Abstract
Background
Adherence to pre-exposure prophylaxis (PrEP) is critical for efficacy. Antiretroviral concentrations are an objective measure of PrEP use and correlate with efficacy. Understanding patterns and correlates of drug detection can identify populations at risk for non-adherence and inform design of PrEP adherence interventions.
Methods
Blood antiretroviral concentrations were assessed among active-arm participants in iPrEx, a randomized, placebo-controlled trial of emtricitabine/tenofovir in men who have sex with men (MSM) and transgender women in 6 countries. We evaluated rates and correlates of drug detection among a random sample of 470 participants at week 8 and a longitudinal cohort of 303 participants through 72 weeks of follow-up.
Results
Overall, 55% (95% CI 49–60%) of participants tested at week 8 had drug detected. Drug detection was associated with older age and varied by study site. In longitudinal analysis, 31% never had drug detected, 30% always had drug detected, and 39% had an inconsistent pattern. Overall detection rates declined over time. Drug detection at some or all visits was associated with older age; indices of sexual risk, including condomless receptive anal sex; and responding "don't know" to a question about belief of PrEP efficacy (0–10 scale).
Conclusions
Distinct patterns of study-product use were identified, with a significant proportion demonstrating no drug detection at any visit. Research literacy may explain greater drug detection among populations having greater research experience, such as older MSM in the US. Greater drug detection among those reporting highest-risk sexual practices is expected to increase the impact and cost-effectiveness of PrEP.
Keywords: pre-exposure prophylaxis, drug detection, emtricitabine/tenofovir, adherence, men who have sex with men
INTRODUCTION
The use of antiretroviral pre-exposure prophylaxis (PrEP) for HIV prevention has generated great interest and promise, with four recently completed randomized clinical trials demonstrating its safety and efficacy across different populations.1–4 A key finding from these studies is the critical relationship between adherence and PrEP efficacy.5,6 For example, in the Global iPrEx study, the efficacy of daily oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) was 42% on an intention-to-treat basis, but was estimated to be >90% among those with drug detected at any concentration in blood.4,7 Similarly, in the CAPRISA 004 microbicide study, having a tenofovir (TFV) level of >1000 ng/ml in cervicovaginal fluid was associated with significant efficacy (74%), while a TFV level below this threshold was not protective.7,8 While adherence and efficacy were both high among HIV-uninfected men and women in serodiscordant couples in the Partners PrEP study,1 the FEM-PrEP and VOICE studies were unable to demonstrate PrEP efficacy in African women, due in part to low study product adherence.9,10
Although self-reported and pill-count based adherence data have been commonly used in research settings, including PrEP studies, these measures are subject to social desirability bias and potential manipulation11,12 and have been shown to over-estimate PrEP use across a number of studies.13 The analysis of pharmacologic levels of antiretroviral drugs has emerged as an objective measure of study product use in PrEP trials1–4 and may help identify groups at risk for non-adherence, who may benefit from adherence support, and help identify those most likely to adhere to the PrEP regimen.
The Global iPrEx Study, the only PrEP efficacy trial among men who have sex with men (MSM) and transgender women, was conducted in 6 countries in North and South America, Asia, and Africa.4 We evaluated rates and correlates of PrEP drug detection in blood early in the study and longitudinally among participants in this trial.
METHODS
Study population
The Global iPrEx study was a phase 3, double-blind, randomized, placebo-controlled trial evaluating the safety and efficacy of FTC/TDF PrEP in 2,499 HIV-uninfected MSM and transgender women, with 11 study sites in the Peru, Ecuador, Brazil, Thailand, South Africa, and the United States. Participants returned for follow-up visits every 4 weeks after enrollment for HIV testing, counseling, and medication dispensation. Details of the study design, eligibility criteria, and primary results have been previously published.4
Selection of samples for pharmacologic analysis
We report two analyses of drug levels in active arm participants in iPrEx: 1) a cross-sectional analysis of prevalence and correlates of drug detection at week 8, reflecting initiation and early adherence to or discontinuation of study drug, and 2) a longitudinal analysis of patterns of drug detection across multiple time-points in the trial through 72 weeks of participation, reflecting persistence and consistency of use of study drug in the cohort.
For the cross-sectional analysis, a random sample of serum specimens stored at week 8 was selected for testing, stratified by study site. Specifically, 25% of the active-arm specimens or at least 40 specimens (whichever was larger) were selected from each site. If less than 40 specimens were available for a given site, all week 8 specimens were tested. For the longitudinal analysis, we analyzed all available plasma (stored every 12 weeks) and peripheral blood mononuclear cells (PBMC) samples (stored every 24 weeks) from participants in 1) the DEXA substudy evaluating the impact of FTC/TDF on bone and body composition (from 7 sites in the US, Peru, Brazil, Thailand, and South Africa); and/or 2) matched active-arm controls in the case-control substudy of seroconverters (from 9 iPrEx sites with active arm seroconversions).14
Sample collection and processing
The methods for collection and processing of serum, plasma and PBMC samples have been published previously.7 Briefly, whole blood was collected in EDTA and serum separator tubes and processed within 24 hours of collection (4–12 hours at most sites). Serum and plasma samples were analyzed for TFV and FTC via a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay.15 The linearity of the concentration curves was in the range of 10–1500 ng/ml, and the lower limit of quantification was 10 ng/ml for both analytes.
Procedures for processing viable PBMCs have also been previously described7 (see Supplemental Digital Content 1).
