Abstract
Background
A health technology assessment (HTA) of left ventricular assist devices (LVADs) as destination therapy in patients with end-stage heart failure was commissioned by the Dutch Health Care Insurance Board [College voor Zorgverzekeringen (CVZ)]. In this context, a systematic review of the economic literature was performed to assess the procedure’s value for money.
Methods
A systematic search (updated in December 2013) for economic evaluations was performed by consulting various databases: the HTA database produced by the Centre for Reviews and Dissemination (CRD HTA), websites of HTA institutes, CRD’s National Health Service Economic Evaluation Database (NHS EED), Medline (OVID) and EMBASE. No time or language restrictions were imposed and pre-defined selection criteria were used. The two-step selection procedure was performed by two people. References of the selected studies were checked for additional relevant citations.
Results
Six relevant studies were selected. Four economic evaluations relied on the results of the REMATCH trial to compare a pulsatile-flow LVAD with optimal medical therapy (OMT). These evaluations were performed before the publication of the HeartMate II (HM-II) Destination Therapy Trial which compared a pulsatile-flow with a continuous-flow LVAD. Two more recent economic evaluations combined the results of both trials to make an indirect comparison of a continuous-flow LVAD with OMT.
In all studies, the largest part of the incremental cost was due to the reimplantation cost of an LVAD, with a device cost of €58,000-€75,000 and about €55,000 for the surgical procedure. The survival gain was highest with a continuous-flow LVAD, up to about three life-years gained (LYG) versus OMT in the most optimistic study. Quality of life (QoL) was improved but measures with a generic utility instrument were lacking, making estimates on quality-adjusted life-years (QALYs) gained more uncertain. Incremental cost-effectiveness ratios of the two most recent studies were on average €107,600 and $198,184 (ca.€145,800) per QALY gained.
Conclusions
Although LVAD destination therapy improves survival and QoL, it remains questionable as to whether it offers value for money. This conclusion may alter if the price of the device/procedure decreases sufficiently, in combination with further improved outcomes for mortality, adverse events and QoL.
Keywords: Heart-assist devices, left ventricular assist devices (LVADs), cost-benefit analysis, review
Introduction
Mechanical circulatory support through left ventricular assist devices (LVADs) is increasingly being used as a bridge-to-transplantation (BTT) in patients with end-stage heart failure (1). As the number of patients with end-stage heart failure is growing without an accompanying increase in available donor hearts, LVADs are also being used as destination therapy as an alternative to heart transplantation.
A systematic review revealed two randomized trials which investigated LVADs as destination therapy, in patients with end-stage heart failure who were not candidates for cardiac transplantation (1). In 2001, the REMATCH trial (2), comparing a pulsatile-flow LVAD with optimal medical therapy (OMT), demonstrated improved one-year survival after LVAD support and was the basis for the Food and Drug Administration (FDA) to approve destination therapy in the United States. The relative mortality risk was 0.52 [95% confidence interval (CI), 0.34-0.78; P=0.001]. Survival at one year was 52% versus 28%, in favor of the pulsatile-flow LVAD over OMT (2). At two years, this was 29% versus 13% (3).
The second trial, published in 2009, compared a pulsatile-flow LVAD with a continuous-flow LVAD [HeartMate II (HM-II) Destination Therapy Trial] (4). The relative mortality risk was 0.54 (95% CI, 0.34-0.86; P=0.008). Survival at one year was 68% versus 55%. Survival at two years was 58% versus 24% in favor of the continuous-flow HM-II over the pulsatile-flow LVAD (4). Survival after implantation of a continuous-flow LVAD was thus significantly better than with an older pulsatile-flow device.
Partly based on economic considerations, the Dutch Health Care Insurance Board [College voor Zorgverzekeringen (CVZ)] concluded in 2007 that pulsatile-flow LVADs as destination therapy for end-stage heart failure could not be included in the basic healthcare package (4). Because of technological advances with smaller and better performing continuous-flow LVADs, a new health technology assessment (HTA) report was requested, including a systematic review of published economic evaluations and a primary economic evaluation of these LVADs as destination therapy in patients with end-stage heart failure. For this special issue of Annals of Cardiothoracic Surgery, an update of this systematic review of economic evaluations was performed.
Methods
In December 2013, a systematic search for economic literature on the cost-effectiveness of LVADs was performed by consulting various databases. First, reviews on this topic were searched by consulting the HTA database produced by the Centre for Reviews and Dissemination (CRD HTA) and websites of HTA institutes mentioned on the International Network of Agencies for Health Technology Assessment (INAHTA) website (www.inahta.net). Websites of non-member HTA institutes such as NICE (www.nice.org.uk) were also checked for relevant analyses. Furthermore, the CRD National Health Service Economic Evaluation Database (NHS EED), Medline (OVID) and EMBASE databases were searched to retrieve both full economic evaluations and reviews of full economic evaluations of LVADs as destination therapy. No restrictions on publication date and language were imposed. Details of the original search strategy performed in January 2011 are available in the appendix of the full HTA report (1). The search strategy in these databases was performed by one researcher, transparently reported, and validated afterwards by a second researcher. The update in December 2013 was performed with a similar approach.
