 (33.3 %) | 2 (16.7 %) | 0 |
| Pulmonary thrombosis | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 2 (16.7 %) |
| Urticaria | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 0 |
| Flushing | 1 (16.7 %) | 1 (16.7 %) | 2 (16.7 %) | 0 |
Ten of the 12 patients completed all 6 cycles. Discontinuation due to adverse events occurred in 2 patients (17 %), both in the 20 mg/kg cohort; one patient who had 3 prior regimens including autologous transplant developed myelodysplastic syndrome after 3 cycle of study drug and one with 1 prior regimen had grade 4 neutropenia attributed to bendamustine after cycle 3. The protocol required discontinuation after 2 weeks if the absolute neutrophil counts did not recover. These patients had PR and CR, respectively. In this small study, there was no apparent relationship between dose and toxicity.
Clinical responses
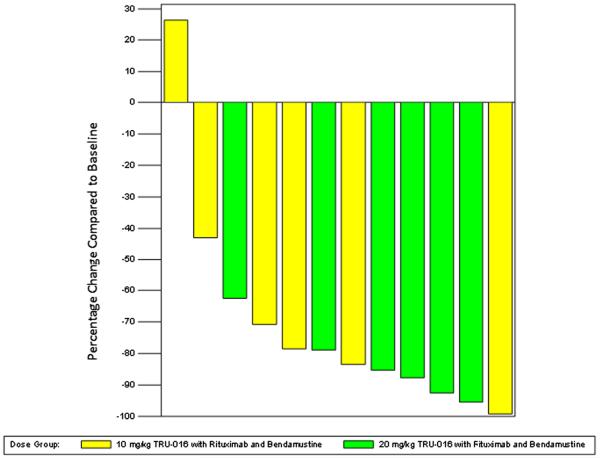
The overall response rate was 83.3 % for all 12 subjects with 4 of 6 (67 %) in the 10 mg/kg cohort and 6 of 6 (100 %) in the 20 mg/kg dose cohort having a response. Three patients in the 10 mg/kg cohort and 1 in the 20 mg/kg cohort had a complete response (CR). Three of the 4 patients with bulky disease and 5 of the 7 patients refractory to last treatment responded to the regimen. All responses were seen in Cycle 2 and these patients had not progressed at the 60 day follow up assessment (Table 2). Reduction in lymph node size was observed in 11 (92 %) patients at completion of study treatment (Fig. 1).
Fig. 1.
Lymph Node Size. Lymph node sum of product diameters from CT Scans obtained during screening were compared to CTscans with the highest reduction in the sum of product diameters
Pharmacokinetics and pharmacodynamics
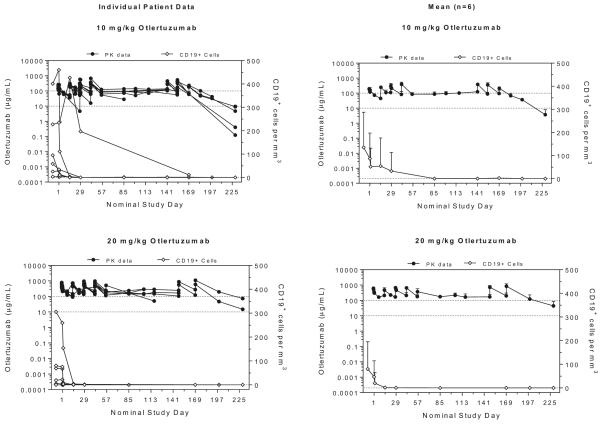
Serum concentrations of otlertuzumab were measured at multiple time points throughout the dosing cycle and mean concentrations and CD19+ cells per mm3 over time are plotted by dose group in Fig. 2. Systemic exposure for subjects, or the AUC, was more variable following multiple doses, because not all subjects completed 6 full treatment cycles. For subjects treated with 10 mg/kg, 5 of 6 subjects were able to complete all 6 treatment cycles, whereas only 2 of 6 subjects dosed with 20 mg/kg completed 6 treatment cycles with otlertuzumab. At these doses and this schedule, concentration was maintained throughout the treatment period. For all but one patient, circulating CD19+ cells decreased to 2 or fewer cells/mm3 by Day 29.
Fig. 2.