Correlates of drug detection variables
Potential correlates of drug detection included socio-demographics; sexual and drug use behaviors; medical conditions and symptoms; and beliefs about HIV risk, treatment assignment and PrEP efficacy collected from visit interviews, clinical assessments or computer-assisted self-interview (CASI). Sociodemographic characteristics included age, location (city), and living situation collected via staff interview, and education level and transgender identity collected via CASI at screening. Sexual behaviors over the previous 3-months were assessed at screening via interview and included numbers of sex partners and reports of receptive anal intercourse without a condom (no condom Receptive Anal Intercourse: ncRAI). ncRAI reported at follow-up visits were also included in the longitudinal analysis of drug detection. Substance use behaviors included CASI-collected frequency of alcohol use and number of drinks per day on days when alcoholic beverages were consumed, and use of stimulants (methamphetamine or cocaine) in the past month. Medical assessments included having a self-reported sexually transmitted infection (STI, e.g. gonorrhea, chlamydia, or syphilis) or depression self-reported or diagnosed at screening by clinician assessment, baseline creatinine clearance, and whether the participant was circumcised. Beliefs about lifetime HIV risk, treatment assignment and PrEP efficacy were assessed via CASI (see supplemental digital content). To assess the impact of a potential "start-up syndrome" previously described4 with PrEP initiation on subsequent drug detection, clinical symptoms recorded via a symptom checklist including nausea, vomiting, diarrhea, abdominal pain, flatulence, and headache were analyzed at weeks 4 and 8 for the week 8 cohort and weeks 12–24 in the longitudinal cohort.
Statistical Analysis
Cross sectional analysis of drug detection
Our primary outcome for the cross-sectional analysis was having any drug detection in serum (either TFV or FTC) at week 8, defined as above the lower limit of quantification of 10 ng/mL. For each of the independent variables evaluated as correlates of drug detection, we calculated frequencies for these categorical variables and determined proportion of drug detection for each of the categories, adjusted and weighted by site to reflect the full cohort. For each outcome, univariable logistic regression models with inclusion of site as a fixed effect and incorporating sampling weights were used to assess the association between the outcome and covariates. For outcomes with more than one significant predictor in univariable models, factors associated with drug detection (P <0.10) were entered into multivariable models.
Longitudinal analyses
Analyses of patterns of drug detection over time were restricted to participants with 2 or more drug levels available in the longitudinal cohort. Drug detection in plasma or PBMC was defined as above the lower limit of quantification, as described above. At time points where both plasma and PBMCs were available, drug was considered detectable if either TFV or FTC were detected in plasma, or TFV-DP or FTC-TP were detected in PBMCs, to provide the most inclusive definition of product use. The proportion of individuals with drug detected at no visits, some visits (inconsistent pattern), and all visits were calculated, adjusting for site differences and number of samples tested. To evaluate correlates of drug detection at some or all visits, a multinomial logistic regression was performed, comparing participants who sometimes or always had drug detected with those who never had drug detected.17 To evaluate persistence of study drug use in this cohort, we determined the proportion of subjects with drug detected at the first visit but had no drug detected at a later time-point and at all subsequent visits, and calculated the median time to stopping drug, using the first date of non-detection that was subsequently followed by consistent non-detection. To evaluate whether persistence of symptoms was associated with drug discontinuation in the longitudinal cohort, we assessed among participants with drug detected at week 12, whether symptoms reported in the subsequent 12 weeks (between week 12 and 24) were associated with drug detection at week 24. To estimate the trajectory of drug detection over time in the overall cohort, we fit a regression model to samples tested in the longitudinal cohort with a restricted cubic spline for week. Plasma drug detection patterns for each individual in the longitudinal cohort were also plotted graphically by visit week.
RESULTS
Study participants
Of 2,499 participants enrolled in iPrEx, 1,251 were assigned to the active arm. Overall, 470 (38%) active arm participants had a sample tested at week 8, and 303 (24%) had specimens tested as part of the longitudinal cohort (Table 1), with 135 (11%) tested in both cohorts. Approximately 2% (30/1638) of specimens were tested at visits when the participant was on a drug interruption (period during which study drug was not dispensed by study staff); drug was detected in only one of these samples. About half of participants were age 25 or younger, and over three-quarters had at least a high school education. At screening, the majority reported ncRAI in the prior 3 months, and over two-thirds perceived they were at moderate or high risk for HIV infection. Participants selected for week 8 and/or for longitudinal testing did not differ significantly from overall active arm participants with respect to variables in Table 1, except there were more STIs reported at baseline in the longitudinal cohort (p<0.001) and a trend towards lower rates of ncRAI in the week 8 cohort (p=0.056). Participants tested at week 8 and in the longitudinal cohort were less likely to be from South America (68%) compared with the overall active arm (83%), reflecting over-sampling of smaller sites outside the Andean region to allow adequate comparison of drug detection by site.