All retrieved references were assessed against pre-defined selection criteria (Table 1). The selection was restricted to patients with end-stage heart failure receiving an LVAD as destination therapy. Patients with an LVAD as BTT were excluded. Only full economic evaluations were included, i.e., the comparative analysis of at least two alternative interventions in terms of both costs and outcomes. Partial evaluations such as cost analysis were excluded.
Table 1. Economic evaluation selection criteria.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population | Patients with end-stage heart failure, NYHA Class IIIB/IV | Other patients |
| Intervention | LVADs as destination therapy | Other interventions |
| Comparator | Optimal medical therapy (OMT), heart transplantation, implantable cardiac resynchronization therapy, LVAD as bridge to transplant | Other interventions |
| Design | Full economic evaluations | Other designs such as cost calculations |
LVADs, left ventricular assist devices; NYHA, New York Heart Association.
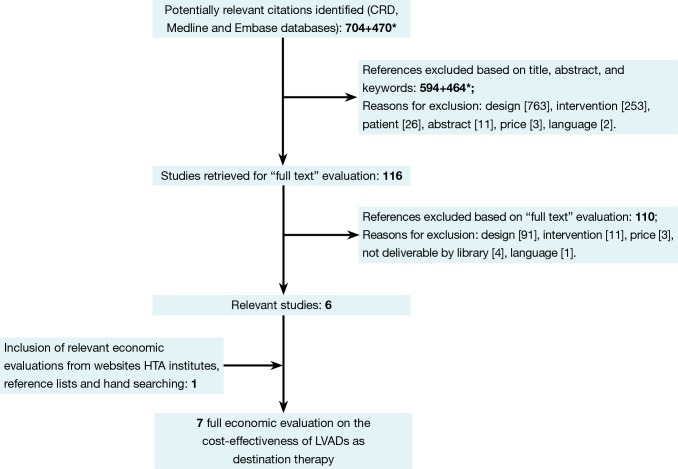
The selection of relevant articles was performed in a two-step procedure: initial assessment of the title, abstract, and keywords, followed by a full-text assessment of the selected references. This procedure was in first instance performed by an economist (MN). To improve the quality of this procedure, a physician (JV) checked the medical selection criteria. In case of doubt, the opinion of a third researcher (AV) was asked. Reference lists of the selected studies were checked for additional relevant citations. Figure 1 provides the flow chart of this process. Most articles were excluded due to not being a full economic evaluation (design). In the end, seven relevant studies were selected (5-11). Two studies represented the same analysis and were discussed as one study (5,8). These full economic evaluations were summarized by a health economist in an in-house developed structured data extraction sheet. These working documents provided the basis of this overview, in which the models’ input variables were compared with the systematically identified evidence and with real-world data from the Dutch University Medical Centre Utrecht.
Figure 1.
Selection of relevant articles. *For this special issue on LVADs, the original search performed in January 2011 was updated in December 2013. Of the seven identified references (5-11), two (5,8) presented the same analysis and were discussed as one. LVADs, left ventricular assist devices; HTA, health technology assessment; CRD, Centre for Reviews and Dissemination.
Results
In the following paragraphs, we provide an overview of all input variables, results and conclusions from the published economic evaluations. Initially, the data are provided as published in the economic evaluations. This information will then be critically appraised in our discussion.
General information
The evaluations were conducted for the UK (2), the Netherlands (2), Italy (1), and the US (1) (Table 2). Four studies (5,6,10,11) used a Markov model to perform a cost-utility analysis (CUA). The other two evaluations applied an alternative method. The study by Girling et al. (7) identified thresholds for survival parameters which would allow the intervention to be cost effective. The Italian study (9) calculated the value of the intervention based on the additional survival in combination with the societal economic counter value for each month of life saved. All models included a lifelong time horizon. Adang et al. (6) mentioned a three-year horizon, but after 35 months, all patients in the model were deceased. Clegg et al. (5) applied a five-year horizon and all patients were deceased in the 21st quarter. The discount rate for costs and effects usually reflected national guidelines and varied between 3-6% for costs and 1.5-3.5% for effects.
