Abstract
Background
People who attempt suicide often display cognitive impairments, particularly poor cognitive control. Could poor cognitive control contribute to high suicide rates in old age? A component of cognitive control, cognitive inhibition – active suppression of task-irrelevant processing – is very sensitive to aging and has been linked to attempted suicide. We investigated cognitive inhibition in older high-lethality suicide attempters, closely resembling suicide victims, as well as low-lethality attempters, and control groups with and without depression and suicidal ideation.
Methods
102 participants aged 60+ (17 psychiatrically healthy control subjects, 38 depressed control subjects, 16 suicide ideators, 14 low-lethality suicide attempters, and 17 high-lethality suicide attempters) underwent comprehensive clinical and cognitive assessments. They completed the Delis–Kaplan Executive Function System Color-Word Interference Test, a validated modification of the Stroop test.
Results
High-lethality suicide attempters demonstrated a distinct pattern of cognitive inhibition deficits. Compared to psychiatrically healthy control subjects and suicide ideators, high-lethality attempters took longer to complete inhibition trials, even after accounting for potential confounding factors (age, education, MMSE score, information processing speed, and accuracy). Compared to non-suicidal depressed and healthy control subjects, low-lethality suicide attempters committed more uncorrected errors; however, this difference was not specific to the inhibition condition.
Conclusions
Older suicide attempters are a cognitively heterogeneous group. Poor cognitive control in high-lethality attempters may undermine their ability to solve real-life problems, precipitating a catastrophic accumulation of stressors. Meanwhile, low-lethality attempters' poor performance may reflect a careless approach to the task or faulty monitoring.
Keywords: Suicidal behavior, Stroop, Neuropsychology, Cognitive Control, Mood Disorders
Introduction
It is becoming increasingly clear that individuals who attempt suicide or die by suicide have a predisposition for this behavior (Mann, 2003, Turecki et al., 2012) - a reduced ability to adapt to stressors, leading them to suicidal ideas and acts. In addition to impulsive aggression (McGirr and Turecki, 2007) and persistent hopelessness (Beck et al., 1985, Szanto et al., 1998), several studies have now reported impairments in cognitive domains such as attentional/cognitive control, episodic memory, working memory, and language fluency (Keilp et al., 2013, Keilp et al., 2001, Raust et al., 2007, Richard-Devantoy et al., 2012) as well as in decision-making (Clark et al., 2011, Dombrovski et al., 2010, Jollant et al., 2005). Cognitive impairments may be particularly prominent in elderly suicide attempters (Dombrovski et al., 2008a, Dombrovski et al., 2010, Erlangsen et al., 2008, Gujral et al., 2012, McGirr et al., 2012). Age-related decline in cognitive control (Amieva et al., 2004, Drag et al., 2010) may be among the factors contributing to the increasing suicide rates in old age (Dombrovski et al., 2008b, Erlangsen et al., 2008).
Cognitive control enables us to flexibly adapt our behavior to meet current demands (Barch et al., 2009), especially in the face of ambiguous, complex, and/or changing environments (Botvinick et al., 2001). Thus, deficient cognitive control has been hypothesized to reduce one's ability to respond adaptively to stressors, increasing the likelihood of seeing suicide as the only obvious solution in vulnerable individuals. Cognitive control is a general ability that underlies performance on tests of task switching, cognitive inhibition, error detection, response conflict and cognitive flexibility (Miller and Cohen, 2001). Cognitive inhibition, a major component of cognitive control and an active suppression process that limits the processing of irrelevant stimuli for the on-going task (Shallice and Burgess, 1991), is very sensitive to aging (Hasher et al., 1999). Aging most notably affects shifting, updating, and inhibition functions (Miyake et al., 2000), non-automatic controlled inhibition processes (Darowski et al., 2008), as well as decision-making (Eppinger et al., 2012), and cognitive control (Tisserand et al., 2004).
Suicide attempts vary in medical severity, from acts that cause no medical damage to those that would have been fatal without rescue. There is increasing evidence that the cognitive and broader biological profile of those individuals who make serious (high medical lethality) versus low-medical lethality suicide attempts are different (Dombrovski et al., 2011b, Keilp et al., 2001, McGirr et al., 2012, Oquendo et al., 2003). We have previously reported a deficit in cognitive inhibition in elderly depressed suicide attempters compared to depressed and healthy subjects (Richard-Devantoy et al., 2012). However, this study did not take into account the heterogeneity of suicidal behavior, nor investigated whether those who contemplated suicide but did not exhibit suicidal behavior differ in cognitive control. It is unclear whether cognitive control deficits specifically characterize only those who carry out serious attempts (those with high medical lethality attempts), and whether they are also present in those who currently contemplate suicide (Marzuk et al., 2005) or carry out low lethality attempts.
Depression is the most common mental disorder in those who die by suicide or attempt suicide (Conwell et al., 1996, Szanto et al., 2001, Waern et al., 2003). It remains unclear to what extent the cognitive control profile of older suicide attempters is qualitatively different from that of other patients with late-life depression. The aim of this study was to examine cognitive inhibition ability in older adults who had serious (near fatal) and low lethality suicide attempts and to compare them to similarly depressed non-suicidal individuals (with no lifetime history of suicide attempt or suicidal ideation) and to those who contemplated suicide but never tried to kill themselves. We hypothesized that both groups of suicide attempters would show a deficit in cognitive inhibition in comparison to the other groups, and that high-lethality attempters would show the most pronounced deficit.