Otlertuzumab concentration and number of CD19+ Cells per mm3
Table 4 and Table 5 contain PK parameter estimates for each patient and include group means, standard deviations, and a measure of precision (coefficient of variation).
Table 4.
PK Parameter Estimates After the Last Doses of 10 or 20 mg/kg
| Subject | Dose mg/kg | Doses of Otlertuzumab | Rsq adjusted | # points lambda z | HL day | Tmax day | Cmax μg/mL | Cmax/Dose kg*μg/mL/mg | AUC day*μg/mL | AUC/dose day*kg*μg/mL/mg | V mL/kg | CL mL/day/kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1103 | 10 | 12 | 0.970 | 5 | 7.80 | 0.20 | 288.95 | 28.90 | 1,240 | 124 | 66.6 | 5.91 |
| 1104 | 10 | 12 | 0.865 | 3 | 10.02 | 0.20 | 264.801 | 26.48 | 1,210 | 121 | 74.9 | 5.18 |
| 1301 | 10 | 12 | 0.971 | 3 | 7.42 | 0.20 | 205.459 | 20.55 | 1,066 | 107 | 71.9 | 6.71 |
| 1302 | 10 | 12 | 0.987 | 4 | 10.71 | 0.20 | 251.469 | 25.15 | 1,361 | 136 | 70.7 | 4.58 |
| 1303 | 10 | 4 | 0.999 | 5 | 5.84 | 0.20 | 167.061 | 16.71 | 1,315 | 132 | 75.6 | 8.98 |
| 1901 | 10 | 12 | 0.943 | 4 | 10.75 | 0.25 | 353.28 | 35.33 | 1,908 | 191 | 33.3 | 2.14 |
| Mean | 8.76 | 255.17 | 25.52 | 1,350 | 135 | 65.48 | 5.58 | |||||
| SD | 2.03 | 64.97 | 6.50 | 292 | 29 | 16.11 | 2.28 | |||||
| CV% | 23.20 | 25.5 | 25.5 | 21.6 | 21.6 | 24.6 | 40.8 | |||||
| 1105 | 20 | 5 | 0.996 | 3 | 11.35 | 0.20 | 586.742 | 29.34 | 2,944 | 147 | 66.7 | 4.07 |
| 1106 | 20 | 10 | 0.993 | 3 | 7.16 | 0.20 | 737.957 | 36.90 | 2,870 | 144 | 55.3 | 5.35 |
| 1107 | 20 | 12 | 1.000 | 3 | 8.51 | 0.20 | 602.977 | 30.15 | 2,753 | 138 | 67.5 | 5.50 |
| 1304 | 20 | 4 | 0.781 | 4 | 16.97 | 0.20 | 393.394 | 19.67 | 2,908 | 145 | 92.6 | 3.78 |
| 1402 | 20 | 6 | 0.955 | 4 | 8.96 | 0.20 | 777.885 | 38.89 | 3,323 | 166 | 50.0 | 3.87 |
| 1902 | 20 | 12 | 0.789 | 5 | 6.98 | 0.20 | 591.573 | 29.58 | 2,626 | 131 | 55.1 | 5.47 |
| Mean | 9.99 | 615.1 | 30.75 | 2,904 | 145 | 64.52 | 4.67 | |||||
| SD | 3.76 | 135.8 | 6.79 | 236 | 12 | 15.40 | 0.85 | |||||
| CV% | 37.69 | 22.1 | 22.1 | 8.1 | 8.1 | 23.9 | 18.1 |
Abbreviations: STD, Standard deviation; CV%, coefficient of variation; RSQ adjusted, Goodness of fit statistic for the terminal elimination phase adjusted for the number of points used in the estimation of λz; # points lambda z=number of points in the terminal elimination phase used to calculate λz; HL, Apparent terminal elimination half life; Tmax, Time to reach Cmax; Cmax, Maximum observed concentration, occurring at Tmax; Cmax/Dose, Maximum observed concentration normalized by dose; V, Volume of distribution based on the terminal phase; CL, Systemic clearance; AUC, Area under the concentration-time curve
Table 5.