Table 2. General information of the selected economic evaluations.
| Reference (country); conflict of interest | Time horizon discount rate | Analytic technique design | Population Intervention and comparator |
|---|---|---|---|
| Pulsatile LVAD | |||
| Clegg et al., 2005 (5) (UK)*; no conflict of interest | 5 years; costs: 6%; effects: 1.5% | CUA; Markov model | ~REMATCH population**; LVAD as long-term chronic support (LTCS) versus medical therapy |
| Adang et al., 2006 (6) (the Netherlands); no conflict of interest mentioned | 3 years; 3% for both costs and effects | CUA; Markov model | Patients with end-stage heart failure (NYHA class IV); LVAD as destination therapy versus optimal medical therapy |
| Girling et al., 2007 (7) (UK); Support through the MATCH Programme (no further details) | Lifetime (not explicitly mentioned); 3.5% for both costs and effects | (Alternative) CUA; health-economic model | ~REMATCH population***; LVAD as destination therapy versus optimal medical management |
| Messori et al., 2009 (9) (Italy); two authors on a single occasion received reimbursement of travel expenses from companies manufacturing LVADs | Lifetime; no discounting in base case scenario (Scenario analysis—costs: 3%; effects: 1.5%) | (Alternative) CEA (base case scenario) and CUA (sensitivity analysis); health-economic model | Patients who received a HeartMate device [68 patients (53 males, 97.1% with NYHA class IV, mean age of 66 years)]; LVAD (HeartMate device) versus no LVAD |
| Continuous-flow LVAD | |||
| Rogers et al., 2012 (10) (US); Thoratec provided funding support. Authors have served as consultants for Thoratec, received a research grant, or are employee of Thoratec | 5 years; 3% for both costs and effects | CUA; Markov model | Patients with predominantly NYHA class IV symptoms and an LVEF of ≤25%. These patients were ineligible for heart transplantation. Continuous-flow LVAD for destination therapy versus optimal medical management |
| Neyt et al., 2013 (11) (the Netherlands); no conflict of interest | Lifetime; costs: 4%; effects: 1.5% | CUA; Markov model | Adults with chronic end-stage heart failure, contraindications for a heart transplant, LVEF of 25 percent or less, and NYHA class IV for at least 90 days despite OMT. Continuous-flow LVAD as destination therapy versus OMT |
*, The journal article of Clegg et al. published in 2007 (8) is the same as the report published in 2005 (5); **, NYHA class IV population with an average age of 66-68 years; ***, patients with chronic end-stage heart failure and NYHA class IV symptoms. LVADs, left ventricular assist devices; OMT, optimal medical therapy; CUA, cost-utility analysis; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The economic evaluations can be divided in two groups. First, four economic evaluations were performed before the results of the HM-II Destination Therapy trial were published and compared a 1st-generation pulsatile LVAD with OMT. Second, two more recent studies were published afterwards and compared a 2nd-generation continuous-flow LVAD with OMT. The populations reflected the characteristics of the underlying clinical trials (Table 2).
Costs
Table 3 provides an overview of the most important cost items and their valuation. The economic evaluations were mostly carried out from a healthcare payer perspective. To reflect a broader societal perspective, Neyt et al. (11) also included travel costs. However, these costs were so small relative to the medical cost that they could be neglected. All analyses included direct medical costs.
Table 3. Cost information.
| Reference | Perspective | Currency & year | Cost information |
|---|---|---|---|
| Neyt et al., 2013 (11) | Societal perspective | €, 2010 | Data from UMC Utrecht (69 patients with HM-II implantation as BTT) |
| LVAD implantation cost: €126,505 (incl. LVAD device of €70,000). | |||
| Cost rehospitalization (excl. LVAD replacement): €8,118 | |||
| Number of repeat hospitalizations: | |||
| HM-II group: 2.64 per patient-year; OMT group: 3.15 per patient-year | |||
| Monthly costs: LVAD €1,261 (incl. rent PBU and LVAD accessories); OMT: €1,047 | |||
| Discounted incremental cost: €299,100 (95% CI, 190,500-521,000) | |||
| Rogers et al., 2012 (10) | Third-party payer perspective | US $, 2009 | LVAD implantation hospital cost: US $193,812 |
| LVAD implantation professional service cost: US $8,841 | |||
| LVAD replacement cost: US $131,430 | |||
| Monthly LVAD replacement rate: 0.005 | |||
| Rehospitalization cost (per event): US $6,850 | |||
| Monthly rehospitalization rate for LVAD: 0.21 | |||
| Monthly rehospitalization rate for OMT: 0.1325 | |||
| Monthly outpatient costs (LVAD & OMT): US $2,331 | |||
| End-of-life cost (LVAD & OMT): US $44,211 | |||
| Total costs (discounted): | |||
| Continuous-flow LVAD: US $360,407 | |||