Table 1.
Baseline characteristics of iPrEx participants with drug levels tested at week 8 or in longitudinal cohort
| Characteristic (n, %) | Active arm participants (N=1,251) |
Active arm participants with week 8 testing (N=470) |
Active arm participants with longitudinal testing (N=303) |
|---|---|---|---|
| Age ≤20 >20–25 >25–30 >30 |
270 (22%) 387 (31%) 236 (19%) 358 (29%) |
99 (21%) 134 (29%) 87 (19%) 150 (32%) |
60 (20%) 94 (32%) 45 (15%) 99 (33%) |
| Education Less than high school Some high school Some college or more |
279 (23%) 675 (55%) 280 (23%) |
105 (23%) 213 (46%) 142 (31%) |
174 (23%) 462 (60%) 138 (18%) |
| Race White Black Mixed race or other Asian |
224 (18%) 117 (9%) 847 (68%) 63 (5%) |
116 (25%) 73 (16%) 238 (51%) 43 (9%) |
49 (16%) 35 (12%) 167 (56%) 47 (16%) |
| Site, by location Lima, Peru (2 sites) Iquitos, Peru Guayaquil, Ecuador Rio de Janeiro, Brazil (2 sites) Sao Paulo, Brazil San Francisco, USA Boston, USA Chiang Mai, Thailand Cape Town, South Africa |
470 (38%) 230 (18%) 150 (12%) 147 (12%) 39 (3%) 70 (6%) 43 (3%) 57 (5%) 45 (4%) |
107 (23%) 56 (12%) 40 (9%) 80 (17%) 35 (7%) 40 (9%) 36 (8%) 40 (9%) 36 (8%) |
136 (46%) 16 (5%) 14 (5%) 30 (10%) -- 32 (11%) -- 44 (15%) 26 (9%) |
| Transgender | 163 (13%) | 61 (13%) | 40 (13%) |
| # sex partners at baseline (prior 3 mo) ≤1 male partner >1–5 partners >5–10 partners >10 partners |
113 (9%) 423 (34%) 264 (21%) 451 (36%) |
45 (10%) 190 (40%) 87 (19%) 148 (31%) |
28 (9%) 89 (30%) 68 (23%) 113 (38%) |
| Unprotected receptive anal sex, at baseline (prior 3 mo) | 732 (58%) | 267 (57%) | 180 (60%) |
| Alcohol use, at baseline (prior mo.) ≥2–3 times/week |
861 (70%) |
318 (67%) |
203 (68%) |
| # drinks per day when drinking (prior mo.) ≥5 |
666 (53%) |
240 (51%) |
135 (45%) |
| Meth or cocaine use, at baseline (prior mo.) | 93(7%) | 55 (12%) | 25 (8%) |
| Sexually transmitted infection at screening | 336 (27%) | 105 (23%) | 83 (28%)† |
| Depression at screening | 60 (5%) | 34(7%) | 18 (6%) |
| Circumcised | 162 (13%) | 95 (21%) | 42 (14%) |
| Perceived likelihood of HIV, at baseline* Not likely Could happen Probably/almost certainly will happen |
213 (19%) 736 (64%) 193 (17%) |
100 (21%) 286 (61%) 54 (11%) |
50 (15%) 288 (68%) 58 (17%) |
| Living situation With male or female sexual partner Alone With family or friends Other |
97 (8%) 191 (15%) 944 (75%) 19 (2%) |
47 (10%) 91 (20%) 313 (68%) 9(2%) |
22 (7%) 49 (16%) 218 (73%) 9 (3%) |
| Concern about a place to live Not concerned Somewhat/very concerned |
569 (47%) 654 (54%) |
222 (49%) 230 (51%) |
129 (44%) 167 (56%) |
| Concern about having a job Not concerned Somewhat/very concerned |
388 (32%) 834 (68%) |
147 (33%) 304 (67%) |
86 (29%) 210 (71%) |
Self-assessment of how likely they will become HIV infected in their lifetime
P<0.05 compared with overall active arm participants
Serum drug detection at week 8
Overall, 55% (95% CI 49–60%) of week 8 samples tested had drug detected. Detection of TFV and FTC were concordant in 99% samples. Rates of drug detection varied significantly by site (Table 2), ranging from 35% in Lima, Peru, to 90% in San Francisco, USA. The proportion of participants with drug detection according to socio-demographic variables and reported behaviors is shown in Table 3. Drug detection was significantly associated with older age (Table 3). Level of education, number of male sex partners, ncRAI, alcohol or drug use, creatinine clearance, perceived treatment assignment and PrEP efficacy, being transgender, living situation, and concern about having a job or place to live were not significantly associated with drug detection at week 8. Reporting gastrointestinal symptoms (nausea, vomiting, diarrhea, flatulence, or abdominal pain) or headache at 4 or 8 weeks after PrEP initiation was not associated with drug detection at week 8 (all p values >0.50).
Table 2.
Age and drug detection at week 8 and longitudinally, by site
| Site | Total N | Median age |
% with drug detected at week 8 |
% with never detection |
% with inconsistent detection |
% with always detection |
|
|---|---|---|---|---|---|---|---|
| Overall | 2,499 | 24 | 55% (95% CI 49% –60%) | 31% | 39% | 30% | |
| Lima, Peru (2 sites) | 940 | (38%) | 25 | 35% (95% CI 27%–45%) | 45% | 37% | 17% |
| Iquitos, Peru | 460 | (18%) | 22 | 55% (95% CI 42%–68%) | 19% | 63% | 19% |
| Guayaquil, Ecuador | 300 | (12%) | 22 | 60% (95% CI 44%–74%) | 14% | 64% | 21% |
| Rio de Janeiro, Brazil (2 sites) | 294 | (12%) | 25 | 71% (95% CI 60%–80%) | 26% | 35% | 39% |
| Sao Paulo, Brazil | 76 | (3%) | 29 | 77% (95% CI 60%–88%) | -- | -- | -- |
| San Francisco, USA | 140 | (6%) | 37 | 90% (95% CI 76%–96%) | 1% | 27% | 67% |
| Boston, USA | 87 | (3%) | 41 | 72% (95% CI 56%–84%) | -- | -- | -- |
| Chiang Mai, Thailand | 114 | (5%) | 21 | 72% (95% CI 56%–84%) | 23% | 41% | 36% |
| Cape Town, South Africa | 88 | (4%) | 23 | 68% (95% CI 52%–80%) | 29% | 32% | 39% |
Total N represents number of participants enrolled at each study site.
Table 3.