Materials and Methods
Population sample
Four groups of participants aged 60+ years (n=102) were recruited: 1) 31 depressed patients with a history of suicide attempt (14 low-lethality suicide attempters, 17 high-lethality suicide attempters) and current suicidal ideation; 2) 16 depressed patients with current suicidal ideation with a specific plan but with no lifetime history of a suicide attempt; 3) 38 depressed control subjects with no lifetime history of a suicide attempt or ideation, and 4) 17 psychiatrically healthy control subjects with no history of any Axis I disorder or a suicide attempt.
Suicide attempters, suicide ideators, and non-suicidal depressed control subjects were all diagnosed with major depression without psychotic features by Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I Disorders (SCID/DSM-IV (First et al., 2002)). Depressed Patients and control subjects were all fluent in English, with a Mini-Mental State Examination (Folstein MF, 1975) score ≥24. Exclusion criteria were bipolar disorder, schizophrenia, schizoaffective disorder, electroconvulsive therapy in the previous six months and neurologic disorders including stroke, epilepsy, brain tumor or brain injury and sensory disorders that precluded participating in cognitive assessment. All participants provided written informed consent. The University of Pittsburgh Institutional Review Board approved the study.
Our study was conducted at a university psychogeriatric inpatient unit and a specialty outpatient research clinic for late-life depression. Since we aimed to capture cognitive control in a state similar to a suicidal crisis, we assessed participants with major depression during an acute depressive episode. Participants were assessed within two weeks of inpatient admission or at the beginning of outpatient treatment. Depressed participants continued to receive psychotropic medications as clinically indicated. We ensured that none were intoxicated or had withdrawal symptoms at the time of assessment. Neuropsychological assessment was overseen by an experienced neuropsychologist (MAB) and took place in one to two sessions over 1 to 3 days. Assessors were blind to clinical history and mood ratings. Suicide attempters had a self-directed injurious act with a clear intent to end one's own life (O'Carroll et al., 1996). To capture a state close to the suicidal crisis, all of these participants were required to have suicidal ideation with a specific plan at the time of study enrollment. These participants displayed a high level of suicidal intent during their attempts and expressed severe suicidal ideation. A psychiatrist (KS or AYD) verified suicide attempt history using a clinical interview, medical records, and information from relatives.
Clinical Assessment
Depression severity was measured with the 17-item Hamilton Rating Scale for Depression (Hamilton, 1960). Burden of physical illness was assessed with the Cumulative Illness Rating Scale adapted for Geriatrics (Miller et al., 1992).
Medical seriousness of attempts was assessed using the Beck Lethality Scale (BLS) (Beck et al., 1975). For participants with multiple attempts, data for the most serious attempt are presented. High-lethality attempts required a medical intervention, resulting in coma, need for resuscitation, unstable vital signs, penetrating wounds of abdomen or chest, third-degree burns, or major bleeding, as defined by a score of 4 on the BLS. None had experienced head injuries directly related to attempt, however, to avoid including attempters who may have suffered brain damage and cognitive dysfunction as a result of the attempt we identified those with potential anoxic-ischemic or toxic brain injury, based on the Beck Lethality Scale, medical records and the clinical interview. Past suicidal behaviour was also characterized using the Suicide Intent Scale (Beck et al., 1974). This instrument characterizes several dimensions related to the suicide attempt with 18 items coded on a three-point scale.
Neuropsychological assessment
We used the Delis–Kaplan Executive Function System (Delis et al., 2001) Color-Word Interference Test (CWIT), which consists of four conditions: color naming, word reading, inhibition, and inhibition/switching.
In the CWIT color naming condition, the participant is asked to name the colors of a series of red, green, and blue squares as quickly as possible without making mistakes. In the second task condition, which is the word reading trial, the participant is presented with a page containing the words “red,” “green,” and “blue” printed in black ink. The participant is asked to read the words aloud as quickly as possible without making mistakes. Then, in the inhibition condition based on the Stroop procedure (Stroop, 1935), the participant is presented with a page containing the words “red,” “green,” and “blue” printed incongruently in red, green, or blue ink. The participant is asked to say the color of the ink in which each word is printed. Last is the inhibition/switching condition, in which the participant is presented with a page containing the words “red,” “green,” and “blue” written in red, green, or blue ink. Half of these words are enclosed within boxes. The participant is asked to say the color of the ink in which each word is printed (as in the third trial), but to read the word aloud (and not name the ink color) when a word appears inside a box.
Performance is measured by completion time and error rates on each of the four conditions. In addition, color naming and word reading times may be summed for a composite score representing component functions. Finally, multiple studies have demonstrated that cognitive control impairments in late-life depression are largely mediated by slowed information processing speed (Butters et al., 2000, Butters et al., 2004a, Sheline et al., 2006). Thus, we controlled for information processing speed in our analyses, to isolate inhibition impairments that distinguish suicide attempters.
Analysis
All analyses were conducted with Statistical Package for the Social Sciences 20.0 (SPSS, Chicago, Illinois). All statistical tests were two sided. We first compared groups on demographic and clinical characteristics using analysis of variance (ANOVA) and chi-square tests. For these and all subsequent ANOVAs, we examined post hoc contrasts using the Tukey honestly significant difference test.
