Abstract
Background
Increased exposure to a broad array of pathogens in children residing in sub-Saharan Africa may lead to heightened immune activation and increased proportions of memory T cells. Changes in the size of these cellular subsets have implications for restoration of normal immune function after treatment with HAART and are not well-characterized in young sub-Saharan African children.
Methods
CD4+ and CD8+ T cell subsets were measured by flow cytometry in 157 HIV-infected Zambian children before and at 3-month intervals during HAART for up to 30 months and in 34 control children at a single study visit.
Results
Prior to HAART, HIV-infected children had higher levels of activated and effector memory (EM) CD4+ and CD8+ T cells, and lower levels of naïve T cells and CD8+ T cells expressing IL-7Rα, compared to control children. The median duration of follow-up was 14.9 months (IQR: 6.4, 23.2) among 120 HIV-infected children with at least one study follow-up visit. Levels of immune activation and EM CD4+ T cells declined within six months of HAART but the percentages of EM CD4+ T cells and effector CD8+ T cells remained elevated through 30 months of HAART. IL-7Rα-expressing CD8+ T cells increased with HAART, suggesting expansion of memory capacity.
Conclusions
HAART significantly reduced levels of immune activation and EM CD4+ T cells, and promoted reconstitution of naïve T cells and IL-7Rα-expressing CD8+ T cells. However, persistently high levels of EM CD4+ T cells in HIV-infected children may reflect chronic perturbations in T cell subset composition.
Introduction
An estimated 2.5 million children are infected with human immunodeficiency virus (HIV) worldwide and the vast majority of these children live in sub-Saharan Africa.1 Children in this region experience high rates of morbidity and mortality due to poverty, poor nutrition, and increased exposure to acute and chronic infections. Infection with HIV further diminishes the immunologic capacity of these children to respond to disease challenges and is characterized by high levels of activated cell phenotypes, depletion of CD4+ T cells, and decreased quantity and quality of memory T and B cells.2–7
As access to highly active antiretroviral therapy (HAART) increases, HIV-infected children are achieving improved health outcomes and prolonged survival.8 Despite this enormous progress, increased exposure to a broad array of pathogens in children residing in sub-Saharan Africa may lead to heightened immune activation and increased proportions of memory CD4+ T cells, with implications for restoration of normal immune function after treatment with HAART. Changes in the size of the memory and activated CD4+ T cell subpopulations after HAART initiation have not been well characterized in young children, particularly among HIV-infected children in sub-Saharan Africa. Identifying the contributions of repopulated cellular subsets after HAART initiation is critical for estimating levels of polyfunctional T cells available for normal immune function and vaccine responses as well as evaluating strategies for HIV remission and cure.
Many studies report total CD4+ memory T cells based on surface expression of CD45RO or the lack of CD45RA expression9 and distinguish central memory T cells from effector memory T cells by expression of CCR7 or CD62L.10 However, only two studies specifically examined central memory CD4+ T cells among children born to HIV-infected mothers, both of which used cross-sectional study designs.11;12 In the few studies measuring T cell subsets before and after HAART initiation in HIV-infected children in sub-Saharan Africa, T cell activation was assessed6;13;14 but changes in T cell subset compositions are largely unknown. To better understand the impact of HAART on T cell composition in HIV-infected children residing in sub-Saharan Africa, particularly activated and memory phenotypes, we measured circulating CD4+ and CD8+ T cell populations in HIV-infected Zambian children before and at 3-month intervals after HAART initiation and compared to levels in control children.
Materials and Methods
Study design and population
HIV-infected children initiating HAART at public clinics in Lusaka, Zambia were recruited into a prospective, observational cohort study from January 20th, 2009 to February 28th, 2012 to assess humoral and cellular immune responses to measles virus.15 Eligible children were 9 months to 10 years of age with a documented history of measles vaccination. Follow-up study visits occurred every three months in concert with routine clinical care. A comparison group of children presenting to the same clinics for routine clinical care were enrolled for a single study visit and considered population controls. The HIV infection status of comparison children was not confirmed but was presumed to be negative based on medical history and clinical assessment.
A questionnaire was administered by trained study nurses to the parent or guardian accompanying the child to record demographic and clinical information, including age, sex, height, weight, history of illness, and vaccination history. A 3–5 mL peripheral blood sample was collected in Vacutainer tubes containing heparin or EDTA (BD Biosciences, Franklin Lakes, NJ, USA). Informed consent was obtained from each child’s parent or guardian and assent was obtained from children aged 7 years and older. The study protocol was approved by the Research Ethics Committee of the University of Zambia and the Institutional Review Boards at the University of Alabama and the Johns Hopkins Bloomberg School of Public Health.
Hematology
Blood arrived in the laboratory within 6 hours of collection and processed immediately. White blood cell (WBC) counts and lymphocyte percentages were measured using 100 µL of whole blood with a Sysmex Kx-21 automated hematology analyzer (Sysmex Corporation, Kobe, Japan). Plasma HIV viral load measurements were not performed.
Immunophenotyping
Immunophenotyping was performed to determine the composition of CD4+ and CD8+ T cell populations. Briefly, 50 µL of heparinized whole blood was mixed with monoclonal antibodies against cell surface markers (described below), red blood cells were lysed with FACSLyse (BD Biosciences, Franklin Lakes, NJ, USA), and cells were washed with PBS and resuspended in 1% paraformaldehyde. Flow cytometry data were acquired with CellQuest Pro (BD Biosciences) on a FACSCalibur flow cytometer and analyzed using FlowJo v7.5 (Treestar, Ashland, OR, USA). The gating strategy for identification of cell subsets is presented in Supplemental Figure 1.
T cells were stained with monoclonal antibodies to CD45RA, CCR7, CD3, and CD4 or CD8 (BD Biosciences) (Supplemental Table 1). Total T cells were defined by expression of the pan-T cell marker CD3. To distinguish T cell subsets, CD4+ and CD8+ T cells were defined as: naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA−), effector (CCR7−CD45RA−), and effector memory (CCR7−CD45RA+). Activated phenotypes were assessed using HLA-DR and CD38,16 and interleukin 7 receptor-α (IL-7Rα) was used to detect CD8+ T cells with the potential to develop into long-lived memory cells.17;18
Data analysis
Children missing date of birth or both total CD4+ and total CD8+ T cell data were excluded from analysis. The Wilcoxon rank-sum test was used to compare medians of continuous outcomes and Fisher’s exact test was used to compare proportions between HIV-infected and control children at enrollment. The percentages of T cell subsets were calculated using the respective parent population (CD3 and CD4 or CD8) as denominator. Absolute counts were calculated as the product: WBC count × lymphocyte percentage/100 × cell subset percentage/100. Percentages and counts of cell subsets were transformed by natural logarithm or square root to approximate normal distributions for regression models.