PK Parameter Estimates After a Single 10 or 20 mg/kg Dose
| Subject | Dose mg/kg | Rsq adjusted | # points lambda z | Lambda z lower day | Lambda z upper day | HL day | Tmax day | Cmax μg/mL | Cmax/Dose kg*μg/mL/mg | AUC day*μg/mL | AUC/dose day*kg*μg/mL/mg | V mL/kg | CL mL/day/kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1104 | 10 | 0.9766 | 3 | 154.2 | 259 | 10.49 | 42.20 | 681.0 | 68.10 | 37,903 | 3,790 | 26.45 | 1.748 |
| 1301 | 10 | 0.9902 | 4 | 167.2 | 226 | 11.14 | 42.20 | 257.0 | 25.70 | 18,360 | 1,836 | 55.80 | 3.471 |
| 1302 | 10 | 0.9998 | 3 | 169.2 | 199 | 12.03 | 155.20 | 337.9 | 33.79 | 23,765 | 2,377 | 43.14 | 2.486 |
| 1901 | 10 | 0.9989 | 3 | 161 | 226 | 15.10 | 42.20 | 495.81 | 49.58 | 32,920 | 3,292 | 36.41 | 1.672 |
| Mean | 12.19 | 442.9 | 44.29 | 28,237 | 2,824 | 40.45 | 2.34 | ||||||
| STD | 2.04 | 187.1 | 18.71 | 8,811 | 881 | 12.32 | 0.84 | ||||||
| CV% | 16.74 | 42.3 | 42.3 | 31.2 | 31.2 | 30.5 | 35.7 | ||||||
| 1105 | 20 | 0.8425 | 3 | 56.2 | 114 | 18.80 | 14.00 | 720.569 | 36.03 | 23,887 | 1,194 | 54.49 | 2.009 |
| 1107 | 20 | 0.9618 | 3 | 162.2 | 220 | 10.76 | 27.20 | 652.373 | 32.62 | 45,822 | 2,291 | 38.75 | 2.495 |
| 1902 | 20 | 0.9065 | 4 | 154.2 | 203 | 13.30 | 154.20 | 1,100.950 | 55.05 | 63,145 | 3,157 | 24.44 | 1.274 |
| Mean | 14.29 | 824.6 | 41.23 | 44,285 | 2,214 | 39.23 | 1.93 | ||||||
| STD | 4.11 | 241.7 | 12.09 | 19,674 | 984 | 15.03 | 0.62 | ||||||
| CV% | 28.75 | 29.3 | 29.3 | 44.4 | 44.4 | 38.3 | 31.9 |
Abbreviations: STD, Standard deviation; CV%, Coefficient of variation; RSQ adjusted, Goodness of fit statistic for the terminal elimination phase adjusted for the number of points used in the estimation of λz; # points lambda z=number of points in the terminal elimination phase used to calculate λz; HL, apparent terminal elimination half life; Tmax, Time to reach Cmax; Cmax, Maximum observed concentration, occurring at Tmax; Cmax/Dose, Maximum observed concentration normalized by dose; V, volume of distribution based on the terminal phase; CL, systemic clearance; AUC, area under the concentration-time curve
After single IV doses of otlertuzumab, a biphasic pattern of decline was apparent in the concentration-time curves, and observed concentrations were consistent with a 2 compartment model. The Cmax or peak concentration for otlertuzumab occurred during or shortly after the first IV infusion for all subjects, and Cmax for each patient normalized by dose was similar for both treatment groups being 28.14 kg*μg/mL/mg. After a single infusion, otlertuzumab exposure normalized by dose (AUC/dose) was also similar for subjects treated with 10 or 20 mg/kg, being 140.2 day*kg*μg/mL/mg. Additional PK estimates such as mean CL and Vz were also similar for both dose levels, being 5.3 mL/day/kg and 65.3 mL/kg, respectively.
The mean terminal T1/2 was calculated to be 8 and 10 days after a single dose of otlertuzumab for NHL patients dosed with 10 and 20 mg/kg, respectively, and it ranged from 6 to 17 days. After multiple doses of otlertuzumab, mean T1/2 increased to 12 and 14 days for subjects dosed with 10 and 20 mg/kg, respectively, and it ranged from 10 to 18 days. Serum T1/2 of otlertuzumab was not significantly affected by dose, when comparing single or multiple doses, as determined using Graphpad Prism Software, version 6.01 to run a one-way analysis of variance (ANOVA) test and Tukey's multiple comparisons test.