We used the general linear model to test the influence of different variables on performance of the DKEFS-CWIT inhibition total time to completion. Then, we used a linear regression model to test the association between the history of high-lethality suicide attempt and inhibition test scores, adjusting for age, global cognitive performance (MMSE score), severity of depression, information processing speed (CWIT naming total time to completion), and CWIT accuracy (measured by uncorrected errors), separately. Then, we used a linear regression model to test the association between group status and DKEFS-CWIT inhibition total time to completion adjusting for age, MMSE score, information processing speed, and CWIT performances, together. Finally, as the distribution of the number of errors was zero-inflated, we used a generalized linear model with a negative binomial log link (which afforded the best fit for the distribution) to examine uncorrected, self-corrected, and total errors. DKEFS Naming Total Time to Completion was used as an information processing speed measure.
Results
Demographic and Clinical Characteristics
The five groups were similar in age, gender, and MMSE scores (Table 1). The four depressed groups did not differ significantly in the severity of depressive symptoms, psychotropic exposure, prevalence of lifetime or current substance use disorders, or burden of medical illness. The suicidal intent of high-lethality and low-lethality suicide attempters was similar.
Table 1. Demographic and clinical characteristics.
| High-lethality suicide attempters (n=17) HSA | Low-lethality suicide attempters (n=14) LSA | Ideators (n=16) SI |
Depressed controls (n=38) D |
Healthy controls (n=17) HC |
F or χ2 | p | Post-hoc Tukey HSD | |
|---|---|---|---|---|---|---|---|---|
| Females, n (%) | 4 (23.5) | 6 (42.9) | 9 (56.3) | 23 (60.5) | 11 (64.7) | 8.3 | .08 | |
| White, n (%) | 17 (100) | 12 (85.7) | 15 (93.8) | 33 (86.8) | 12 (70.6) | 7.4 | .11 | |
| Age, mean±SD | 69.3±6.5 | 64.4±3.5 | 67.4±7.4 | 67.8±6.1 | 71.4±10.2 | 2.1 | .08 | |
| Education (years), mean±SD | 13.0±3.5 | 14.3±2.7 | 14.8±3.1 | 14.6±2.4 | 15.1±1.8 | 1.5 | .19 | |
| MMSE, mean±SD | 27.5±1.8 | 28.5±1.7 | 28.7±1.2 | 28.6±1.9 | 28.9±1.8 | 1.2 | .19 | |
| HAMD-17, mean±SD | 11.2±7.9 | 17.3±6.7 | 16.4±6.6 | 15.7±5.5 | 2.3±2.2 | 18.7 | <.001 | HSA, LSA, SI, D > HC |
| Suicide Ideation Scale, mean±SD | 17.2±12.0 | 18.4±12.7 | 11.4±8.5 | - | - | 26.7 | <.001 | HSA, LSA, SI > D |
| Suicide Intent Scale, mean±SD | 16.4±4.8 | 18.1±6.3 | - | - | - | 0.67 | .42 | |
| Medical Illness Burden (CIRSG), mean±SD | 9.8±4.4 | 8.9±3.3 | 9.6±5.7 | 10.8±3.3 | - | 0.83 | .48 | |
| Lifetime substance use disorders, n (%) | 5 (31.3) | 3 (25) | 1 (7.1) | 3 (8.8) | - | 10.9 | .08 |
HAMD-17 items: Hamilton Depression Inventory 17-items; CIRSG: Cumulative Illness Rating Scale-Geriatrics; MMSE: Mini-Mental State Examination.
HSA: High-Lethality Suicide Attempters, LSA: Low-Lethality Suicide Attempters, SI: Suicide Ideators, D: Depressed controls, HC: Healthy Controls.
Cognitive Inhibition
As we hypothesized that both groups of suicide attempters would show a deficit in cognitive inhibition in comparison to the other groups, and that high-lethality attempters would show the most pronounced deficit, we first compared attempters' performance with controls, and then we separated the attempter group based on the medical severity of the attempt. Compared to healthy and depressed control subjects, suicide attempters demonstrated a higher rate of DKEFS-CWIT inhibition uncorrected (Wald χ2(3)=14.8, p=.002) but not self-corrected errors (Wald χ2(3)=3.7, p=.29, both controlling for age and education), and took more time to complete the DKEFS-CWIT inhibition condition (F(3, 96)=4.9, p=.003, ηp2=.134, controlling for age and education).
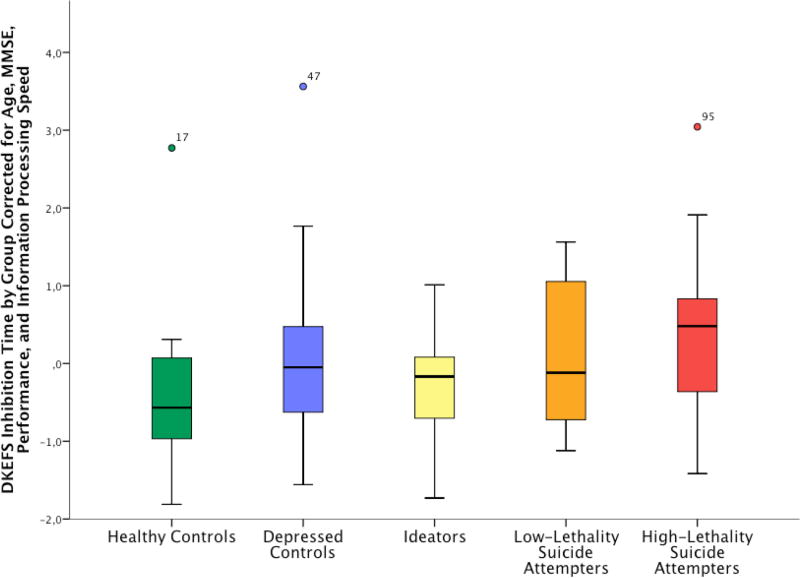
Cognitive Inhibition in High- and Low-Lethality Attempters (Figure 1)
Figure 1. DKEFS Inhibition Time by Group Corrected for Age, MMSE, Accuracy and Information Processing Speed.