| Medically treated patients: $62,856 | |||
| Incremental cost: US $297,551 | |||
| Messori et al., 2009 (9) | Not explicitly stated | €, not explicitly mentioned (exception: cost surgery in 2004) | Individual reimbursement = €0 if bLE > indST |
| Individual reimbursement = €5,000 per extra month if bLE < indST | |||
| The average value was compared with the price of the HeartMate device (€75,000) | |||
| Whereby: | |||
| IndST: individual survival time | |||
| bLE: baseline life expectancy | |||
| €5,000 = MCV: monthly countervalue | |||
| (i.e., the societal economic countervalue for each month of life saved) | |||
| Cost of LVAD surgery (excl. price device): ~€50.000 | |||
| Girling et al., 2007 (7) | Perspective of healthcare provider (costs from study of Clegg et al., 2005) (5) | £, 2003 (i.e., costs mainly from study Clegg et al., 2005) (5) | Base case device cost: £60,000. Results are presented for a range of costs for the unit, ranging from free to £80,000 |
| Initial hospitalisation cost: LVAD success: £27,821; LVAD Failure: £63,989 | |||
| Cost hospital readmission: £16,170 per month in hospital for both LVAD and OMT | |||
| Outpatient cost per visit: £99 for both LVAD and OMT | |||
| Adang et al., 2006 (6) | Perspective of healthcare payer (not explicitly stated) | €, 2006 | Data from UMC Utrecht (52 patients, LVAD as BTT) |
| Hospitalisation: €49,896 (16 IC days: €26,944; 40 hospitalisation days: €19,040; 6 hours surgery room: €3912) | |||
| Material: €71,129 (LVAD: €69,600; blood products: €1,529) | |||
| After hospital discharge, up to 1 year: €5165 (15 consulations: €1,500; 1.1 readmissions: €3,665) | |||
| Total (1st year): €126,190 | |||
| Medication OMT group (per month): €348,50 | |||
| Rehospitalisation: LVAD group: 0.22; OPT group: 0.15 | |||
| Total costs (discounted): | |||
| Average cost LVAD: ca. €141.000 per patient | |||
| Average cost OPT: ca. €22.000 per patient | |||
| Incremental cost: €119.000 per patient | |||
| Clegg et al., 2005 (5) | NHS perspective | £, 2003 | LVAD costs were from one of the UK centers (16 patients) |
| LVAD costs start model: | |||
| Assessments: £2,891; Implant operation: £36,986; Cost device: £48,000 (HeartMate VE LVAD) | |||
| LVAD follow-up cost: £4,192 per quarter (outpatient visit and readmission) | |||
| No cost for medication in LVAD arm | |||
| Medical treatment: £1,382 per quarter | |||
| Total cost (discounted): | |||
| LVAD group: £106,756 per patient; Medical therapy: £4,758; Incremental cost: £101,998 |
LVADs, left ventricular assist devices; OMT, optimal medical therapy; HM-II, HeartMate II; BTT, bridge-to-transplantation; CI, confidence interval; IC, intensive care; PBU, power base unit; UMC, university medical center. Exchange rates January 9, 2014: US $1 = €0.736; £1 = €1.211 (source: www.xe.com).
The cost of the device ranged between £48,000 (ca.€58,000, exchange rate January 9, 2014) and €75,000 (Table 3). One analysis varied this price between offering the device for free and €80,000 (7). The cost of the initial surgery (excluding the device) was about €55,000 in the most recent Dutch evaluation and £39,877 (€48,300) in the UK studies. The study of Girling et al. (7) made a distinction between successful implantations [£27,821 (€33,700)] and failures [£63,989 (ca.€77,500)]. Messori et al. (9) only included the cost of the device in the baseline scenario. In a sensitivity analysis, costs for the surgery were also included and approximate €50,000. As such, the total cost for the initial hospitalization including the device cost ranged from £85,000 (5) (ca.€102,900) to US $193,800 (10) (ca.€142,600).
The cost of outpatient visits and rehospitalization was expressed in different ways: a quarter (5), up to one year after discharge from hospital (6), per month in hospital (7) or per event (10,11) (Table 3). These costs varied across countries and are difficult to compare. The Italian study did not include these costs. For an overview of other included cost items, we refer to Table 3.
Survival
The four economic evaluations published between 2005 and 2009 (5-7,9) relied on the results of the REMATCH trial, which compared a pulsatile-flow LVAD with OMT, to model outcomes. The Italian study (9) did not mention this explicitly, but based on the description of LVAD implantations in 68 patients it is very likely that the authors also referred to this trial. Trials have a limited follow-up period and extrapolation to a lifetime horizon is necessary to calculate the number of quality-adjusted life-years (QALYs) gained. The study of Clegg et al. (5) showed that the largest part of the total survival benefit is already achieved within the trial follow-up period. In this study, the survival benefit in the LVAD arm versus the comparator was 6.8 months, of which 90% (6.2 months) was already achieved within the trial follow-up period.