Maximum serum drug concentration values after multiple doses of otlertuzumab were determined using direct inspection of concentration data, and values were higher after multiple doses (Table 4) when compared to Cmax values from a single dose (Table 5). For each dose group (10 and 20 mg/kg) there was not a significant difference between otlertuzumab Cmax values following single or multiple doses (p<0.05) as determined by the same comparison tests run above. However, when comparing Cmax values from subjects dosed with 10 or 20 mg/kg otlertuzumab, differences were statistically significant (p<0.05) between dose levels after single and multiple doses. Time to reach Cmax ranged from 14 to 155 days following multiple doses of otlertuzumab, and once again Cmax normalized by dose was very similar for both dose levels being 43 kg*μg/mL/mg. Both volume and clearance estimates decreased after multiple doses of otlertuzumab, as would be expected when clearance mechanisms become saturated.
Subject systemic exposure to otlertuzumab or the AUC demonstrated greater variability following multiple doses, because not all subjects completed 6 full treatment cycles. For subjects treated with 10 mg/kg, 5 of 6 subjects were able to complete all 6 treatment cycles, whereas only 2 of 6 subjects dosed with 20 mg/kg completed 6 treatment cycles with otlertuzumab. However, data still show that with increasing doses of otlertuzumab, there appeared to be a proportional increase in AUC and Cmax after single or multiple doses of otlertuzumab, even though AUC after a single dose is more likely to better characterize the dose response during dose escalation.
Discussion
In this multicenter phase Ib study in patients with relapsed/refractory NHL we have shown that otlertuzumab in combination with bendamustine and rituximab was tolerated at a dose of 20 mg/kg with reductions in lymph node size as measured by standard response criteria. Clinical efficacy was observed in this subject population with relapsed indolent lymphoma, with an objective response in most patients. All responses were observed early after 2 treatment cycles.
No DLTs were observed in either dose cohort; therefore, on this dosing schedule and in this subject population, the MTD of otlertuzumab when combined with rituximab and bendamustine was found to be at least 20 mg/kg.
Ten of 12 patients completed all 6 cycles of treatment. Although 83 % of patients had a grade 3 or 4 adverse event, most did not lead to discontinuation of study drug. Two events of pulmonary thrombosis were reported in this study. Pulmonary thrombosis, deep vein thrombosis, or retinal vein thrombosis are reported in elderly subjects and in those subjects with lymphoma. Caruso et al.[27] performed a meta-analysis of 29 independent cohorts including 18,018 subjects and 1,149 thrombotic events and found an incidence rate of symptomatic thrombosis of 6.5 % in subjects with NHL. No literature could be found on asymptomatic cases of thrombosis in lymphoma subjects, but repeated use of modern high-resolution, contrast enhanced CT imaging may have increased our ability to detect small emboli when compared to historical experiences. The two cases with pulmonary thrombosis were discovered incidentally on routine CT scans for disease assessment. Both subjects were treated and completed therapy with study drugs. One case of deep vein thrombosis and one of retinal vascular occlusion have been reported in over 170 subjects with CLL treated with otlertuzumab.
The mean terminal elimination half-life for otlertuzumab was 8 and 10 days following a single dose of otlertuzumab, and 12 and 14 days following multiple doses of 10 or 20 mg/kg respectively. This is similar to the half-life of approximately 9.5 days seen in NHL patients and the half-life of approximately 9 days seen in CLL patients when treated with single agent otlertuzumab. The observed kinetics should yield continuous saturation of all available CD37 sites on B-cells throughout the treatment course
Biopsies of the malignant tissues were not obtained so it may be possible that CD37 was not adequately expressed. However, the literature notes that CD37 is present on most but not all B-cell NHL tumors [28–34]
The proposed mechanism of action of otlertuzumab is two-fold; antibody dependent cytotoxicity and direct apoptosis. CD37 ligation results in phosphorylation and activation of the ITIM-like motif at the N-t of CD37 by LYN kinase, leading to SHP1 recruitment and FoxO3a-dependent BIM upregulation with subsequent mitochondrial depolarization and cell death [35]. This mechanism of action for direct apoptosis is unique and different from the mechanism of action for rituximab [36], bendamustine [37], and other agents on the market. Consequently, other combinations may be active and should be explored. For example, the combination of SMIP-016 (murine version of otlertuzumab) and the PI3Kδ isoform-specific inhibitor idelalisib (CAL-101) demonstrated in vitro synergy [35].