Multivariable linear regression (MLR) was used to compare cellular subsets between HIV-infected and control children at enrollment. Linear mixed effects (LME) models with unstructured variance-covariance structures and a random intercept for each child were used to estimate changes in each cellular subset after HAART initiation in HIV-infected children. Both MLR and LME models adjusted for age at enrollment (in months) and sex. The LME models accounted for repeated measures in the same individual over time and included a fixed effect for each 3-month interval of HAART exposure up to 30 months. LME models incorporating a random intercept for each study participant and a random slope for each 12-month age category were also explored. Standard deviations of random slopes indicated a difference of less than 0.01% between age categories in the rate of change in cell subsets and models with random slopes produced higher Akaike's Information Criteria values compared to models with individual-level random intercepts alone, indicating models with only random intercepts represented the data better than models with both random intercepts and slopes.19
Predictions of T cell subset percentages and counts were estimated for each child at each time point from LME models and used to compute means and 95% confidence intervals (CI), which were then back-transformed to the linear scale. The means and 95% CI for each subset from regression models are presented in Supplemental Tables and original data presented in Supplemental Figures. Given that model predictions were drawn from normal distributions, the percentages and counts of T cell subsets do not necessarily sum to 100% and total T cell counts, respectively, at each time point. Statistical analyses were performed using Stata version 10.1 (Stata Corp., College Station, TX, USA) and R v.3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).20 Data are presented as the mean (95% confidence interval) unless otherwise noted. The Benjamini and Hochberg method was used to assess the false discovery rate due to multiple comparisons (Supplemental Figure 2).21 Of the 224 statistically significant differences we observed among 364 statistical comparisons, 214 remained significant after adjustment for multiple comparisons
Results
Characteristics of study children
One hundred and sixty-nine HIV-infected children were enrolled on the day of HAART initiation between January 2009 and February 2012. Eleven HIV-infected children were missing both CD4+ and CD8+ T cell data at baseline, five of whom did not have subsequent follow-up data, and one child was excluded due to missing date of birth, resulting in 157 HIV-infected children eligible for baseline analysis. One-hundred and twenty HIV-infected children had at least one study follow-up visit and the median duration of follow-up was 14.9 months (interquartile range [IQR]: 6.4, 23.2). Approximately half (46%) of children were female and the median age at study enrollment was 26.0 months (Table 1). The unadjusted median CD4+ and CD8+ T cell percentages on the day of HAART initiation were 11.8% and 43.7% (Figures 1a and 1b). The median CD4+ T cell percentage was slightly higher among HIV-infected girls (12.8%) than boys (10.3%) (p=0.08), but no significant differences in median CD8+ T cell percentages were observed by sex (p=0.93). Children returning for follow-up study visits did not differ significantly by age, sex, or CD4+ and CD8+ T cell percentages compared to children completing only baseline study visits.
Table 1.
Baseline characteristics of HIV-infected and control children
| HIV-infected Children |
Control Children |
p-value* | |||
|---|---|---|---|---|---|
| N | N | ||||
| Age (months) | 157 | 26.0 (18.3, 51.3) | 34 | 24.2 (18.3, 38.3) | 0.311 |
| ≤12 | 10 | 9.6 (9.6, 10.6) | 2 | 10.2, 11.6 (--) | |
| >12 – 24 | 57 | 17.9 (14.5, 21.0) | 15 | 18.1 (12.9, 19.7) | |
| >24 – 36 | 33 | 28.3 (24.9, 32.0) | 7 | 30.1 (26.7, 34.2) | |
| >36 – 48 | 14 | 42.6 (40.5, 83.1) | 6 | 42.0 (37.8, 44.0) | |
| >48 – 60 | 18 | 54.3 (50.9, 58.5) | 0 | -- | |
| >60 – 120 | 25 | 82.9 (73.4, 93.2) | 4 | 83.6 (63.0, 111) | |
| Female | 157 | 73 (46%) | 34 | 16 (47%) | 0.953 |
Dichotomous characteristics are expressed as N (%), continuous characteristics as median (interquartile range).
p-values are from Wilcoxon rank-sum or χ2 tests.
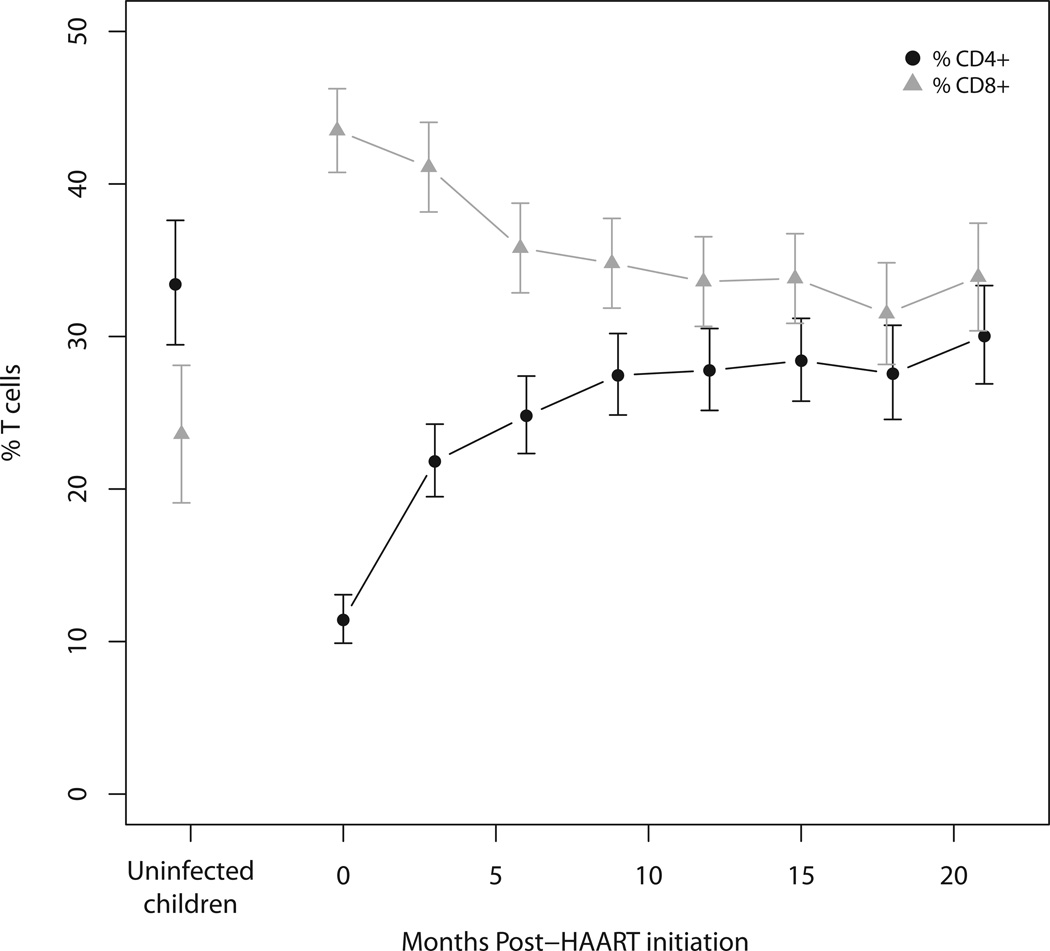
Figure 1.