The two most recent studies (10,11) noticed that no direct comparison between a continuous-flow LVAD and OMT has ever been performed in a trial. Both studies made an indirect comparison between OMT and a continuous-flow LVAD. The survival for the OMT arm was also based on the results of the REMATCH trial, while the outcomes for the continuous-flow LVAD were based on the HM-II Destination Therapy Trial. The indirect comparison was performed unadjusted because of the comparable inclusion criteria and similar outcomes with the pulsatile-flow LVADs in the two trials (11).
The survival gain with continuous-flow LVADs was much higher than with pulsatile LVADs. Whereas the survival gain of pulsatile LVADs versus OMT was 6.8 months per person in the study of Clegg et al. (5), this was already almost two years and more than three years in the two most recent studies evaluating continuous-flow LVADs (Table 4). The relatively large difference in survival gain between these two studies lies within the extrapolation period. Rogers et al. extrapolated survival beyond 24 months based on an exponential survival curve using the constant hazard rate observed within 24 months. In contrast, Neyt et al. used the monthly mortality during the second year for extrapolation purposes. The reason for the latter is that mortality is very different when comparing the 30-day, one-year and two-year survival after continuous-flow LVAD implantation: 10.1% 30-day mortality (1,15), and 32% and 42% mortality after one and two years (4) respectively. These numbers indicate that the first month is critical and surviving this period and the first year is the biggest hurdle for LVAD patients.
Table 4. Information on survival.
| Reference | Survival |
|---|---|
| Clegg et al., 2005 (5) | Kaplan-Meier (KM) survival curve using censored data from the REMATCH randomised trial + extrapolation |
| The survival gain of the LVAD arm over medical therapy was 6.84 months per person | |
| Adang et al., 2006 (6) | Survival based on the results of the REMATCH trial |
| Girling et al., 2007 (7) | Exponential (constant hazard) distribution for patient survival with mean survival as suggested by the REMATCH trial |
| For OMT: mean survival 7.8 months (Samson et al., 2004) (12) | |
| For LVAD patients: | |
| LVAD failure: mean survival 2 months | |
| Life expectancy LVAD group depends on: | |
| Proportion of LVAD success/failure (π) | |
| Mean survival time for successes (μS) | |
| In the REMATCH trial (1st generation device): | |
| π is estimated as 0.33 (=17/51) (Oz et al., 2003) (13) | |
| μS is estimated as 35 months | |
| Based on expert opinion: | |
| Median survival: 25 months (range, 12-40 months) | |
| 30-day mortality: 10% (range, 3-16%) | |
| Messori et al., 2009 (9) | Baseline life expectancy without LVAD: 150 days |
| Individual survival times after LVAD implantation for the published 68 patients receiving the HeartMate device were derived by a computerized analysis of the original KM curve (Messori et al., 2008) (14) | |
| An additional survival time of 12 months was determined by extrapolating the published survival curves | |
| Rogers et al., 2012 (10) | OMT: KM survival curve from the REMATCH trial |
| Continuous-flow LVAD: KM survival curve from the HeartMate II Destination Therapy trial | |
| Extrapolation past 24 months: based on exponential survival curve using the constant hazard rate observed within 24 months | |
| OMT: 0.105 per month | |
| Continuous-flow LVAD: 0.023 per month (base case analysis) | |
| LVAD vs. OMT: 2.42 versus 0.64 life years | |
| Neyt et al., 2013 (11) | OMT: survival from the REMATCH trial |
| Continuous-flow LVAD: survival from the HeartMate II Destination Therapy trial | |
| Extrapolation past 24 months (base case scenario): | |
| OMT group: 2-year survival of 13%; no survival after 3 years | |
| Continuous-flow LVAD: the monthly mortality during the second year is used to extrapolate results. | |
| Age and gender-adjusted increase in monthly mortality risk is applied according to Dutch life table | |
| Discounted incremental effect: 3.23 life-years gained (LYG) (95% CI, 2.18-4.49) |
LVADs, left ventricular assist devices; OMT, optimal medical therapy.
Quality of life (QoL)
Clegg et al. (5) reported that utility weights were measured in the REMATCH trial. Unfortunately, these results have not been published. As an alternative, the utility weights were estimated using an expert panel. Data from the REMATCH trial were linked to the Minnesota Living with Heart Failure Questionnaire (MLHFQ), which contained 21 items across five domains and is specifically designed to measure the impact of heart failure on QoL. A summary score of 105 can be achieved with a lower score indicating better health. The REMATCH MLHFQ trial reported an average score at the beginning of the study (75/105), and after one year for both the LVAD (41/105) and the OMT arm (58/105). A panel of 12 members allocated utility weights to these scores. The median value for QoL was 0.655, 0.7 and 0.925, respectively at baseline and for the OMT and LVAD group (Table 5).