The importance of dual B-cell antigen targeting with otlertuzumab and rituximab along with bendamustine in the context of developing novel approaches for patients with indolent B-NHL stems from the potential to improve response rates and possibly outcomes without an appreciable increase in toxicity. Our data suggest that adverse event rates were comparable to those reported with bendamustine-rituximab combinations [38, 1, 39]. Likewise, response rates were also encouraging, although larger trials will be necessary to validate these findings.
In summary, otlertuzumab combined with rituximab and bendamustine was tolerated with predictable and manageable toxicity and showed clinical efficacy in patients with indolent relapsed lymphoma, with a 100 % objective response rate in the 20 mg/kg dose cohort. Further evaluation of otlertuzumab combined with rituximab and bendamustine in Phase 2 studies is warranted.
Acknowledgments
AKG is a Scholar in Clinical Research of the Leukemia and Lymphoma Society and is supported in part by the Fred Hutchinson Cancer Research Center-University of Washington Cancer Consortium Cancer Center Support Grant of the National Institutes of Health (P30 CA015704), a grant from the Life Sciences Discovery Fund of Washington State and a philanthropic gift from Frank and Betty Vandermeer. DJG is supported by a mentored career development award (K08) from the NCI (CA151682).
NCI Clinical Trials Network registration: NCT01317901.
Footnotes
Presented at ASH Annual Meeting 2012
Conflict of interest AJE and SCS are employees of Emergent Biosolutions. The other authors have no conflict of interest.
References
- 1.Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, Josten KM, Durk H, Rost A, Neise M, von Grunhagen U, Chow KU, Hansmann ML, Hoelzer D, Mitrou PS. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(15):3383–3389. doi: 10.1200/JCO.2005.08.100. doi:10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 2.Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, Schwartzberg LS, Lemieux B, Cheson BD. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(27):4473–4479. doi: 10.1200/JCO.2008.17.0001. doi:10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- 3.Fowler NH, Advani RH, Sharman JP, Smith SM, McGreivy J, Kunkel L, Troung V, Zhou C, Boyd TE. The Bruton's Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Is Active and Tolerated in Relapsed Follicular Lymphoma American Society of Hematology Annual Meeting; December 7–10, 2013; New Orleans, LA: 2013. [Google Scholar]
- 4.Witzig TE. Current treatment approaches for mantle-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(26):6409–6414. doi: 10.1200/JCO.2005.55.017. doi:10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, Kaplan H, Voralia M, Pietronigro D, Takeshita K, Ervin-Haynes A, Zeldis JB, Vose JM. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(32):5404–5409. doi: 10.1200/JCO.2008.21.1169. doi:10.1200/JCO.2008.21.1169. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, Porrata LF, Ansell SM, Colgan JP, Jacobsen ED, Ghobrial IM, Habermann TM. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25(2):341–347. doi: 10.1038/leu.2010.226. doi:10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobinai K, Ogura M, Maruyama D, Uchida T, Uike N, Choi I, Ishizawa K, Itoh K, Ando K, Taniwaki M, Shimada N, Kobayashi K. Phase I study of the oral mammalian target of rapamycin inhibitor everolimus (RAD001) in Japanese patients with relapsed or refractory non-Hodgkin lymphoma. Int J Hematol. 2010;92(4):563–570. doi: 10.1007/s12185-010-0707-5. doi:10.1007/s12185-010-0707-5. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B. Clinical efficacy and management of temsirolimus in patients with relapsed or refractory mantle cell lymphoma. Clinical lymphoma, myeloma & leukemia. 2013;13(4):351–359. doi: 10.1016/j.clml.2013.04.003. doi:10.1016/j.clml.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Bouabdallah K, Ribrag V, Terriou L, Soria JC, Delarue R. Temsirolimus in the treatment of mantle cell lymphoma: frequency and management of adverse effects. Curr Opin Oncol. 2013;25(Suppl 2):S1–12. doi: 10.1097/CCO.0b013e32835de8ee. doi:10.1097/CCO.0b013e32835de8ee. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Tang H, Kurtin PJ, Koenig PA, Inwards DJ, Shah K, Ziesmer SC, Feldman AL, Rao R, Gupta M, Erlichman C, Witzig TE. Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. The lancet oncology. 2011;12(4):361–368. doi: 10.1016/S1470-2045(11)70062-6. doi:10.1016/S1470-2045(11)70062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal AK, Kahl BS, DeVos S, Wagner-Johnston ND, Schuster SJ, Blum KA, Wojciech JJ, Flinn IW, Flowers CR, Martin P, Viardot A, Goy A, Davies A, Zinzani PL, Dreyling M, Holes LM, Li D, Dancey R, Godfrey WR, Salles GA. Mature response data from a phase 2 study of PI3K-delta inhibitor idelalisib in patients with double (rituximab and alkylating agent)-refractory indolent B-cell Non-Hodgkin lymphoma (iNHL) Blood. 2013;122(21):85. [Google Scholar]
- 12.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24(2):203–216. doi: 10.1016/j.beha.2011.02.009. doi:10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd J, Lapalombella R, Ramanunni A, Andritsos L, Flynn J, Baum P, Thompson P, Muthusamy N. Effect of CD37 small modular immuno-pharmaceutical (SMIP) on direct apoptosis in chronic lymphocytic leukemia cells via transcriptional up-regulation of the BH3 family member BIM. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:15S. [Google Scholar]
- 14.Barrena S, Almeida J, Yunta M, Lopez A, Fernandez-Mosteirin N, Giralt M, Romero M, Perdiguer L, Delgado M, Orfao A, Lazo PA. Aberrant expression of tetraspanin molecules in B-cell chronic lymphoproliferative disorders and its correlation with normal B-cell maturation. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2005;19(8):1376–1383. doi: 10.1038/sj.leu.2403822. doi:10.1038/sj.leu.2403822. [DOI] [PubMed] [Google Scholar]
- 15.Belov L, de la Vega O, dos Remedios CG, Mulligan SP, Christopherson RI. Immunophenotyping of leukemias using a cluster of differentiation antibody microarray. Cancer Res. 2001;61(11):4483–4489. [PubMed] [Google Scholar]
- 16.Campo E, Cardesa A, Alos L, Palacin A, Cobarro J, Traserra J, Montserrat E. Non-Hodgkin's lymphomas of nasal cavity and paranasal sinuses. An immunohistochemical study American journal of clinical pathology. 1991;96(2):184–190. doi: 10.1093/ajcp/96.2.184. [DOI] [PubMed] [Google Scholar]
- 17.Carbone A, Pinto A, Gloghini A, Volpe R, Zagonel V. B-zone small lymphocytic lymphoma: a morphologic, immunophenotypic, and clinical study with comparison to “well-differentiated” lymphocytic disorders. Hum Pathol. 1992;23(4):438–448. doi: 10.1016/0046-8177(92)90092-h. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, Baum PR, Lin TS, Jarjoura D, Lehman A, Kussewitt D, Lee RJ, Caligiuri MA, Tridandapani S, Muthusamy N, Byrd JC. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110(7):2569–2577. doi: 10.1182/blood-2006-12-062927. doi:10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112(3):830–835. doi: 10.1182/blood-2008-01-132142. doi:10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jilani I, O'Brien S, Manshuri T, Thomas DA, Thomazy VA, Imam M, Naeem S, Verstovsek S, Kantarjian H, Giles F, Keating M, Albitar M. Transient down-modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood. 2003;102(10):3514–3520. doi: 10.1182/blood-2003-01-0055. doi:10.1182/blood-2003-01-0055. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, Foley PL, Taylor RP. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101(3):1071–1079. doi: 10.1182/blood-2002-03-0876. doi:10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 22.Baum PR, Cerveny C, Gordon B. Evaluation of the effect of TRU-016, an anti-CD37 directed SMIP in combination with other therapeutic drugs in models of non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(15):8571. [Google Scholar]
- 23.Algate PW,J, Nilsson C, Sho M, Chao D, Starling GC, Byrd JC, Gordon B. TRU-016, An Anti-CD37 SMIPTM Biologic, In Combination with Other Therapeutic Drugs In Models of Non-Hodgkin's Lymphoma. Blood. 2010;116:3931. [Google Scholar]