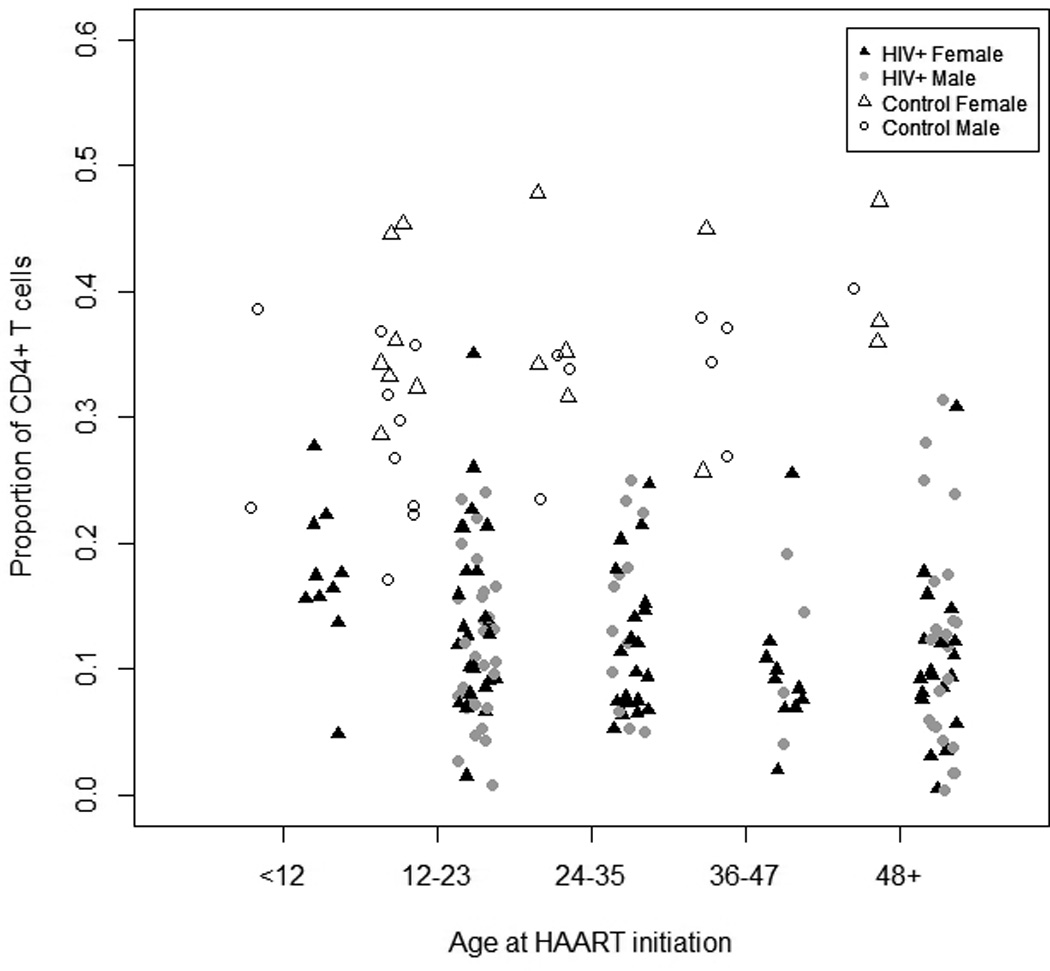
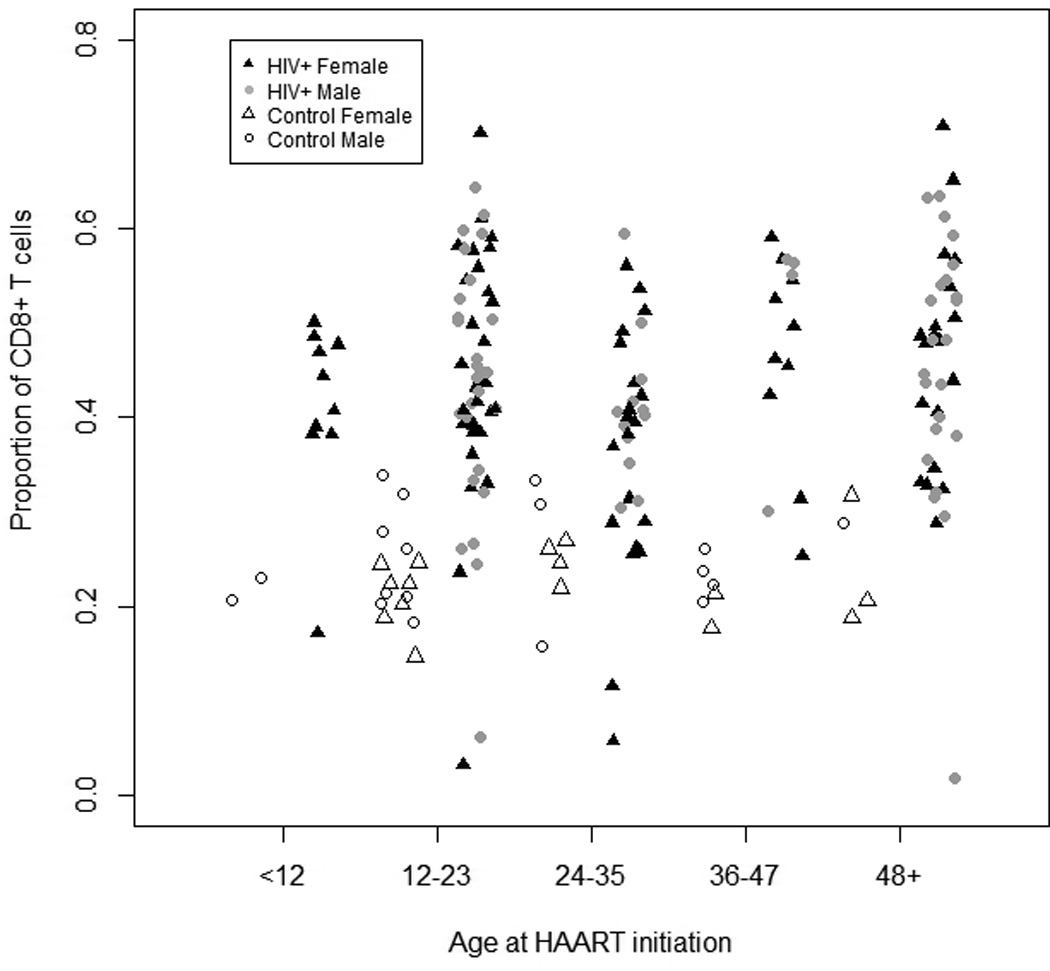
Unadjusted a) total CD4+ and b) total CD8+ T cells stratified by age (in months) in HIV-infected and control Zambian children at baseline. Filled and open symbols represent HIV-infected and control children, respectively.
c) Total CD4+ and CD8+ T cell percentages in HIV-infected Zambian children at 3-month intervals following HAART initiation and in control children adjusted for age and sex. Error bars represent 95% confidence intervals.