High-lethality suicide attempters demonstrated a distinct pattern of cognitive inhibition deficits. Compared to psychiatrically healthy control subjects and suicide ideators, high-lethality attempters took longer to complete inhibition trials, even after accounting for potential confounding factors (age, education, MMSE score, information processing speed, and accuracy). Compared to non-suicidal depressed and healthy control subjects, low-lethality suicide attempters committed more uncorrected errors; however, this difference was not specific to the inhibition condition.
High-lethality suicide attempters demonstrated a distinct pattern of cognitive inhibition deficits. The groups differed in time to complete the inhibition condition of the task (F(4, 97)=3.93, p=.005; ηp2=.081), with high lethality attempters displaying poorer performance compared to depressed and healthy control subjects, and suicide ideators. Compared to depressed control subjects, low-lethality attempters demonstrated an increased rate of DKEFS-CWIT uncorrected errors (Wald χ2(4)=17.5, p=.002, controlling for age and education). No significant difference was found between low- and high-lethality suicide attempters on any of the DKEFS-CWIT inhibition variables. Finally, the groups differed in time to execute the DKEFS-CWIT naming condition (F(4, 97)=4.3, p=.003, ηp2=.154), with high lethality attempters displaying poorer performance compared to healthy and depressed control subjects. We found no significant differences between groups in other DKEFS-CWIT conditions: word- reading, color-naming, and inhibition-switching.
Compared to patients with high-lethality suicide attempts, ideators and healthy control subjects were faster in the inhibition condition, even after controlling for age, MMSE score, information speed processing, and accuracy (Table 2). This difference remained after taking into account the severity of depression.
Table 2. Group Differences in DKEFS Total Time to Completion by Condition, with High-Lethality Suicide Attempters as a Reference Group (n=102).
| Model 1: age-corrected | Model 2: age, MMSE, and information processing speed-corrected* |
Model 3: age, MMSE, information processing speed, and accuracy- corrected*,** |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Independent variable |
Standardized coefficient |
P-Value | ηp2 | Post-hoc | Model R2 |
Standardized coefficient |
P-Value | ηp2 | Post-hoc | Model R2 |
Standardized coefficient |
P-Value | ηp2 | Post-hoc | Model R2 |
| Inhibition Total Time to Completion | |||||||||||||||
|
| |||||||||||||||
| 0.276 | 0.521 | 0.521 | |||||||||||||
|
| |||||||||||||||
| Group | 0.37 | <.001 | .173 | HSA> SI, D, HC | 0.18 | .019 | .084 | HSA> SI, HC | 0.18 | .025 | .081 | HSA> SI, HC | |||
| Age | 0.42 | <.001 | .172 | - | 0.27 | <.001 | .118 | - | 0.27 | .001 | .112 | - | |||
| MMSE | - | - | - | -0.10 | .15 | .017 | - | -0.10 | .18 | .013 | - | ||||
| Color naming time to completion | - | - | - | 0.5 | <.001 | .321 | - | 0.50 | <.001 | .318 | - | ||||
| Uncorrected errors | - | - | - | - | - | - | 0.02 | .76 | .003 | - | |||||
|
| |||||||||||||||
| Inhibition-Switching Total Time to Completion | |||||||||||||||
|
| |||||||||||||||
| 0.165 | 0.530 | 0.547 | |||||||||||||
|
| |||||||||||||||
| Group | 0.26 | .006 | .083 | HSA> D, HC | 0.05 | .47 | .020 | NS | 0.04 | .58 | .014 | NS | |||
| Age | 0.34 | .001 | .107 | - | 0.15 | .038 | .049 | - | 0.16 | .028 | .053 | - | |||
| MMSE | - | - | - | -0.20 | .006 | .082 | - | -0.18 | .014 | .064 | - | ||||
| Color naming time to completion | - | - | - | 0.59 | <.001 | .392 | - | 0.57 | <.001 | .383 | - | ||||
| Uncorrected errors | - | - | - | - | - | - | 0.13 | .063 | .034 | - | |||||
HSA: High-Lethality Suicide Attempters, LSA: Low-Lethality Suicide Attempters, SI: Suicide Ideators, D: Depressed control subjects, HC: Healthy Control subjects.
NS: Non significant.
ηp2: Partial Eta squared
N=100 (one healthy control and one low-lethality suicide attempter were missing the MMSE score).
Gender and education did not explain significant variance in any of the models.
Finally, group differences in inhibition self-corrected errors were diminished when accuracy in the color-naming condition was taken into account, indicating that low-lethality attempters' poor performance was not specific for the inhibition condition (Table 3).