Table 5. Quality of life (QoL) data.
| Reference | QoL |
|---|---|
| Clegg et al., 2005 (5) | Mapping of MLHFQ scores to utilities from the summarized data reported in the pivotal REMATCH study via panel: |
| Baseline: MLHFQ score 75/105→utility score panel: 0.55 | |
| LVAD: MLHFQ score 41/105→utility score panel: 0.925 | |
| OPT: MLHFQ score 58/105→utility score panel: 0.7 | |
| Total QALYs: | |
| LVAD group: 1.04 QALY per patient | |
| Medical treatment: 0.44 QALY per patient | |
| Incremental effect: 0.60 QALY per patient | |
| Adang et al., 2006 (6) | % patients in NYHA class III/IV: (Samson et al., 2004) (12) [LVAD; Medication] |
| Month 0: [100; 100] | |
| Month 1: [46; 100] | |
| Month 3: [32; 97] | |
| Month 6: [20; 91] | |
| Month 9: [18; 100] | |
| Month 12: [29; 100] | |
| Month 18: [56; 100] | |
| Month 24: [29; 67] | |
| Valuation health state (Moskowitz et al., 1997) (16) | |
| NYHA class III/IV: 0.55 | |
| NYHA class I/II: 0.81 | |
| Total QALYs: | |
| OMT group: 0.27 QALYs | |
| LVAD group: 1.34 QALYs | |
| Incremental effect: 1.07 QALY per patient | |
| Girling et al., 2007 (7) | QoL (Moskowitz et al., 1997) (16) |
| LVAD: 0.81 | |
| OMT: 0.55 | |
| Messori et al., 2009 (9) | Base-case analysis: no adjustment for QoL |
| Alternative scenario: (Moskowitz et al., 1997) (16) | |
| QoL LVAD: 0.809 (0.673-0.945) | |
| Rogers et al., 2012 (10) | Mean utility values of 0.855, 0.771, 0.673, and 0.532 for NYHA classes I, II, III, and IV. Probability of belonging to a specific NYHA class: Monthly estimates obtained from the REMATCH and HeartMate II Destination Therapy trials for the OMT and LVAD arms (probabilities of being in NYHA I-IV at 0, 1, 3, 6, 9, 12, 18 and 24 months in original text) |
| LVAD vs. OMT: 1.87 versus 0.37 QALYs | |
| Neyt et al., 2013 (11) | QoL (Moskowitz et al., 1997) (16) |
| LVAD: 0.809 (95% CI, 0.745-0.873) | |
| OMT: 0.548 (95% CI, 0.389-0.708) | |
| Discounted incremental effect: 2.83 QALYs gained (95% CI, 1.91-3.90) |
MLHFQ, Minnesota Living with Heart Failure Questionnaire; LVADs, left ventricular assist devices; QALYs, quality-adjusted life-years; OMT, optimal medical therapy; CI, confidence interval; NYHA, New York Heart Association.
The study of Adang et al. (6) combined the probability that patients are in NYHA class III/IV or I/II and a utility weight for these states. The probability of being in a NYHA class was taken from the study of Samson et al. (12). Utility weights of 0.55 and 0.81 were assigned to NYHA class III/IV and I/II, respectively, based on the study of Moskowitz et al. (16) (Table 5). Girling et al. (7) assigned these utility weights of 0.55 and 0.81 to the OMT and LVAD group, respectively. Messori et al. (9) did not take QoL into account in the baseline analysis.
The two most recent publications also did not identify good measures of QoL in the relevant patient group. Rogers et al. (10) mapped NYHA classes with utilities and Neyt et al. (11) applied the results from the study of Moskowitz (see Table 5). We will address this limitation again in our discussion.
Uncertainty and sensitivity analysis
Most input parameters are surrounded by uncertainty and can be described by a probability distribution, rather than a point estimate. Guidelines for economic evaluations require the use of probabilistic sensitivity analysis (PSA). In this approach, applying Monte Carlo simulation, the parameter uncertainty is translated into the imprecision around the cost-effectiveness. Only a couple of studies applied this technique. For example, the most recent study included probability distributions for mortality, QoL and cost variables in which transition probabilities and utilities were modelled as beta distributions and cost variables as gamma distributions (11). On the other hand, all studies performed one- or multi-way sensitivity analysis changing, e.g., cost of an LVAD device, the discount rate, utility weights, rehospitalization probabilities, life expectancy and extrapolation scenarios. The most determining variables are mentioned in the results section.