Thirty-four control children with one study visit each were enrolled. Forty-seven percent of these children were female and their median age was 24.2 months (Table 1). As expected, the control children had a significantly higher median CD4+ T cell percentage (33.4%, range: 17%, 48%) (Figure 1a) and lower CD8+ T cell percentage (23.6%, range: 0.4%, 35%) (Figure 1b) than HIV-infected children, consistent with few, if any, of the control children being HIV-infected.
T cell subsets at baseline
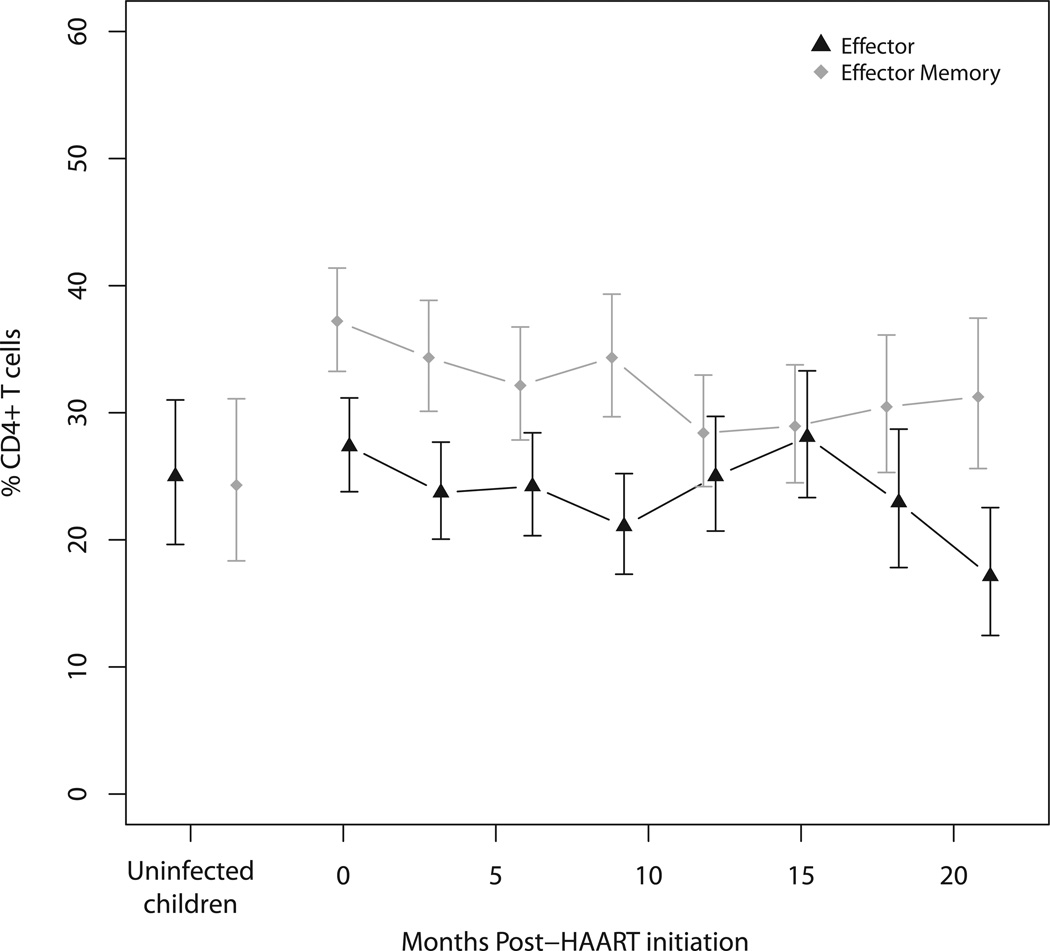
Before HAART initiation, effector memory cells constituted the largest mean percentage of CD4+ T cells in HIV-infected children (37.2%), followed by effector (27.4%), naïve (19.4%) and central memory CD4+ T cells (4.6%) after adjusting for age and sex (Figures 2a and 2b; Supplemental Table 2a). In contrast, naïve cells were the largest subset in control children and comprised one-third of CD4+ T cells. While statistically significant differences in CD4+ T cell subsets between age categories and sex were observed among HIV-infected and control children, these differences were less than 1% (Supplemental Figure 3 and Supplemental Table 3a).
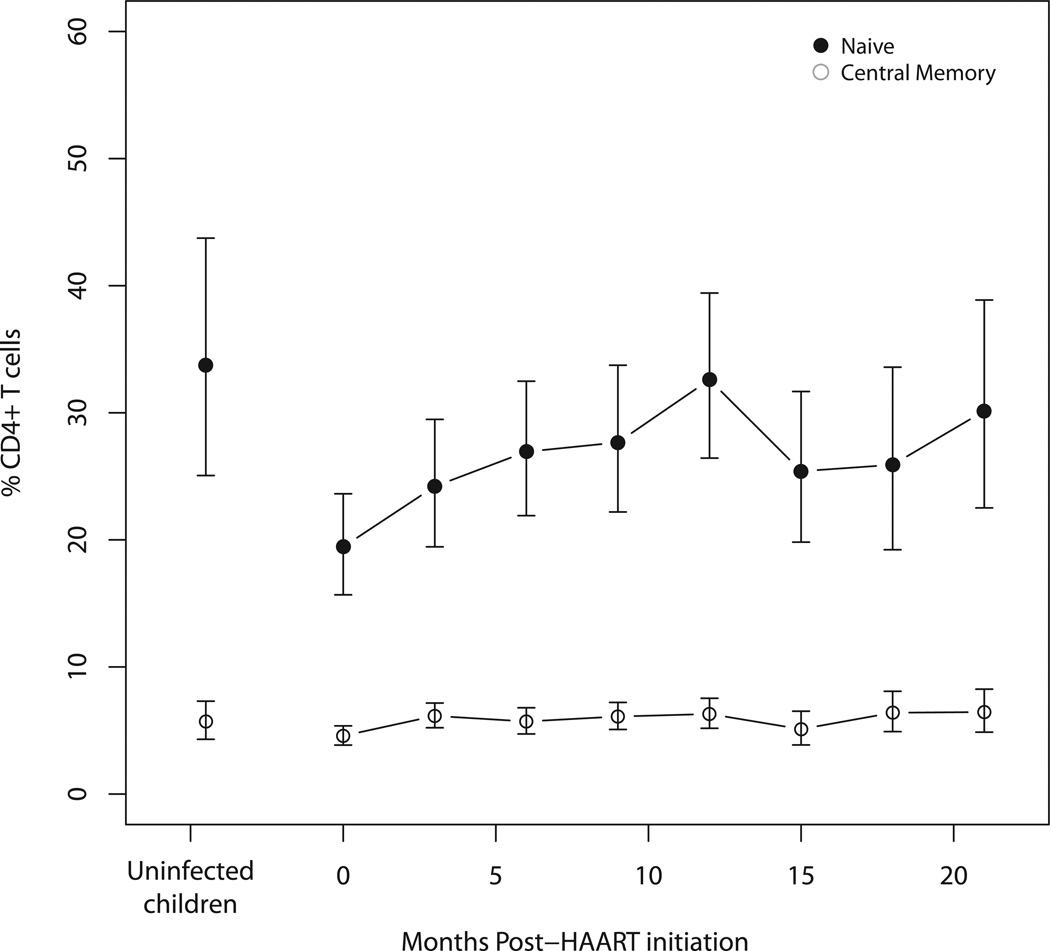
Figure 2.