Table 3. Group Differences in DKEFS Inhibition Errors, with High-Lethality Suicide Attempters as a Reference Group (n=100).
| Model 1: age-corrected | Model 2: age-, and MMSE-corrected** | Model 3: age-, MMSE-, and color-naming accuracy-corrected**,*** | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Independent variable | chi2**** | P-Value | ηp2 | Post-hoc | AIC | chi2 | P-Value | ηp2 | Post-hoc | AIC | chi2 | P-Value | ηp2 | Post-hoc | AIC |
| Inhibition Self-Corrected Errors | |||||||||||||||
|
| |||||||||||||||
| 346 | 343 | 337 | |||||||||||||
|
| |||||||||||||||
| Group | 9.5 | .050 | .123 | HSA> D, SI, HC | 5.3 | .26 | .089 | NS | 7.0 | .14 | .093 | NS | |||
| Age | 0.8 | .38 | .006 | - | 0.22 | .64 | .002 | - | 0.0 | .83 | .004 | - | |||
| MMSE | - | - | - | 5.0 | .026 | .083 | - | 4.8 | .029 | .086 | - | ||||
| Color-naming self-corrected errors | - | - | - | - | - | - | 6.3 | .012 | .004 | - | |||||
|
| |||||||||||||||
| Inhibition Uncorrected Errors | |||||||||||||||
|
| |||||||||||||||
| 245 | 239 | 235 | |||||||||||||
|
| |||||||||||||||
| Group | 21.4 | <.001 | .116 | LSA> D, HC HSA>D | 18.1 | .001 | .126 | LSA> HC, D | 9.3 | .055 | .122 | NS | |||
| Age | 6.3 | .012 | .032 | - | 4.2 | .039 | .026 | - | 3.1 | .077 | .020 | - | |||
| MMSE | - | - | - | 9.1 | .003 | .077 | - | 5.9 | .016 | .071 | - | ||||
| Color-naming uncorrected errors | - | - | - | - | - | - | 4.6 | .031 | .005 | - | |||||
HSA: High-Lethality Suicide Attempters, LSA: Low-Lethality Suicide Attempters, SI: Suicide Ideators, D: Depressed control subjects, HC: Healthy Control subjects.
NS: Non significant.
ηp2: Partial Eta squared
Generalized linear model using a negative binomial log link.
Two participants with missing MMSE scores were excluded from every model to compare fits.
Gender and time to completion did not explain additional variance in any of the models. Any variance explained by education was absorbed by the MMSE.
df: group, 4; continuous predictors, 1.
Discussion
In the present study, high-lethality suicide attempters displayed impaired cognitive inhibition when compared with depressed control subjects, healthy control subjects, and suicide ideators. High-lethality attempters took longer to complete the inhibition condition of the task compared to all other groups, except low-lethality attempters, who demonstrated an intermediate performance. This difference remained after taking into account possible confounders including education, global cognitive performance, and information processing speed. Interestingly, low-lethality suicide attempters tended to make more uncorrected errors in all conditions, suggesting a careless approach to the task or a lack of monitoring. These results indicate that people with a history of attempted suicide display a considerable cognitive heterogeneity, which can be parsed to some extent by grouping them into high- vs. low-lethality attempters (Dombrovski et al., 2011a, Keilp et al., 2001, McGirr et al., 2012). Finally, we found no differences between groups for DKEFS-CWIT inhibition-switching, which it is not surprising in view of low sensitivity of this condition to individual differences in cognitive control ability (Lippa and Davis, 2010).
There is accumulating evidence that people vulnerable to suicide display deficits in various aspects of cognitive control. We have previously reported that, in an overlapping sample of older people with depression, a history of high-lethality attempts predicted poor performance on the Wisconsin Card Sort (McGirr et al., 2012). In a US study of younger adults, Keilp and colleagues (Keilp et al., 2013) found an impairment in Stroop performance in suicide attempters compared to both patient and healthy control subjects. Our finding of a high uncorrected error rate in low-lethality attempters parallels that of Brazilian study by Malloy-Diniz and colleagues (Malloy-Diniz et al., 2009) who reported a positive correlation between the number of suicide attempts and the number of errors on the Stroop test in younger bipolar I patients. Findings, however, are mixed with respect to the motor component of cognitive inhibition, captured by the Go/No-Go test (Keilp et al., 2013, Raust et al., 2007, Richard-Devantoy et al., 2012). The only previous study of cognitive inhibition in elderly suicide attempters (Richard-Devantoy et al., 2012) had found significant impairments in access to relevant information and deletion of irrelevant information in comparison to both depressed and healthy control groups. That study, however, lacked the power to subdivide suicide attempters according to lethality of suicide attempt, nor did it include a comparison group of suicide ideators. The intermediate performance of suicide ideators in the current sample suggests a dose-response relationship between cognitive control deficits and the progression of the suicidal process. This dose-response relationship, however, was lacking in our previous analysis of a screening measure of cognitive control, EXIT25, in a larger, overlapping sample of older adults (Gujral et al., 2012), where suicide ideators performed as poorly as suicide attempters.