- 24.Byrd JC, Pagel JM, Awan FT, Forero A, Flinn IW, Deauna-Limayo DP, Spurgeon SE, Andritsos LA, Gopal AK, Leonard JP, Eisenfeld AJ, Bannink JE, Stromatt SC, Furman RR. A phase 1 study evaluating the safety and tolerability of otlertuzumab, an anti-CD37 mono-specific ADAPTIR therapeutic protein in chronic lymphocytic leukemia. Blood. 2014;123(9):1302–1308. doi: 10.1182/blood-2013-07-512137. doi:10.1182/blood-2013-07-512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on L Revised response criteria for malignant lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. doi:10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Ellis S, Carroll KJ, Pemberton K. Analysis of duration of response in oncology trials. Contemporary clinical trials. 2008;29(4):456–465. doi: 10.1016/j.cct.2007.10.008. doi:10.1016/j.cct.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Caruso V, Di Castelnuovo A, Meschengieser S, Lazzari MA, de Gaetano G, Storti S, Iacoviello L, Donati MB. Thrombotic complications in adult patients with lymphoma: a meta-analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood. 2010;115(26):5322–5328. doi: 10.1182/blood-2010-01-258624. doi:10.1182/blood-2010-01-258624. [DOI] [PubMed] [Google Scholar]
- 28.Al-Sharabati M, Chittal S, Duga-Neulat I, Laurent G, Mazerolles C, Al-Saati T, Brousset P, Delsol G. Primary anterior mediastinal B-cell lymphoma. A clinicopathologic and immunohistochemical study of 16 cases. Cancer. 1991;67(10):2579–2587. doi: 10.1002/1097-0142(19910515)67:10<2579::aid-cncr2820671030>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Link MP, Bindl J, Meeker TC, Carswell C, Doss CA, Warnke RA, Levy R. A unique antigen on mature B cells defined by a monoclonal antibody. J Immunol. 1986;137(9):3013–3018. [PubMed] [Google Scholar]
- 30.Moore K, Cooper SA, Jones DB. Use of the monoclonal antibody WR17, identifying the CD37 gp40–45 Kd antigen complex, in the diagnosis of B-lymphoid malignancy. J Pathol. 1987;152(1):13–21. doi: 10.1002/path.1711520103. doi:10.1002/path.1711520103. [DOI] [PubMed] [Google Scholar]
- 31.Norton AJ, Isaacson PG. Detailed phenotypic analysis of B-cell lymphoma using a panel of antibodies reactive in routinely fixed wax-embedded tissue. The American journal of pathology. 1987;128(2):225–240. [PMC free article] [PubMed] [Google Scholar]
- 32.Picker LJ, Weiss LM, Medeiros LJ, Wood GS, Warnke RA. Immunophenotypic criteria for the diagnosis of non-Hodgkin's lymphoma. The American journal of pathology. 1987;128(1):181–201. [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz-Albiez R, Dorken B, Hofmann W, Moldenhauer G. The B cell-associated CD37 antigen (gp40–52) Structure and sub-cellular expression of an extensively glycosylated glycoprotein Journal of immunology. 1988;140(3):905–914. [PubMed] [Google Scholar]
- 34.Schuurman HJ, van Baarlen J, Huppes W, Lam BW, Verdonck LF, van Unnik JA. Immunophenotyping of non-Hodgkin's lymphoma. Lack of correlation between immunophenotype and cell morphology The American journal of pathology. 1987;129(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- 35.Lapalombella R, Yeh YY, Wang L, Ramanunni A, Rafiq S, Jha S, Staubli J, Lucas DM, Mani R, Herman SE, Johnson AJ, Lozanski A, Andritsos L, Jones J, Flynn JM, Lannutti B, Thompson P, Algate P, Stromatt S, Jarjoura D, Mo X, Wang D, Chen CS, Lozanski G, Heerema NA, Tridandapani S, Freitas MA, Muthusamy N, Byrd JC. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21(5):694–708. doi: 10.1016/j.ccr.2012.03.040. doi:10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–123. doi: 10.1053/j.seminhematol.2010.01.011. doi:10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48(Suppl 1):S12–23. doi: 10.1053/j.seminhematol.2011.03.003. doi:10.1053/j.seminhematol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Cheson BD, Rummel MJ. Bendamustine: rebirth of an old drug. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(9):1492–1501. doi: 10.1200/JCO.2008.18.7252. doi:10.1200/JCO.2008.18.7252. [DOI] [PubMed] [Google Scholar]
- 39.Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Durk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W, Study group indolent L Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. doi: 10.1016/S0140-6736(12)61763-2. doi:10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]