a) Effector (CCR7− CD45RA−) and effector memory (CCR7− CD45RA+) and b) naive (CCR7+ CD45RA+) and central memory (CCR7+ CD45RA−) CD4+ T cell subsets in HIV-infected Zambian children at 3-month intervals after HAART initiation and in control children adjusted for age and sex. Error bars represent 95% confidence intervals.
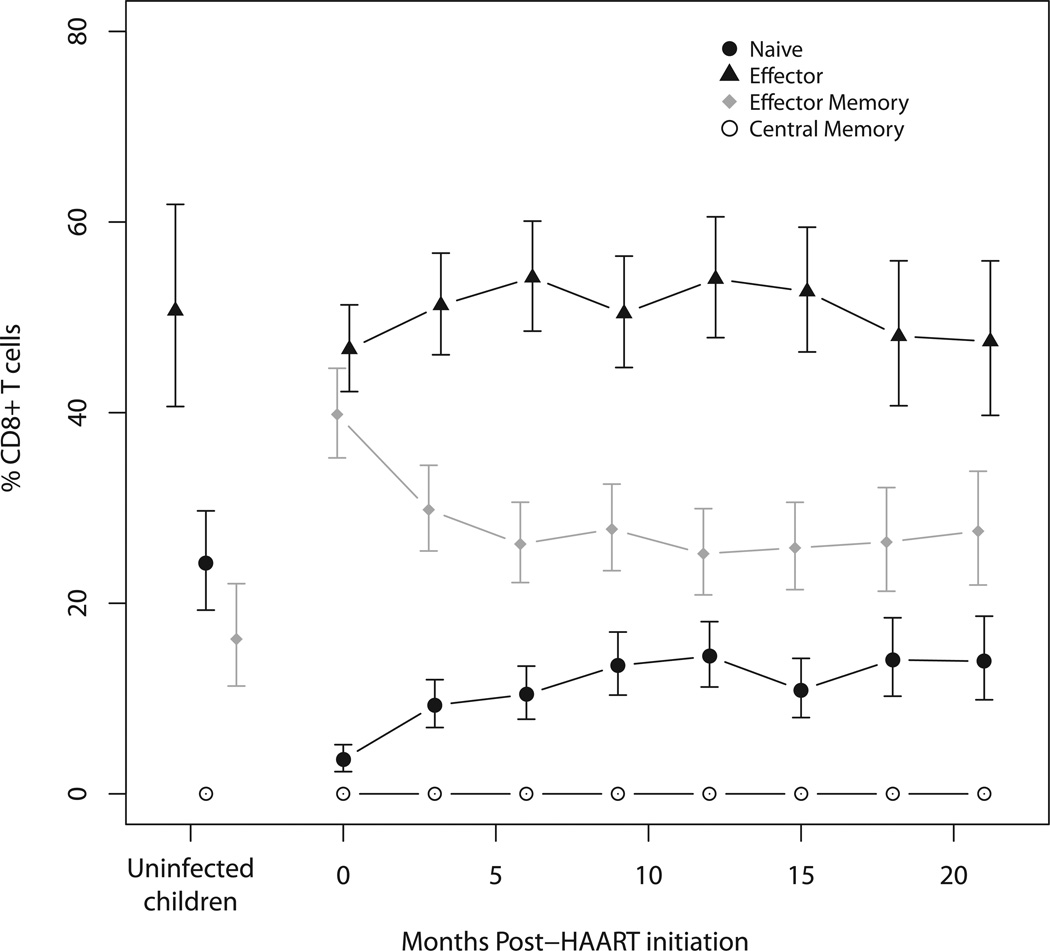
A majority of CD8+ T cells were of the effector phenotype in both HIV-infected (46.6%) and control children (50.7%) (Figure 3; Supplemental Table 2b). While naïve cells comprised the next largest percentage of CD8+ T cells in control children (24.2%), effector memory CD8+ T cells were the second largest percentage in HIV-infected children (39.8%). Central memory cells constituted a substantially smaller percentage of CD8+ T cells in the circulation of both HIV-infected and control children at less than 0.1%, reflecting recruitment to the bone marrow.22
Figure 3.
Naive (CCR7+ CD45RA+), effector (CCR7− CD45RA−), effector memory (CCR7− CD45RA+) and central memory (CCR7+ CD45RA−) CD8+ T cell subsets in HIV-infected Zambian children at 3-month intervals after HAART initiation and in control children, adjusted for age and sex. Error bars represent 95% confidence intervals.