How exactly do cognitive control deficits contribute to suicidal behavior? Cognitive control can be thought of as the ability to organize information in a way that helps achieve the best outcome. It underlies certain (but not all) aspects of decision-making, which in turn appears impaired in younger (Jollant et al., 2005) and older (Dombrovski et al., 2010, Dombrovski et al., 2011a) suicide attempters. Decisions that involve uncertainty, options with multiple features, and changes over time place particularly high demands on cognitive control (Walton et al., 2010)(Dombrovski et al., under review). Since the suicidal process involves 1) a catastrophic accumulation of stressors (Mathew and Nanoo, 2013), such as widowhood (Erlangsen et al., 2004) and physical illness (Juurlink et al., 2004), 2) an inability to find alternative solutions (Pollock and Williams, 2001, Pollock and Williams, 2004), and 3) the disregard for the tragic consequences of suicide (Baumeister, 1990, Williams and Pollock, 2001), future experiments need to elucidate the contribution of poor cognitive control to these failures of real-life problem-solving and decision-making. We propose the hypothesis that the inability to find and implement alternative solutions in a suicidal crisis is the most direct consequence of cognitive control deficit.
Deficits of cognitive inhibition in suicidal older patients may be related to the dysfunction of the lateral prefronto-parietal network (Alichniewicz et al., 2013, Coderre and van Heuven, 2013, Harrison et al., 2005). Cognitive control abilities in general depend on the associative cortices comprising the lateral frontoparietal and cinguloopercular networks (Milham et al., 2002)(Dombrovski et al., under review). Studies have implicated that the dorsolateral prefrontal cortex is crucial in working memory processes and in the ability to inhibit responses (Kane and Engle, 2002, McDowd et al., 1995). Indeed, initial results from FDG-PET (Sublette et al., 2013) and fMRI studies (Jollant et al., 2011) point to frontoparietal and cinguloopercular alterations in attempted suicide. FDG-PET studies have found lower regional cerebral metabolic rates of glucose in right dorsolateral prefrontal regions of suicide attempters compared to depressed non-suicidal subjects (Sublette et al., 2013). Deficits in cognitive control and the putative frontoparietal and cinguloopercular alterations in attempted suicide appear distinct from impairments in value-based decision-making paralleled by paralimbic and particularly vmPFC dysfunction (Glascher et al., 2012, Noonan et al., 2010) and anterior cingulate cortex (Glascher et al., 2012). These latter deficits are illustrated by our recent findings of paralimbic and particularly vmPFC disruptions during value-based decision-making in older suicide attempters, which correlated with high impulsivity, a neglect of decision-relevant information, and poorly planned suicide attempts (Dombrovski et al., 2013). Thus, we would argue for the existence of two independent vulnerability pathways, marked by cognitive control/frontoparrietal vs. value/paralimbic dysfunction. The first pathway, illustrated by the current behavioral findings, may involve an inability to find and implement alternative solutions in a crisis, and can be further probed by cognitive control tasks and paradigms involving uncertainty. The second, “value/paralimbic” pathway, may involve impulsivity, a low threshold for suicidal acts, and a disregard of deterrents, and can be probed by paradigms that involve value comparisons, such as gambling or intertemporal choice tasks. .
It has also been shown that alterations of cognitive inhibition in depressed elderly patients were associated with poor antidepressant response (Alexopoulos et al., 2005, Alexopoulos et al., 2009, Butters et al., 2004b, Murphy and Alexopoulos, 2006). Early identification of inhibition impairments would not only facilitate recognition of individuals at risk of suicide (especially high-lethality attempters) but also inform compensatory therapeutic approaches (Alexopoulos et al., 2009).
Limitations
Our findings are limited by a case-control design and retrospective assessment of suicidal behavior. Another limitation is the relatively modest group size, as we dichotomized the group of suicide attempters based on lethality. While the design dissociates the role of major depression, it does not identify whether the deficits in cognitive control are stable or state-dependent.
Conclusion
Clinicians need useful predictors of late-life suicide attempts (Alexopoulos et al., 2009), above and beyond correlates of depression. The consistency with which cognitive control deficits are implicated in suicidal behavior across samples and age groups highlights their potential clinical predictive utility. Of all facets of cognitive control, poor cognitive inhibition shows perhaps the strongest association with suicidal behavior. Finally, an understanding of cognitive vulnerability to suicide could guide clinicians in developing remedial and compensatory interventions for older people at risk (Kiosses et al., 2010, McLennan and Mathias, 2010).
Key points.
Cognitive control was impaired in elderly suicide attempters.
Older high-lethality suicide attempters displayed an exaggerated Stroop effect, even after accounting for a number of possible confounders.
Low-lethality, but not high-lethality, suicide attempters committed more uncorrected errors across conditions, suggesting a careless approach to the task.
Inhibition deficits in high-lethality attempters may undermine their ability to solve real-life problems, precipitating a catastrophic accumulation of unresolved problems culminating in a suicidal crisis.
Acknowledgments
None.
Role of funding source: This study was supported by grants from the National Institute of Mental Health (NIMH) to Katalin Szanto (R01 MH05436 and K23 MH070471) and Alexandre Dombrovski (K23 MH086620). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflicts of interest: Meryl Butters has received remuneration from GlaxoSmithKline for performing neuropsychological assessment services. No other author declares that they have no conflicts of interest.
Contributors: KS and AD obtained funding. KS, AD, and MB designed the study and oversaw subject recruitment and data collection. SRD performed the statistical analyses with statistical review by AD. SRD drafted the manuscript, which was edited and finalized by AD, MB, JK, & KS.