Results of the identified economic evaluations
Table 6 provides an overview of the results of the identified economic evaluations. The study by Clegg et al. (5) presented a base case incremental cost-effectiveness ratio (ICER) for LVADs as destination therapy of £170,616 (€206,600) per QALY gained, i.e., 0.6 QALYs per person at an additional cost of £102,000 (€123,500) per patient over a period of five years. Comparing this with the ICER threshold in the UK of £30,000 (€36,300) per QALY, the authors concluded that LVADs as destination therapy does not appear to be cost-effective for patients with end-stage heart failure. This ICER was not sensitive to changes in the discount rate, costs or changes in the utility assumptions: the cost per QALY remained well above generally accepted norms (5).
Table 6. Results of identified economic evaluations for LVADs as destination therapy.
| Reference | Result |
|---|---|
| Clegg et al., 2005 (5) | ICER “base-case scenario”: £170,616/QALY (0.6 QALYs gained and additional cost of £102,000 per patient) ICER “future scenario”: £44,339/QALY If 60% improvement in survival versus REMATCH trial and lower device cost of £35,000 |
| Adang et al., 2006 (6) | ICER: ca.€112,000/QALY (1.07 QALYs gained and additional cost of €119,000 per patient) |
| Girling et al., 2007 (7) | LVAD therapy is extremely unlikely to be cost-effective at current UK QALY valuations of around £30,000 if the device costs as much as £60,000 |
| Messori et al., 2009 (9) | Mean reimbursement base-case analysis: €82,426 (range, €0 to €250,000) Mean reimbursement in scenario with utility of 0.809: €66,683 |
| Rogers et al., 2012 (10) | ICER: $198,184/QALY (1.5 QALYs gained and additional cost of $297,551) and $167,208/LYG (1,78 LYG and additional cost of $297,551) |
| Neyt et al., 2013 (11) | ICER: €107,600/QALY (95% CI, 66,700-181,100) (2.83 QALYs gained and additional cost of €299,100) and €94,100/LYG (95% CI, 59,100-160,100) (3.23 LYG and additional cost of €299,100) |
ICER, Incremental cost-effectiveness ratios; QALYs, quality-adjusted life-years; LYG, life-years gained. Exchange rates January 9, 2014: US $1 = €0.736; £1 = €1.211 (source: www.xe.com).
Adang et al. (6) obtained an ICER of about €112,000 per QALY gained (Table 6). Based on their cost-effectiveness acceptability curve (which showed the probability that an intervention is cost-effective compared to alternative interventions, depending on decision-makers’ willingness to pay for a QALY), LVAD as destination therapy had a zero probability of being cost-effective when the maximum threshold is below €90,000 per QALY. The authors concluded that a survival benefit has been demonstrated for patients with LVAD as destination therapy compared to drug therapy; however, the additional cost was considerably higher in comparison to other accepted interventions.
Girling et al. (7) calculated ICERs depending on device cost, proportion of LVAD failures and median survival under LVAD. Using UK established thresholds (£30,000/QALY), cost-effectiveness probabilities of LVAD were found to be very low. Moreover, sensitivity analysis showed that the intervention could not represent a cost-effective therapy at current UK QALY valuations at any positive value of the device cost. The future cost-effectiveness will mainly depend on the improved survival achieved with next-generation devices.
The alternative study of Messori et al. (9) calculated an average value of €82,426 for LVAD as destination therapy, which was close to the price of the HeartMate device of €75,000. However, this value was not sufficient to cover the costs for the surgical procedure of about €50,000.
The study of Rogers et al. (10) revealed a significant reduction in the ICER/QALY, from US $802,700 (€590,800) with a pulsatile-flow LVAD to US $198,184 (€145,900) with a continuous-flow LVAD when comparing with OMT. The authors mentioned that this change is explained by significant improvements in survival and functional status and by the reduction in implantation costs. However, although this improvement is encouraging, they also remarked that this ICER is still significantly higher than the traditionally used threshold of US $50,000 when considering therapies to be cost-effective.
Finally, the most recent study (11) calculated an average ICER of €94,100 per life-year gained or €107,600 per QALY gained (Table 6). Sensitivity analyses showed these results were robust. The authors concluded that although LVAD destination therapy improved survival and QoL, it remained a relatively expensive intervention, which renders the reimbursement of this therapy questionable.
In general, none of the identified economic evaluations calculated a favorable ICER.