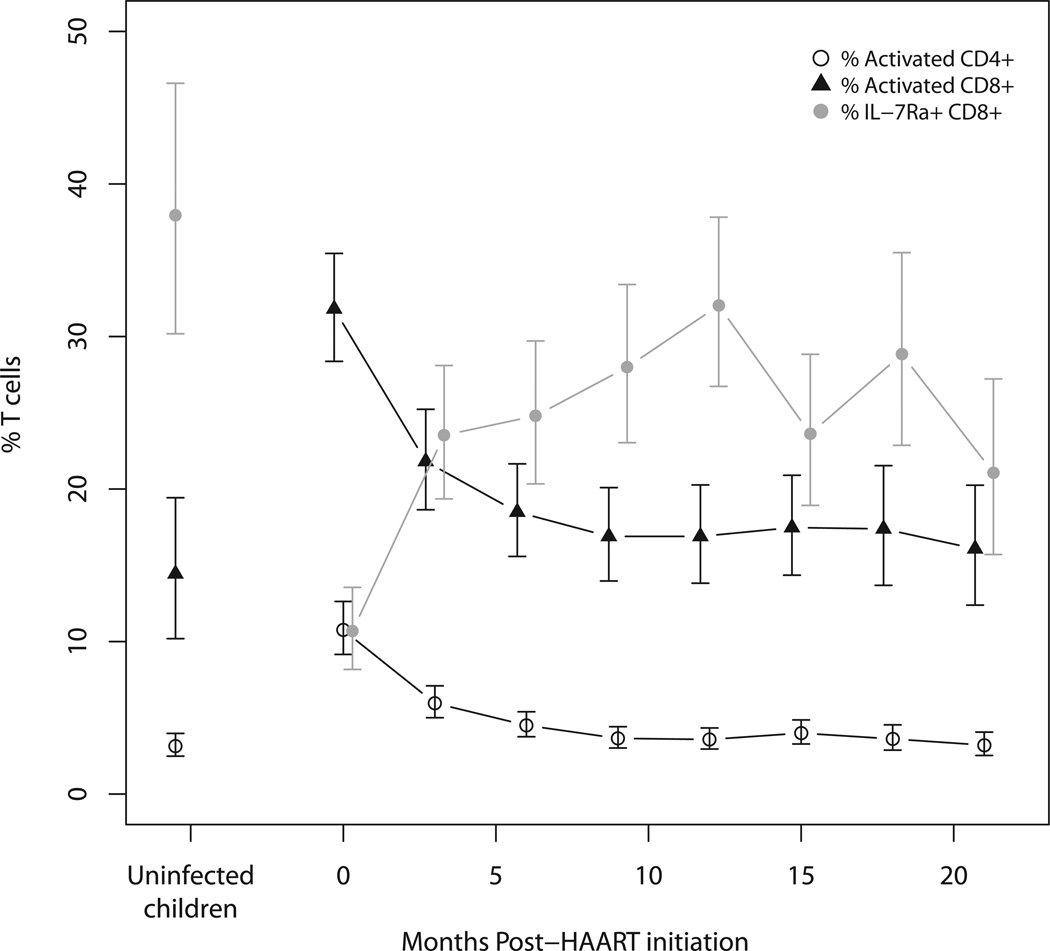
Consistent with increased cellular immune activation during HIV infection, the percentages of activated CD4+ and CD8+ T cells in HIV-infected children were 3- and 2-fold higher, respectively, than those of control children (Figure 4 and Supplemental Figure 5). Additionally, the memory capacity of CD8+ T cells, as measured by expression of IL-7Rα, was 27 percentage points lower in HIV-infected children than in control children. Similar to CD4+ T cells, differences among CD4+ T cell subsets by age categories and sex were less than 1% for HIV-infected and control children except for naive CD8+ T cells, which were 2.75% (95% CI: 0.16, 8.51) higher in female compared to male control children (Supplemental Figure 4 and Supplemental Table 3b).
Figure 4.
CD4+ and CD8+ T cells displaying HLA-DR and CD38 (activation markers) and CD8+ T cells displaying IL-7Rα (memory marker) in HIV-infected Zambian children at 3-month intervals after HAART initiation and in control children, adjusted for age and sex. Error bars represent 95% confidence intervals.
Changes in CD4+ T cell subsets after HAART
Almost three-quarters (73%) of HIV-infected children achieved at least 20% CD4+ T cells within six months of HAART initiation. After adjusting for age and sex, the mean percentage of total CD4+ T cells increased significantly within three months of starting HAART and continued to increase through nine months of treatment, reaching 27.5% of the T cell population, after which no statistically significant changes occurred (Figure 1c; Supplemental Table 2a). The rate at which total CD4+ T cell percentages increased from baseline to 9 months after HAART did not differ between age groups (Supplemental Figure 6a).
During the first 6 months of HAART, naïve and central memory CD4+ T cells increased significantly and maintained percentages similar to those of control children for the remainder of study follow-up (Figure 2a). There were no differences in the rates of naive and central memory CD4+ T cell increases after HAART initiation between age groups (Supplemental Figures 6b and 6c). Effector memory cells decreased in HIV-infected children on HAART, although not to percentages observed in control children (Figure 2b), and the rate of decrease did not differ with age (Supplemental Figure 6d). Effector cell percentages remained similar to those of control children (Figure 2b). Changes in the mean absolute counts of CD4+ T cell subsets showed patterns similar to CD4+ T cell subset percentages after children began HAART (Supplemental Table 4a). These findings suggest increases in CD4+ T cells were driven primarily by gains in naïve CD4+ T cells.
Changes in CD8+ T cell subsets after HAART
While the mean percentage of total CD8+ T cells in HIV-infected children decreased significantly after 6 months of HAART, this percentage remained higher (34%) than in control children (24%) for the remainder of follow-up (Figure 1c). As observed among CD4+ T cell subsets, naïve CD8+ T cell percentages in HIV-infected children increased significantly after HAART initiation but maintained mean percentages between 10% and 15% over study follow-up, slightly lower than 24% of CD8+ T cells among control children (Figure 3; Supplemental Table 2b). After HAART, percentages of central memory and effector cells remained similar in HIV-infected and control children, comprising <0.1% and 50%, respectively, of the total CD8+ T cell population. In contrast, the mean effector memory CD8+ T cell percentage in HIV-infected children decreased significantly during HAART but remained higher than that observed among control children, with effector memory T cells largely responsible for the decline in total CD8+ T cells. Similar changes in absolute CD8+ T cell counts were observed in response to HAART (Supplemental Table 4b). Sex and age were not associated with differences in the rates of change in any CD8+ T cell subsets among HIV-infected children (Supplemental Figures 7a–7e).
Cellular activation and memory capacity
Activated CD4+ T cells decreased to less than half of baseline levels six months after starting HAART and approached levels observed in control children (Figure 4). Activated CD8+ T cells demonstrated a similar decline following HAART initiation but remained slightly higher than was observed in control children (Figure 4; Supplemental Table 3b). Memory capacity of CD8+ T cells, as measured by IL-7Rα expression, more than doubled after 6 months of HAART, but remained lower than the mean percentage observed in control children. Sex and age were not associated with changes in activated CD4+ or CD8+ T cell subsets or IL-7Rα expression by CD8+ T cells (Supplemental Figures 8a–8c and Supplemental Table 3).