Proportion and factors associated with drug detection in serum, week 8
| Drug detected† | ||||
|---|---|---|---|---|
| Characteristic | % of samples |
% with drug detected* |
OR (95% CI) | P value |
| Age ≤20 21–25 26–30 >30 |
21% 29% 19% 32% |
40% 59% 56% 62% |
(ref) 2.44 (1.24 to 4.77) 2.18 (1.06 to 4.49) 2.86 (1.36 to 6.03) |
0.009 0.035 0.006 |
| Education Less than high school High School Graduate Some college or more |
23% 46% 31% |
60% 49% 64% |
(ref) 0.63 (0.36 to 1.12) 1.21 (0.59 to 2.49) |
0.114 0.609 |
| Transgender No Yes |
87% 13% |
54% 55% |
(ref) 1.03 (0.55 to 1.96) |
0.918 |
| # sex partners at baseline (prior 3 mo) ≤1 male partner >1–5 partners >5–10 partners >10 partners |
10% 40% 19% 32% |
51% 53% 51% 59% |
(ref) 1.06 (0.46 to 2.45) 0.97 (0.38 to 2.46) 1.42 (0.55 to 3.64) |
0.9 0.941 0.464 |
| Unprotected receptive anal intercourse at baseline (prior 3 mo) No Yes |
43% 57% |
51% 57% |
(ref) 1.31 (0.81 to 2.13) |
0.273 |
| Alcohol use, at baseline (prior month) <2–3 times/week ≥2–3 times/week, but less than daily |
32% 68% |
53% 55% |
(ref) 1.10 (0.68 to 1.79) |
0.7 |
| Alcohol use, at baseline (drinks/day) <5 ≥5 Missing |
46% 51% 3% |
54% 54% 69% |
(ref) 0.97 (0.60 to 1.57) 2.05 (0.54 to 7.81) |
0.91 0.293 |
| Meth/cocaine use (prior mo.) No Yes |
64% 36% |
55% 53% |
(ref) 0.90 (0.55 to 1.49) |
0.689 |
| Perceived treatment assignment (week 12) Placebo Don’t know Truvada |
11% 62% 27% |
45% 58% 54% |
(ref) 1.85 (0.81 to 4.21) 1.55 (0.63 to 3.77) |
0.141 0.337 |
| Perception of PrEP efficacy (week 12) <50% effective 50–99% effective 0% effective Don't know |
9% 28% 10% 54% |
60% 66% 53% 52% |
(ref) 1.32 (0.47 to 3.69) 0.73 (0.22 to 2.38) 0.70 (0.27 to 1.83) |
0.595 0.602 0.466 |
| Perceived likelihood of HIV infection Not likely Could happen Probably/almost certain will happen |
23% 65% 12% |
47% 55% 61% |
(ref) 1.42 (0.73 to 2.77) 1.91 (0.78 to 4.65) |
0.299 0.155 |
| STI, at baseline No Yes |
77% 23% |
52% 60% |
(ref) 1.40 (0.81 to 2.42) |
0.223 |
| Depression, at baseline No Yes |
93% 7% |
54% 72% |
(ref) 2.38 (0.63 to 9.08) |
0.203 |
| Circumcised No Yes |
79% 21% |
53% 68% |
(ref) 2.03 (0.94 to 4.40) |
0.073 |
| Living situation With male or female sexual partner Alone With family/friends Other |
10% 20% 68% 2% |
58% 58% 54% 58% |
(ref) 0.99 (0.37 to 2.63) 0.82 (0.36 to 1.91) 0.99 (0.15 to 6.54) |
0.988 0.651 0.995 |
| Concern about a place to live Not concerned Somewhat/very concerned |
49% 51% |
55% 56% |
(ref) 1.05 (0.66 to 1.66) |
0.839 |
| Concern about having a job Not concerned Somewhat/very concerned |
33% 68% |
53% 56% |
(ref) 1.16 (0.71 to 1.90) |
0.544 |
| Symptoms reported at week 4 No GI symptoms Any GI symptom‡ No Headache Headache Symptoms reported at week 8 No GI symptoms Any GI symptom‡ No Headache Headache |
71% 29% 84% 16% 82% 18% 85% 15% |
56% 65% 58% 56% 56% 56% 57% 51% |
(ref) 1.54 (0.86 to 2.77) (ref) 0.92 (0.46 to 1.86) (ref) 1.01 (0.49 to 2.07) (ref) 0.75 (0.36 to 1.57) |
0.147 0.824 0.986 0.45 |
Proportions are adjusted for site and weighted by sampling frame
Detection of either tenofovir or emtricitabine in serum
Any GI symptom includes nausea, vomiting, abdominal pain, or flatulence.
Patterns of drug detection over time
For the 303 study participants included in the longitudinal analyses, the average number of samples tested was 3.8 (range 2–6). Out of 1168 visits tested, 467 (40%) visits had both PBMC and plasma samples available for testing, 688 (59%) visits had only plasma available, and 13 (1%) had PBMCs only. Overall, 31% did not have drug detected in any sample, 30% had drug detected in all samples, and 39% had inconsistent drug detection (at some time-points but not others). Patterns of drug detection varied by site: San Francisco had the highest proportion with drug detection at all time-points; Lima, Peru had the highest proportion never having drug detected; and Iquitos, Peru and Guayaquil, Ecuador had the highest proportion with inconsistent drug detection pattern (Table 2). In univariable analyses, having drug detection at some or all visits was associated with older age, baseline ncRAI, and responding "don't know" to a question on PrEP efficacy, when compared with those who never had drug detected (Table 4). Having drug detected at all visits was also associated with reporting ≥2 sex partners and reporting an STI at baseline, and was marginally associated with greater HIV risk perception. Results were similar in multivariable analyses, except that reporting an STI was no longer significant for always having drug detection. In a model adjusting for site and baseline ncRAI, there was a trend towards increased drug detection at follow-up visits where ncRAI was also reported (OR 1.60, 95% CI 0.98–2.62).
Table 4.