References
- Alexopoulos GS, Katz IR, Bruce ML, Heo M, Ten Have T, Raue P, Bogner HR, Schulberg HC, Mulsant BH, Reynolds CF, 3rd PROSPECT Group. Remission in depressed geriatric primary care patients: a report from the PROSPECT study. Am J Psychiatry. 2005;162:718–724. doi: 10.1176/appi.ajp.162.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Reynolds CF, 3rd, Bruce ML, Katz IR, Raue PJ, Mulsant BH, Oslin DW, Ten Have T. PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166:882–890. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alichniewicz KK, Brunner F, Klunemann HH, Greenlee MW. Neural correlates of saccadic inhibition in healthy elderly and patients with amnestic mild cognitive impairment. Front Psychol. 2013;4:467. doi: 10.3389/fpsyg.2013.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Lafont S, Rouch-Leroyer I, Rainville C, Dartigues JF, Orgogozo JM, Fabrigoule C. Evidencing inhibitory deficits in Alzheimer's disease through interference effects and shifting disabilities in the Stroop test. Arch Clin Neuropsychol. 2004;19:791–803. doi: 10.1016/j.acn.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: executive control. Schizophr Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97:90–113. doi: 10.1037/0033-295x.97.1.90. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- Beck AT, Schuyler D, Herman I. Development of Suicidal Intent Scales. In: Beck AT, Resnik P, Lettier AJ, editors. The Prediction of Suicide. Bowie, Maryland: Charles Press Publishing; 1974. [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142:559–563. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF., 3rd Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004a;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004b;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Clark L, Dombrovski AY, Siegle GJ, Butters MA, Shollenberger CL, Sahakian BJ, Szanto K. Impairment in risk-sensitive decision-making in older suicide attempters with depression. Psychol Aging. 2011;26:321–330. doi: 10.1037/a0021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre EL, van Heuven WJ. Modulations of the executive control network by stimulus onset asynchrony in a Stroop task. BMC Neurosci. 2013;14:79. doi: 10.1186/1471-2202-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 1996;153:1001–1008. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- Darowski ES, Helder E, Zacks RT, Hasher L, Hambrick DZ. Age-related differences in cognition: the role of distraction control. Neuropsychology. 2008;22:638–644. doi: 10.1037/0894-4105.22.5.638. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis–Kaplan Executive Function System (D-KEFS): Examiner's manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dombrovski AY, Butters MA, Reynolds CF, 3rd, Houck PR, Clark L, Mazumdar S, Szanto K. Cognitive Performance in Suicidal Depressed Elderly: Preliminary Report. Am J Geriatr Psychiatry. 2008a;16:109–115. doi: 10.1097/JGP.0b013e3180f6338d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Butters MA, Reynolds CF, 3rd, Houck PR, Clark L, Mazumdar S, Szanto K. Cognitive performance in suicidal depressed elderly: preliminary report. The American Journal of Geriatric Psychiatry. 2008b;16:109–115. doi: 10.1097/JGP.0b013e3180f6338d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Clark L, Siegle GJ, Butters MA, Ichikawa N, Sahakian BJ, Szanto K. Reward/Punishment reversal learning in older suicide attempters. Am J Psychiatry. 2010;167:699–707. doi: 10.1176/appi.ajp.2009.09030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Clark L, Reynolds CF, Siegle GJ. Reward Signals, Attempted Suicide, and Impulsivity in Late-Life Depression. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Siegle GJ, Wallace ML, Forman SD, Sahakian B, Reynolds CF, 3rd, Clark L. Lethal forethought: delayed reward discounting differentiates high- and low-lethality suicide attempts in old age. Biol Psychiatry. 2011a;70:138–144. doi: 10.1016/j.biopsych.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Siegle GJ, Wallace ML, Forman SD, Sahakian B, Reynolds CFr, 3rd, Clark L. Lethal forethought: delayed reward discounting differentiates high- and low-lethality suicide attempts in old age. Biol Psychiatry. 2011b;70:138–144. doi: 10.1016/j.biopsych.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA, Langenecker SA, Greenfield LJ. Cognitive functioning, retirement status, and age: results from the Cognitive Changes and Retirement among Senior Surgeons study. J Am Coll Surg. 2010;211:303–307. doi: 10.1016/j.jamcollsurg.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlangsen A, Jeune B, Bille-Brahe U, Vaupel JW. Loss of partner and suicide risks among oldest old: a population-based register study. Age Ageing. 2004;33:378–383. doi: 10.1093/ageing/afh128. [DOI] [PubMed] [Google Scholar]
- Erlangsen A, Zarit SH, Conwell Y. Hospital-diagnosed dementia and suicide: a longitudinal study using prospective, nationwide register data. Am J Geriatr Psychiatry. 2008;16:220–228. doi: 10.1097/JGP.0b013e3181602a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen. New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- Folstein MF, F S, Mc Hugh PR. “Mini-mental state”. A pratical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral S, Dombrovski AY, Butters M, Clark L, Reynolds CF, 3rd, Szanto K. Impaired Executive Function in Contemplated and Attempted Suicide in Late Life. Am J Geriatr Psychiatry. 2012 doi: 10.1016/j.jagp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Shaw M, Yucel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olver JS, Nathan PJ, Pantelis C. Functional connectivity during Stroop task performance. Neuroimage. 2005;24:181–191. doi: 10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, Cynthia PM. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance XVII. Cambridge: MIT Press; 1999. [Google Scholar]
- Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. Am J Psychiatry. 2005;162:304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World J Biol Psychiatry. 2011;12:319–339. doi: 10.3109/15622975.2011.556200. [DOI] [PubMed] [Google Scholar]
- Juurlink DN, Herrmann N, Szalai JP, Kopp A, Redelmeier DA. Medical illness and the risk of suicide in the elderly. Arch Intern Med. 2004;164:1179–1184. doi: 10.1001/archinte.164.11.1179. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, Mann JJ. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2013;43:539–551. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. 2001;158:735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- Kiosses DN, Arean PA, Teri L, Alexopoulos GS. Home-delivered problem adaptation therapy (PATH) for depressed, cognitively impaired, disabled elders: A preliminary study. Am J Geriatr Psychiatry. 2010;18:988–998. doi: 10.1097/JGP.0b013e3181d6947d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa SM, Davis RN. Inhibition/switching is not necessarily harder than inhibition: an analysis of the D-KEFS color-word interference test. Arch Clin Neuropsychol. 2010;25:146–152. doi: 10.1093/arclin/acq001. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz LF, Neves FS, Abrantes SS, Fuentes D, Correa H. Suicide behavior and neuropsychological assessment of type I bipolar patients. J Affect Disord. 2009;112:231–236. doi: 10.1016/j.jad.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Marzuk PM, Hartwell N, Leon AC, Portera L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112:294–301. doi: 10.1111/j.1600-0447.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Mathew A, Nanoo S. Psychosocial stressors and patterns of coping in adolescent suicide attempters. Indian J Psychol Med. 2013;35:39–46. doi: 10.4103/0253-7176.112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowd JM, Oseas-Kreger DM, Filion DL. Inhibitory processes in cognition and aging. In: Dempster FN, Brainerd CJ, editors. Interference and inhibition in cognition. San diego: Academic Press; 1995. [Google Scholar]
- McGirr A, Dombrovski AY, Butters MA, Clark L, Szanto K. Deterministic learning and attempted suicide among older depressed individuals: cognitive assessment using the Wisconsin Card Sorting Task. J Psychiatr Res. 2012;46:226–232. doi: 10.1016/j.jpsychires.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Turecki G. The relationship of impulsive aggressiveness to suicidality and other depression-linked behaviors. Current Psychiatry Reports. 2007;9:460–466. doi: 10.1007/s11920-007-0062-2. [DOI] [PubMed] [Google Scholar]
- McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. International Journal of Geriatric Psychiatry. 2010;25:933–944. doi: 10.1002/gps.2431. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Miyake A, Emerson MJ, Friedman NP. Assessment of executive functions in clinical settings: problems and recommendations. Semin Speech Lang. 2000;21:169–183. doi: 10.1055/s-2000-7563. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Alexopoulos GS. Attention network dysfunction and treatment response of geriatric depression. Journal of Clinical and Experimental Neuropsychology. 2006;28:96–100. doi: 10.1080/13803390490918101. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll PW, Berman AL, Maris RW, Moscicki EK, Tanney BL, Silverman MM. Beyond the Tower of Babel: a nomenclature for suicidology. Suicide Life Threat Behav. 1996;26:237–252. [PubMed] [Google Scholar]
- Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, Kegeles LS, Cooper TB, Parsey RV, van Heertum RL, Mann JJ. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- Pollock LR, Williams JM. Effective problem solving in suicide attempters depends on specific autobiographical recall. Suicide Life Threat Behav. 2001;31:386–396. doi: 10.1521/suli.31.4.386.22041. [DOI] [PubMed] [Google Scholar]
- Pollock LR, Williams JM. Problem-solving in suicide attempters. Psychol Med. 2004;34:163–167. doi: 10.1017/s0033291703008092. [DOI] [PubMed] [Google Scholar]
- Raust A, Slama F, Mathieu F, Roy I, Chenu A, Koncke D, Fouques D, Jollant F, Jouvent E, Courtet P, Leboyer M, Bellivier F. Prefrontal cortex dysfunction in patients with suicidal behavior. Psychol Med. 2007;37:411–419. doi: 10.1017/S0033291706009111. [DOI] [PubMed] [Google Scholar]
- Richard-Devantoy S, Jollant F, Kefi Z, Turecki G, Olie JP, Annweiler C, Beauchet O, Le Gall D. Deficit of cognitive inhibition in depressed elderly: a neurocognitive marker of suicidal risk. J Affect Disord. 2012;140:193–199. doi: 10.1016/j.jad.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;6:643–661. [Google Scholar]
- Sublette ME, Milak MS, Galfalvy HC, Oquendo MA, Malone KM, Mann JJ. Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Arch Suicide Res. 2013;17:434–447. doi: 10.1080/13811118.2013.801813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto K, Holly G, Reynolds C., III Suicide in the elderly. Clinical Neuroscience Research. 2001;1:366–376. [Google Scholar]
- Szanto K, Reynolds CF, 3rd, Conwell Y, Begley AE, Houck P. High levels of hopelessness persist in geriatric patients with remitted depression and a history of attempted suicide. J Am Geriatr Soc. 1998;46:1401–1406. doi: 10.1111/j.1532-5415.1998.tb06007.x. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Turecki G, Ernst C, Jollant F, Labonte B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Waern M, Rubenowitz E, Wilhelmson K. Predictors of suicide in the old elderly. Gerontology. 2003;49:328–334. doi: 10.1159/000071715. [DOI] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Pollock LR. Psychological aspects of the suicidal process. In: van Heeringen K, editor. Understanding suicidal behaviour. Chichester: WS: Wiley; 2001. [Google Scholar]