Discussion
The cellular immune activation status and levels of memory T cell subsets in HIV-infected Zambian children were substantially altered after starting HAART. As expected, untreated HIV-infected children had lower percentages of naïve T cells and higher percentages of effector memory and activated T cells than control children, consistent with changes in T cell composition during chronic HIV infection and persistent immune activation.23;24 HAART significantly reduced levels of cellular immune activation and effector memory CD4+ T cells, and promoted reconstitution of naïve T cells and IL-7Rα-expressing CD8+ T cells. Interestingly, CD4+ and CD8+ effector T cell percentages did not differ between HIV-infected and control children. However, increased CD8+ effector T cell percentages at HAART initiation were significantly associated with an increased risk of mortality in this cohort of children,25 possibly reflecting T cell exhaustion and loss of polyfunctional cytokine responses commonly observed in HIV-infected individuals.26
Most HIV-infected children require lifelong treatment with HAART, as the major barrier to cure is the development of a cellular reservoir that harbors latent infectious virus.27 Current approaches for HIV cure include purging the latent reservoir through re-activation of resting cells;28 thus, understanding both the relative contributions and maximum counts and percentages of cellular subsets is important for assessing reservoir size, calculating HIV clearance kinetics and estimating the potential impact of cure strategies. The cellular distribution of the proviral reservoir in HIV-infected children has not been thoroughly investigated but evidence suggests it is comprised of resting memory CD4+ T cells, including long-lived central memory T cells29 that predominantly home to lymphoid organs.30 Whether the increase in central memory T cells observed in Zambian children represents increased memory cell levels that typically develop with aging or redistribution of central memory T cells in circulating blood relative to those sequestered in secondary lymphoid organs is unclear. In healthy children younger than 10 years of age, the proportions of naïve T cells decrease as the immune system matures and encounters antigens, while memory T cell proportions increase.5 Since control children were enrolled for only a single visit, our ability to differentiate between phenomena associated with aging, such as antigen exposure, and HAART duration was limited when comparing cellular subsets among HIV-infected children. We attempted to control for this by adjusting for age in statistical models and believe a large part of the considerable changes in T cell subsets can be attributed to HAART, as observed upon stratification of cell subset trajectories by age and sex. Additionally, the proportion of central memory T cells that developed in response to HIV and non-HIV antigens was not determined.
This is one of the largest studies to assess long-term changes in T cell subsets in HIV-infected children, particularly in sub-Saharan Africa. Of 13 studies assessing cellular subsets among HIV-infected children, ten included fewer than 50 children.2;11–14;31–38 Only two prior studies assessed T cell activation status among HIV-infected children before HAART initiation in sub-Saharan Africa but these studies used cross-sectional study designs.14;31 After HAART initiation, T cell subsets of European and American HIV-infected children demonstrated patterns of change similar to those of Zambian children.32;38 Among European children starting HAART, both naïve and total memory CD4+ T cells increased within three months of HAART but only naïve CD4+ T cells sustained increases after 12 months.32 Similarly, naïve CD4+ and CD8+ T cell percentages increased after 144 weeks of HAART in American children, while memory cell percentages decreased or remained unchanged.38 The shift in T cell composition after HAART initiation, most notably the increase in naïve T cells and reduction in effector memory T cells, is consistent with control of HIV-mediated T cell stimulation and differentiation.39
While the pattern of T cell subset changes after HAART initiation was similar between HIV-infected children in Zambia and those in developed countries, Zambian children had lower levels of naïve T cells and higher levels of effector memory T cells. Among HIV-infected American and Spanish children, for example, naïve cells constituted 55–56% and 30–38% of the CD4+ and CD8+ T cell populations, respectively, prior to HAART initiation,33;37;38 while the corresponding percentages in HIV-infected Zambian children constituted only 19% and 4%. These differences are underscored by the older median ages of 7 to 10 years in the American and Spanish cohorts, as older age typically is associated with lower naïve T cell percentages. Similarly, control Zambian children had lower proportions of CD4+ and CD8+ naïve T cells than HIV-uninfected American, Brazilian and Cameroonian children.11;36;40 Naïve T cells comprised 54% of CD4+ and 49% of CD8+ T cells in 12–24 month old Cameroonian children40 compared to only 34% and 24% in Zambian children. These differences are more pronounced upon comparison to HIV-uninfected American children who had CD4+ and CD8+ T cell percentages of 75% and 58%, respectively,36 possibly reflecting more frequent exposures to pathogens that induce cellular differentiation in the Zambian children.
A limitation of this work was the inability to measure T cell subsets for all children at later study visits due to short duration of follow-up. We performed sensitivity analyses to determine the extent to which estimates change after restricting analyses to HIV-infected children with at least 12 months of follow-up and no more than one missing visit, resulting in 87 HIV-infected children and 34 control children. However, the estimates did not differ between the original and sensitivity analyses. Moreover, inferences regarding differences between HIV-infected and control children remained the same. Although we were unable to adjust for HIV viral load changes after HAART initiation, the decline in the percentages of CD4+ effector memory cells and cellular immune activation are consistent with decreased HIV stimulation as effector memory cells migrate to non-lymphoid tissues to quickly respond to cognate antigens41 and turnover rapidly.42
HAART significantly reduced levels of cellular immune activation and effector memory CD4+ T cells, and promoted reconstitution of naïve T cells and IL-7Rα-expressing CD8+ T cells. To our knowledge, this is the first study to report the relative proportions and sizes of T cell subsets, specifically the central memory CD4+ T cell population, in HIV-infected children from sub-Saharan Africa before and after HAART initiation.
Supplementary Material
Acknowledgements
WJM, CBM and MMM conceived of the study, CBM and MMM led collection of clinical data, KRL performed statistical analyses, HN optimized and performed laboratory analyses, and KRL and WJM drafted the manuscript. We thank the participants of this study and the clinicians and study staff who collected data and cared for the participating children. We also thank Fred Menendez and Tricia Niles for their assistance with optimizing the flow cytometry.
Sources of Funding: This study was funded by the U.S. National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R01AI070018].
Footnotes
Conflicts of Interest: We declare that we have no conflicts of interest.
Presented in part at the Keystone Symposium “Immunological Mechanisms of Vaccination” in Seattle, Washington, October 27th – November 1st, 2010.