Proportion and factors associated with sometimes and always (vs. never) drug detection over time*
| Characteristic | % never detected |
% sometimes detected |
% always detected |
OR (some vs. never) (95% CI) |
P value | OR (always vs. never) (95% CI) |
P value |
|---|---|---|---|---|---|---|---|
| Age ≤20 21–25 26–30 >30 |
58% 28% 32% 16% |
29% 45% 44% 29% |
13% 27% 24% 55% |
Ref 4.04 (1.66 to 9.85) 3.42 (1.21 to 9.67) 5.13 (1.87 to 14.07) |
0.002 0.02 0.001 |
Ref 6.32 (2.09 to 19.09) 4.74 (1.26 to 17.76) 33.24 (9.91 to 111.45) |
0.001 0.021 <0.001 |
| Education Less than high school High School Graduate Some college or more |
24% 36% 27% |
53% 36% 38% |
23% 28% 35% |
Ref 0.42 (0.18 to 0.97) 0.63 (0.23 to 1.68) |
0.042 0.353 |
Ref 0.75 (0.28 to 2.03) 1.39 (0.46 to 4.16) |
0.577 0.561 |
| Transgender No Yes |
31% 32% |
35% 50% |
35% 18% |
Ref 1.41 (0.59 to 3.36) |
0.444 |
Ref 0.44 (0.14 to 1.38) |
0.16 |
| # sex partners at baseline (prior 3 mo) ≤1 male partner >1–5 partners >5–10 partners >10 partners |
41% 27% 35% 29% |
39% 36% 34% 39% |
20% 37% 32% 32% |
Ref 1.54 (0.49 to 4.88) 1.08 (0.31 to 3.78) 1.54 (0.43 to 5.48) |
0.462 0.908 0.508 |
Ref 3.41 (1.00 to 11.65) 2.16 (0.57 to 8.29) 2.70 (0.69 to 10.61) |
0.05 0.259 0.155 |
| ncRAI*, at baseline (prior 3 mo) No Yes |
46% 22% |
25% 43% |
28% 34% |
Ref 4.29 (2.11 to 8.72) |
<0.001 |
Ref 3.25 (1.54 to 6.85) |
0.002 |
| Alcohol use, at baseline (prior month) <2–3 times/week ≥2–3 times/week, but < daily |
33% 30% |
37% 37% |
30% 33% |
Ref 1.13 (0.60 to 2.12) |
0.708 |
Ref 1.29 (0.64 to 2.57) |
0.475 |
| Alcohol use, at baseline (drinks/day) <5 ≥5 Missing |
28% 32% 73% |
37% 39% 0% |
35% 29% 27% |
Ref 0.94 (0.51 to 1.73) 0.00 (0.00 to .) |
0.847 0.98 |
Ref 0.70 (0.36 to 1.37) 0.22 (0.02 to 2.42) |
0.3 0.214 |
| Meth/cocaine use (prior mo.) No Yes |
31% 28% |
36% 46% |
33% 26% |
Ref 1.49 (0.50 to 4.40) |
0.47 |
Ref 0.86 (0.24 to 3.05) |
0.818 |
| Perceived treatment assignment (week 12) Placebo Don’t know Truvada |
25% 30% 33% |
39% 37% 37% |
35% 33% 31% |
Ref 0.79 (0.25 to 2.43) 0.71 (0.20 to 2.49) |
0.677 0.588 |
Ref 0.75 (0.23 to 2.47) 0.63 (0.17 to 2.36) |
0.634 0.491 |
| Perception of PrEP efficacy (week 12) <50% effective 50–99% effective 100% effective Don't know |
46% 32% 37% 24% |
35% 32% 30% 41% |
20% 36% 33% 35% |
Ref 1.44 (0.51 to 4.06) 1.12 ((0.33 to 3.76) 2.51 (0.97 to 6.53) |
0.489 0.855 0.058 |
Ref 3.17 (0.92 to 10.95) 2.49 (0.62 to 9.99) 4.40 (1.35 to 14.32) |
0.068 0.197 0.014 |
| Perceived likelihood of HIV infection Not likely Could happen Probably/almost certain will happen |
38% 30% 26% |
98% 35% 38% |
22% 35% 36% |
Ref 1.21 (0.48 to 3.00) 1.60 (0.52 to 4.93) |
0.688 0.417 |
Ref 2.45 (0.93 to 6.46) 3.01 (0.83 to 10.90) |
0.07 0.093 |
| STI, at baseline No Yes Depression, at baseline No Yes Circumcised No Yes |
32% 28% 31% 40% 30% 39% |
38% 34% 38% 17% 38% 28% |
30% 38% 31% 44% 32% 32% |
Ref 1.05 (0.52 to 2.10) Ref 0.32 (0.04 to 2.39) Ref 0.54 (0.14 to 2.08) |
0.897 0.264 0.369 |
Ref 1.58 (0.71 to 3.51) Ref 1.11 (0.22 to 5.74) Ref 0.72 (0.20 to 2.58) |
0.264 0.898 0.616 |
| Living situation With male or female sexual partner Alone With family/friends Other |
29% 19% 33% 53% |
38% 37% 36% 28% |
33% 44% 31% 19% |
Ref 1.56 (0.28 to 8.78) 0.83 (0.18 to 3.90) 0.34 (0.01 to 9.96) |
0.613 0.812 0.534 |
Ref 2.29 (0.40 to 12.96) 0.77 (0.16 to 3.69) 0.22 (0.01 to 5.57) |
0.349 0.74 0.356 |
| Concern about a place to live Not concerned Somewhat/very concerned Concern about having a job Not concerned Somewhat/very concerned |
33% 30% 38% 35% |
34% 41% 25% 38% |
33% 29% 37% 37% |
Ref 1.73 (0.87 to 3.46) Ref 1.79 (0.94 to 3.43) |
0.12 0.078 |
Ref 0.84 (0.42 to 1.68) Ref 0.80 (0.43 to 1.49) |
0.622 0.484 |
All analyses adjusted for site
ncRAI: no condom with receptive anal sex
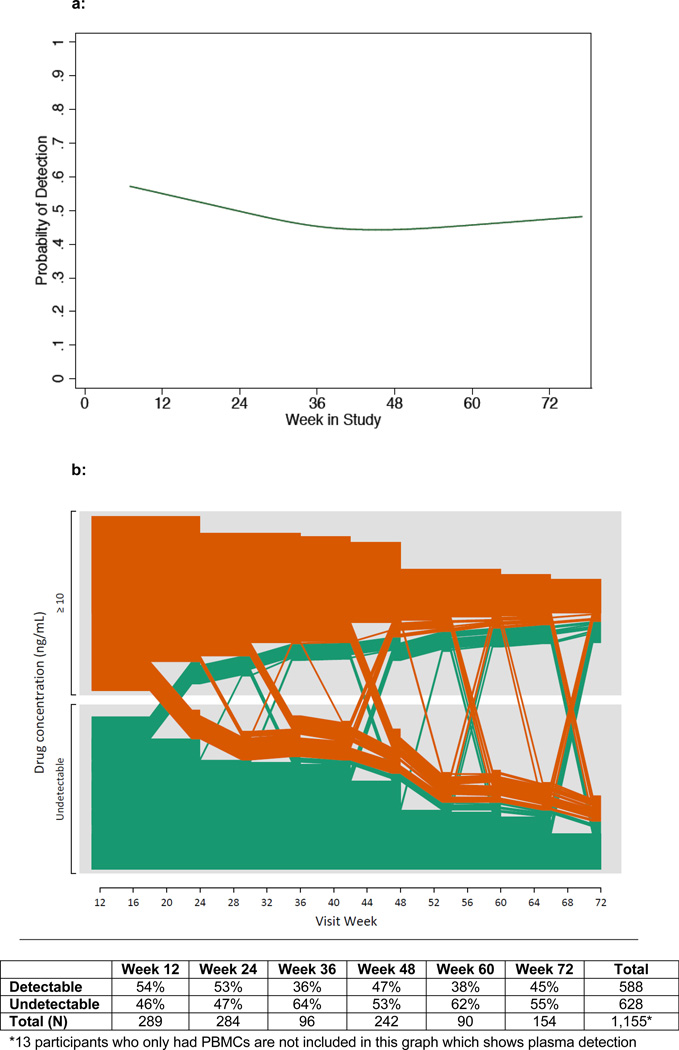
The proportion of participants with drug detection declined over the first 72 weeks of study participation, from a detection rate of 59% at week 8 to 44% at week 72 (Figure 1a). Trajectories of drug detection over time for individual participants in the longitudinal cohort are shown graphically in Figure 1b. Among the 163 participants with drug detected at the first visit (orange lines), 53 (32%) showed a pattern of discontinuation (drug detection stopped and remained undetectable at subsequent visits), with a median time of stopping of 24 weeks. The remaining two-thirds (68%) had drug detection at some or all subsequent follow-up visits. Among participants who had drug detected at week 12, reporting GI symptoms between weeks 12 and 24 was positively associated with drug detection at week 24 (AOR 4.32, 95% CI 1.07–17.49, p=0.04), while reporting headache during this time period was not significantly associated with week 24 detection (p=0.36). Of the 139 participants who had no drug detected at the first time-point (green lines), the majority (68%) did not have drug detection at any subsequent time-point.
Figure 1.
a. Proportion of participants with drug detection over time, by visit week
b: Longitudinal drug detection in plasma in the iPrEx cohort, by individual participant and visit week
Lines in orange and green represent participants who had drug detected and not detected respectively at the earliest follow-up visit with drug level testing. Plasma was tested every 12 weeks. By design, participants in iPrEx had a variable duration of follow-up, based on date of study entry. Overall declining Ns reflect fewer individuals with longer duration of follow-up due to enrolling on a later date, as well as loss to follow-up (approximately 24% of participants had an early termination visit).
DISCUSSION
In this analysis of drug detection among MSM and transgender women enrolled in the iPrEx randomized trial, TFV and/or FTC were detected in approximately half of participants in the active arm early in the study. Furthermore, approximately one-third of participants did not have drug detection at any visit in the longitudinal analysis. These findings suggest that a subgroup of study participants chose not to initiate study drug or discontinued drug very early during follow-up. As PrEP cannot be effective if not used, these patterns of drug detection explain the lower overall intention-to-treat efficacy of 42% observed in this trial, compared with 63%-75% efficacy seen in serodiscordant couples in the Partners PrEP study, which showed higher rates of drug detection.1 We also observed that drug was always detected in approximately one-third of participants, indicating that a substantial proportion of the study cohort initiated and sustained study drug use over the course of the trial.
Drug detection at week 8 and longitudinally varied considerably by site and was highest in San Francisco, USA, and also moderately high in Brazil, Thailand, and South Africa, demonstrating that substantial levels of uptake and adherence are achievable in clinical trials. However, drug detection was considerably lower at a number of sites, particularly in the Andes. These disparities may reflect regional differences in motivations for study participation, participants' relationships with the study staff and clinic, and research literacy, including understanding of the rationale for blinded and placebo-controlled trials. For example, San Francisco has a long history of conducting HIV treatment and prevention trials, including trials with placebo comparison groups, and many participants reported joining the study to give back to their community and help advance HIV prevention science,18 while Andean participants reported socialization, information, and study incentives as key motivations for study participation.19 While a range of incentives in addition to travel reimbursement were provided as part of study participation in the Andes, these were not provided to iPrEx participants in Brazil due to local regulations. The finding that consistent drug detection was associated with reporting "don't know" to a question about PrEP efficacy may further support this research literacy hypothesis, as at the time of the trial, PrEP efficacy was not yet known, and all participants were informed about the goals of the study to determine the safety and efficacy of FTC/TDF PrEP. Individuals who answered "don't know" may have had higher “research literacy,” including a better understanding of clinical equipoise and the importance of taking study product for scientific investigation, even if it may be a placebo or an active agent having no proven benefit. Differences in medication beliefs and socio-cultural contexts may also explain variations in drug detection by site. For example, in formative work conducted in South America, MSM in Peru were more skeptical about PrEP and had significant concerns about side effects, while MSM in Brazil were more open to PrEP and believed it would alleviate concerns about the risk of acquiring HIV (S. Lippman, personal communication, June 16, 2014).
Younger age strongly correlated with lower drug detection in iPrEx, both at week 8 and longitudinally. This finding does not appear to be explained by faster drug clearance in younger persons, as estimated creatinine clearance was not associated with drug detection. Younger age has been associated with lower adherence to antiretroviral treatment in HIV-infected individuals;20 in preventive fields including oral contraceptives,21 tuberculosis prophylaxis,22 and cardiovascular prevention;23–25 and to study drug use in HIV-negative individuals in other PrEP trials, including young women in the VOICE trial10 and serodiscordant couples in Partners PrEP.26 As HIV incidence is particularly high among young MSM27,28 and PrEP could play an important role in reducing HIV acquisition in this population if taken consistently, interventions to support PrEP adherence among young MSM and transgender women may be particularly relevant. Alcohol and drug use, living situation, being transgender, and having concerns about employment and/or housing were not associated with lower drug detection in iPrEx, suggesting these individuals should not be excluded from being prescribed PrEP due to concerns about non-adherence.