Reference List
- 1.UNAIDS. Report on the Global AIDS Epidemic 2010. 2011 Available at: http://www.unaids.org/globalreport/Global_report.htm. [Google Scholar]
- 2.Prendergast A, O'Callaghan M, Menson E, Hamadache D, Walters S, Klein N, Goulder P. Factors influencing T cell activation and programmed death 1 expression in HIV-infected children. AIDS Res Hum Retroviruses. 2012;28:465–468. doi: 10.1089/AID.2011.0113. [DOI] [PubMed] [Google Scholar]
- 3.Puissant-Lubrano B, Combadiere B, Duffy D, Wincker N, Frachette MJ, Ait-Mohand H, Verrier B, Katlama C, Autran B. Influence of antigen exposure on the loss of long-term memory to childhood vaccines in HIV-infected patients. Vaccine. 2009;27:3576–3583. doi: 10.1016/j.vaccine.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Elrefaei M, McElroy MD, Preas CP, Hoh R, Deeks S, Martin J, Cao H. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173:2184–2189. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 5.Rainwater-Lovett K, Moss WJ. Immunologic basis for revaccination of HIV-infected children receiving HAART. Future Virol. 2011;6:59–71. doi: 10.2217/fvl.10.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ssewanyana I, Baker CA, Ruel T, Bousheri S, Kamya M, Dorsey G, Rosenthal PJ, Charlebois E, Havlir D, Cao H. The Distribution and Immune Profile of T Cell Subsets in HIV-Infected Children from Uganda. AIDS Res Hum Retroviruses. 2009;25:65–71. doi: 10.1089/aid.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rainwater-Lovett K, Nkamba H, Mubiana-Mbewe M, Bolton MC, Margolick J, Moss W. Antiretroviral therapy restores age-dependent loss of resting memory B cells in young HIV-infected Zambian children. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 9.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. %19. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Ono E, Nunes dos Santos AM, de Menezes Succi RC, Machado DM, de Angelis DS, Salomao R, Kallas EG, de Moraes-Pinto MI. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res. 2008;41:700–708. doi: 10.1590/s0100-879x2008000800011. [DOI] [PubMed] [Google Scholar]
- 12.Resino S, Navarro J, Bellon JM, Gurbindo D, Leon JA, Munoz-Fernandez MA. Naive and memory CD4+ T cells and T cell activation markers in HIV-1 infected children on HAART. Clin Exp Immunol. 2001;125:266–273. doi: 10.1046/j.1365-2249.2001.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruel T, Ssewanyana I, Achan J, Gasasira A, Kamya MR, Kekitiinwa A, Wong JK, Cao H, Havlir D, Charlebois ED. Dynamics of T cell activation accompanying CD4 recovery in antiretroviral treated HIV-infected Ugandan children. Clin Immunol. 2009;131:410–414. doi: 10.1016/j.clim.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ssewanyana I, Elrefaei M, Dorsey G, Ruel T, Jones NG, Gasasira A, Kamya M, Nakiwala J, Achan J, Charlebois E, Havlir D, Cao H. Profile of T cell immune responses in HIV-infected children from Uganda. J Infect Dis. 2007;196:1667–1670. doi: 10.1086/522013. [DOI] [PubMed] [Google Scholar]
- 15.Rainwater-Lovett K, Nkamba HC, Mubiana-Mbewe M, Bolton-Moore C, Moss WJ. Changes in Measles Serostatus Among HIV-Infected Zambian Children Initiating Antiretroviral Therapy Before and After the 2010 Measles Outbreak and Supplemental Immunization Activities. J Infect Dis. 2013;208:1747–1755. doi: 10.1093/infdis/jit404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 18.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, Ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 19.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. 2014 Available at: http://www.R-project.org/. [Google Scholar]
- 21.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 22.Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann GR, Zaunders J, Murray J, Kelleher AD, Lewin SR, Solomon A, Smith D, Cooper DA. Relative significance of different pathways of immune reconstitution in HIV type 1 infection as estimated by mathematical modeling. AIDS Res Hum Retroviruses. 2001;17:147–159. doi: 10.1089/08892220150217238. %20. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rainwater-Lovett K, Nkamba HC, Mubiana-Mbewe M, Moore CB, Moss WJ. Immunologic risk factors for early mortality after starting antiretroviral therapy in HIV-infected Zambian children. AIDS Res Hum Retroviruses. 2013;29:479–487. doi: 10.1089/aid.2012.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 27.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van LJ, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013;381:2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott-Algara D, Rouzioux C, Blanche S, Burgard M, Didier C, Riviere Y, Buseyne F. In untreated HIV-1-infected children, PBMC-associated HIV DNA levels and cell-free HIV RNA levels are correlated to distinct T-lymphocyte populations. J Acquir Immune Defic Syndr. 2010;53:553–563. doi: 10.1097/QAI.0b013e3181cf060f. [DOI] [PubMed] [Google Scholar]
- 32.Gibb DM, Newberry A, Klein N, De RA, Grosch-Woerner I, Babiker A. Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Lancet. 2000;355:1331–1332. doi: 10.1016/s0140-6736(00)02117-6. [DOI] [PubMed] [Google Scholar]
- 33.Resino S, Seoane E, Perez A, Ruiz-Mateos E, Leal M, Munoz-Fernandez MA. Different profiles of immune reconstitution in children and adults with HIV-infection after highly active antiretroviral therapy. BMC Infect Dis. 2006;6:112–112. doi: 10.1186/1471-2334-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resino S, Galan I, Bellon JM, Navarro ML, Leon JA, Munoz-Fernandez MA. Characterizing the immune system after long-term undetectable viral load in HIV-1-infected children. J Clin Immunol. 2003;23:279–289. doi: 10.1023/a:1024536816684. [DOI] [PubMed] [Google Scholar]
- 35.Hainaut M, Ducarme M, Schandene L, Peltier CA, Marissens D, Zissis G, Mascart F, Levy J. Age-related immune reconstitution during highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 2003;22:62–69. doi: 10.1097/00006454-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Jordan KA, Furlan SN, Gonzalez VD, Karlsson AC, Quigley MF, Deeks SG, Rosenberg MG, Nixon DF, Sandberg JK. CD8 T cell effector maturation in HIV-1-infected children. Virology. 2006;347:117–126. doi: 10.1016/j.virol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Navarro J, Resino S, Bellon JM, Abad ML, Gurbindo D, Fernandez-Cruz E, Munoz-Fernandez MA. Association of CD8+ T lymphocyte subsets with the most commonly used markers to monitor HIV type 1 infection in children treated with highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:525–532. doi: 10.1089/08892220151126607. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg A, Dickover R, Britto P, Hu C, Patterson-Bartlett J, Kraimer J, Gutzman H, Shearer WT, Rathore M, McKinney R. Continuous improvement in the immune system of HIV-infected children on prolonged antiretroviral therapy. AIDS. 2008;22:2267–2277. doi: 10.1097/QAD.0b013e3283189bb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guihot A, Tubiana R, Breton G, Marcelin AG, Samri A, Assoumou L, Goncalves E, Bricaire F, Costagliola D, Calvez V, Rouzioux C, Autran B, Katlama C, Carcelain G. Immune and virological benefits of 10 years of permanent viral control with antiretroviral therapy. AIDS. 2010;24:614–617. doi: 10.1097/QAD.0b013e32833556f3. %20. [DOI] [PubMed] [Google Scholar]
- 40.Sagnia B, Ateba NF, Ndiang Moyo TS, Ndongo TJ, Cairo C, Domkam I, Agbor G, Mve E, Tocke O, Fouda E, Ouwe MO-B, Colizzi V. Reference values of lymphocyte subsets in healthy, HIV-negative children in Cameroon. Clin Vaccine Immunol. 2011;18:790–795. doi: 10.1128/CVI.00483-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 42.Macallan DC, Wallace D, Zhang Y, De LC, Worth AT, Ghattas H, Griffin GE, Beverley PC, Tough DF. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J Exp Med. 2004;200:255–260. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.