In the longitudinal analyses, markers of risky sexual behavior (e.g. reporting ncRAI, ≥2 sexual partners, or having an STI) were associated with more frequent drug detection at some or all study visits, suggesting that participants who had some level of risk were more likely to attempt to take the study medication. Similarly, study drug adherence was lower during periods of no sexual activity in the Partners PrEP study, suggesting that adherence to PrEP may be lower during periods of low perceived risk.26 Greater drug detection among those engaging in the highest risk sexual practices is expected to increase the impact and cost-effectiveness of PrEP.29 It has been previously reported in iPrEx that higher PrEP efficacy was observed among those who reported ncRAI at baseline.4 The observation that ncRAI was associated with drug detection may partially explain this efficacy interaction, although other factors (e.g. lower seroincidence in participants not reporting ncRAI) may also contribute to this finding. Interestingly, risk perception in iPrEx was not associated with drug detection at week 8 and only marginally associated with always having drug detected in the longitudinal cohort, although this one-item measure was a global measure of long-term risk perception (e.g. risk of being HIV-infected in their lifetime) and may not have adequately captured the participant's actual risk perception. Additionally, participants' personal risk assessment may be imperfect and may not accurately reflect their true risk of HIV acquisition and their need to take PrEP, as recently described by Gallagher et al.30 Additional research is needed to better understand the relationship between sexual behavior, perceived risk, and PrEP use. As sexual behavior can fluctuate over time, studies are also needed to determine whether and how best to stop and restart PrEP during periods of changing sexual and partnering practices.
Our study demonstrated an overall decrease in drug detection rates over time. Approximately one-third of participants with drug detected early in the study had a pattern of non-persistence at subsequent visits. Stopping drug was not explained by a lack of risk behavior (data not shown) or reported side effects, suggesting that other factors, such as medication or trial fatigue, may explain waning tablet-use over time. This pattern was also observed in other PrEP trials26,31 and has been described in the literature for other medical treatments.32 The positive association between drug detection at week 24 and symptoms reported in the prior 3 months indicates that while PrEP use may be associated with GI symptoms, these symptoms did not lead to an increase in drug discontinuation. The median time to drug discontinuation was 24 weeks, suggesting the importance of developing adherence strategies to sustain PrEP use over time, particularly during the first 6 months of PrEP use. Most PrEP trials utilized monthly visits to closely monitor for HIV infection, which may have increased fatigue; current recommendations for less frequent monitoring may mitigate this challenge.
This study had several limitations. First, serum and plasma levels of FTC and TFV represent relatively short windows of drug exposure (e.g. dosing over the last 2–3 days), based on the moderately rapid half-lives of 10 and 15 hours, respectively, and were analyzed dichotomously (drug detected vs. undetected).33 Therefore, drug detection in these matrices may not accurately reflect usage patterns over the entire period between visits and do not serve as a quantitative measure of dosing during this period. However, we have previously reported high concordance (>95%) in drug detection between plasma and PBMC samples in iPrEx.4 As intracellular drug levels reflect dosing over one to two weeks given the 5 to 10-fold longer intracellular half-lives,34 this high concordance suggests that plasma drug detection may approximate dosing over a longer time interval in this cohort. Second, drug levels were available only among a random sample at week 8 and among the DEXA and case-control subset for longitudinal analyses, and therefore may not be fully representative of the entire active arm. However, characteristics of participants in these samples were similar to the overall cohort. Third, drug levels were only available for testing and analysis in the active arm. As analyses of factors impacting PrEP efficacy are optimal if they take advantage of the randomization to minimize bias,35 future studies should consider incorporating strategies to measure adherence in the active and placebo arms (e.g. analyzing tagged-placebo tablets for ingestion). Finally, drug levels were measured in the setting of a randomized, placebo-controlled trial, and may not be representative of adherence in open-label contexts.
In terms of study strengths, this analysis presents a comprehensive evaluation of patterns and correlates of drug detection in a large cohort of MSM and transgender women in a multinational PrEP efficacy trial and can provide important recommendations to optimize adherence in future PrEP programs. For future trials evaluating the safety and efficacy of novel oral and topical PrEP regimens, it will be critical to develop strategies to help participants fully understand the value of participation in placebo-controlled trials and the importance of all participants taking the study product for the scientific integrity of the trial. These strategies could include educational and motivational activities to increase research literacy at the individual and community level and foster a sense of ownership and relevance of study participation. Real-time testing of drug levels, particularly at early time-points, may be useful in identifying individuals who do not initiate study product. For open-label demonstration projects and implementation programs, strategies to help individuals decide whether PrEP may be an appropriate prevention strategy are needed, as well as approaches to address fatigue with medication and clinic visits. Additional research is also required to validate biomarkers that reflect PrEP usage over longer periods, can serve as quantitative measures of adherence, and can be easily implemented in real-world settings. Measuring antiretroviral levels in dried blood spots36 and hair37 are two novel biomarkers of PrEP adherence currently under investigation. Validated biological measures of adherence could be used to evaluate adherence rates and correlates in PrEP implementation programs, assess the impact of PrEP adherence interventions, help explain PrEP failures, and determine potential drug exposure thresholds needed for protection. Addressing these important scientific questions will help optimize PrEP adherence and efficacy and maximize PrEP’s public health impact.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Justin Brantley for assistance in preparation of the graphical figure, Alexa Burrell for assistance with manuscript preparation, and the study volunteers who participated in this study.
Sources of Funding:
Drs. Liu and Buchbinder have received honoraria from Clinical Care Options. Dr. Amico's institution has received a research grant from Gilead Sciences. Dr. Schechter has received payment for Board membership at Gilead. Dr. Grant has served as a consultant for Siemens Healthcare on their guidelines panel and has served as a consultant and a site investigator for VIIV. PLA has received study drug and contract work from Gilead Sciences.
This research was supported by funds from the National Institutes of Health (NIAID UO1 AI064002, U01 AI084735 and RO1 AI062333; NIMH R01 MH095628), and the Bill and Melinda Gates Foundation. Study drug was supplied by Gilead Sciences, which also provided funding to support some staff travel to annual investigator meetings, and the creation of a video regarding participant experience.
Footnotes
Conflicts of Interest:
For the remaining authors, no conflicts of interest were declared.
Publisher's Disclaimer: Disclaimers:
The views expressed herein do not necessarily reflect the official policies of the City and County of San Francisco; nor does mention of the San Francisco Department of Public Health imply its endorsement.
Listing of Supplemental Digital Content
Supplemental Digital Content1.docx
Supplemental Digital Content2.docx
REFERENCES
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrix CW. Exploring Concentration Response in HIV Pre-Exposure Prophylaxis to Optimize Clinical Care and Trial Design. Cell. 2013 Oct 24;155:515–518. doi: 10.1016/j.cell.2013.09.030. 2013. [DOI] [PubMed] [Google Scholar]
- 6.Koenig LJ, Lyles C, Smith DK. Adherence to antiretroviral medications for HIV pre-exposure prophylaxis: lessons learned from trials and treatment studies. American journal of preventive medicine. 2013 Jan;44(1 Suppl 2):S91–S98. doi: 10.1016/j.amepre.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012 Sep 12;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011 Jul 16;378(9787):279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrazzo j, Ramjee G, Nair G. Pre-exposure prophylaxis for HIV in Women: Daily Oral Tenofovir, Oral Tenofovir/Emtricitabine, or Vaginal Tenofovir Gel in the VOICE Study (MTN 003); Paper presented at: Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 11.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS Behavior. 2013;17:284–287. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. Journal of acquired immune deficiency syndromes (1999) 2006 Dec 1;43(Suppl 1):S79–S87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muchomba FM, Gearing RE, Simoni JM, El-Bassel N. State of the science of adherence in pre-exposure prophylaxis and microbicide trials. Journal of acquired immune deficiency syndromes (1999) 2012 Dec 1;61(4):490–498. doi: 10.1097/QAI.0b013e31826f9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012 Sep 12;12(4):151. doi: 10.1126/scitranslmed.3004006. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2009 Jul 1;877(20–21):1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushman LR, Kiser JJ, Rower JE, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. Journal of pharmaceutical and biomedical analysis. 2011 Sep 10;56(2):390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agresti A. An introduction to categorical data analysis. New York Wiley. 1996;135 [Google Scholar]
- 18.Gilmore H, Liu A, Koester K, et al. Participant Experiences and Facilitators and Barriers to Pill-Use Among Men who Have Sex with Men in the iPrEx Pre-exposure Prophylaxis Trial in San Francisco, United States. AIDS Patient Care STDS. 2013 doi: 10.1089/apc.2013.0116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goicochea LP, J L, Vargas L. Barriers and Facilitators to PrEP adherence in iPrEx: a prevention trial that started in 3 Andean cities; Conference on Retroviral and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 20.Nachega JB, Hislop M, H N, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J. Acquir Immune Defic Syndr. 2009 May 1; doi: 10.1097/QAI.0b013e318199072e. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clare C, Fraser C. Contraception Adherence among East Harlem Adolescents. Gynecol Obstet. 2013;3(177) 2161-0932.100017. [Google Scholar]
- 22.Pilote L, JP T, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless. A trial to improve adherence to referral. Arch Intern Med. 1996;22(156):161–165. [PubMed] [Google Scholar]
- 23.Hyre AD, Krousel-Wood MA, Muntner P, Kawasaki L, DeSalvo KB. Prevalence and predictors of poor antihypertensive medication adherence in an urban health clinic setting. J Clin Hypertens (Greenwich) 2007 Mar 9;3:179–186. doi: 10.1111/j.1524-6175.2007.06372.x. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naderi S, Bestwick J, Wald D. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012 Sep 1;125(9):882–887. doi: 10.1016/j.amjmed.2011.12.013. 2012. [DOI] [PubMed] [Google Scholar]
- 25.Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013 May;165(5):665–678. 678 e661. doi: 10.1016/j.ahj.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLOS Medicine. 2013 Sep 10; doi: 10.1371/journal.pmed.1001511. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balaji AB, Bowles KE, Le BC, Paz-Bailey G, Oster AM, Group NS. High HIV incidence and prevalence and associated factors among young MSM, 2008. Aids. 2013 Jan 14;27(2):269–278. doi: 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. Aids. 2013 Nov 13;27(17):2665–2678. doi: 10.1097/01.aids.0000432449.30239.fe. [DOI] [PubMed] [Google Scholar]
- 29.Wheelock A, Eisingerich AB, Ananworanich J, et al. Are Thai MSM willing to take PrEP for HIV prevention? An analysis of attitudes, preferences and acceptance. PLoS One. 2013;8(1):e54288. doi: 10.1371/journal.pone.0054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher T, Link L, Ramos M, Bottger E, Aberg J, Daskalakis D. Self-Perception of HIV Risk and Candidacy for Pre-Exposure Prophylaxis Among Men Who Have Sex with Men Testing for HIV at Commercial Sex Venues in New York City. LGBT Health. 2014 doi: 10.1089/lgbt.2013.0046. [DOI] [PubMed] [Google Scholar]
- 31.Hosek SG, Siberry G, Bell M, Lally M, Kapogiannis B, Green K. The Acceptability and Feasibility of an HIV Preexposure Prophylaxis (PrEP) Trial With Young Men Who Have Sex With Men. J Acquir Immune Defic Syndr. 2013;62:447–456. doi: 10.1097/QAI.0b013e3182801081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annual review of pharmacology and toxicology. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 33.Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol. 2007 Jun;47(6):751–759. doi: 10.1177/0091270007300951. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins T, Veikley W, St Claire RL, 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. Journal of acquired immune deficiency syndromes (1999) 2005 Aug 1;39(4):406–411. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 35.Dai JY, Gilbert PB, Hughes JP, Brown ER. Estimating the efficacy of preexposure prophylaxis for HIV prevention among participants with a threshold level of drug concentration. J Epidemiol. 2013 Feb 1;177(3):256–263. doi: 10.1093/aje/kws324. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo-Mancilla JR, Zheng JH, JE R, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013 Feb 29;29(2):384–390. doi: 10.1089/aid.2012.0089. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu A, Yang Q, Huang Y, et al. Strong Relationship between Oral Dose and Tenofovir Hair Levins in a Randomized Trial: Hair as a Potential Adherence Measure for Pre-Exposure Prophylaxis(PrEP) PLoS ONE. 2013 doi: 10.1371/journal.pone.0083736. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.