Discussion
Treatment with the continuous-flow HM-II results in a significantly better survival and QoL in comparison with optimal medical treatment. From a medical point of view, the improvements are significant and clinically relevant. Unfortunately, from an economic point of view, published economic evaluations of 1st and 2nd generation LVADs as destination therapy show that the ratio of incremental costs versus incremental benefits is relatively high. One of the aims of policy makers might be to create as much value as possible for society. Choices have to be made given the limited resources. Based on the opportunity cost, i.e., the value of the best alternative forgone, spending money on relatively expensive interventions might do more harm than good for society as a whole, by not being able to provide other interventions that give a higher value for money.
Next-generation LVADs will become smaller and require less energy, which may have a positive influence on the durability of the device and life expectancy of the battery. Technical improvements, like transcutaneous energy transfer, may also result in a lower risk of adverse events such as infections, bleeding, and neurological events (17).
Next to these outcomes, further research should also try to capture the impact of LVAD implantation on QoL and functional recovery (11,18,19). Several studies relied on data from a single study (16) that dates from 1997. This study included only a small group of patients (n=29) with an LVAD as bridging therapy, and could not measure QoL in the most debilitated patients (i.e., informative missing values) (1,11). Other studies mapped NYHA classes to utilities. However, this indirect approach is subject to major weaknesses. First, assigning a NYHA class II or III is very subjective. Second, QoL is very dependent on co-morbidities, and similar changes in NYHA class may result in very different changes in QoL depending on the presence of these co-morbidities (11). Applying a generic utility instrument in clinical studies, in addition to disease-specific instruments, should be encouraged to support QALY calculations for future economic evaluations (20).
Future research should be performed in an appropriate research setting, preferably a randomized controlled trial, and try to avoid undue financial burden on patients, hospitals or the general healthcare system. A major challenge might be to finance this research. Governments might provide support without increasing their health care budgets by bearing the costs of the alternative interventions that would be provided to these patients if they did not participate in the trial. In exchange for this partial contribution, further agreements could be made, such as on the trial design, the comparator, price of the device, measurement of outcomes, patient follow-up. In one of the economic evaluations, the authors anticipated that continued refinement of patient selection criteria, technological advances, and improvements in management strategies will ultimately result in the demonstration of LVADs as an economically effective treatment option for patients with advanced heart failure (10). Evidence is needed to confirm or refute this prediction. The described ‘partial coverage with evidence generation’ might be a possibility to stimulate further research.
Acknowledgements
Disclosure: This work was supported by the Dutch Health Care Insurance Board (CVZ, College voor zorgverzekeringen) with project number 660/012/2010 to Mattias Neyt, Ann Van den Bruel, Yolba Smit, and Joan Vlayen. One of the included studies is performed by the authors of this review. The other authors report they have no potential conflicts of interest.
References
- 1.Neyt M, Smit Y, Van den Bruel A, et al. Left ventricular assist device (LVAD) toegepast als bestemmingstherapie bij patiënten met eindstadium hartfalen: ME-TA, medical evaluation and technology assessment, 2011.
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. [Summary for patients in J Card Fail. 2002 Apr;8(2):59-60; PMID: 12016626] [Summary for patients in J Card Fail. 2002 Apr;8(2):61-2; PMID: 12016627]. N Engl J Med 2001;345:1435-43. [DOI] [PubMed] [Google Scholar]
- 3.Dembitsky WP, Tector AJ, Park S, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg 2004;78:2123-9; discussion 2129-30. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [DOI] [PubMed] [Google Scholar]
- 5.Clegg AJ, Scott DA, Loveman E, et al. The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation. Health Technol Assess 2005;9:1-132, iii-iv. [DOI] [PubMed] [Google Scholar]
- 6.Adang E, Groenewoud H, van Hees F, et al. eds. Invoering van kunst-en steunhart als bestemmingstherapie voor patiënten met eindstadium hartfalen- Gevolgen voor ziektelast en kosten van behandeling. Nijmegen: Universitair Medisch Centrum St Radboud, 2006. [Google Scholar]
- 7.Girling AJ, Freeman G, Gordon JP, et al. Modeling payback from research into the efficacy of left-ventricular assist devices as destination therapy. Int J Technol Assess Health Care 2007;23:269-77. [DOI] [PubMed] [Google Scholar]
- 8.Clegg AJ, Scott DA, Loveman E, et al. Clinical and cost-effectiveness of left ventricular assist devices as destination therapy for people with end-stage heart failure: a systematic review and economic evaluation. Int J Technol Assess Health Care 2007;23:261-8. [DOI] [PubMed] [Google Scholar]
- 9.Messori A, Trippoli S, Bonacchi M, et al. Left ventricular assist device as destination therapy: application of the payment-by-results approach for the device reimbursement. J Thorac Cardiovasc Surg 2009;138:480-5. [DOI] [PubMed] [Google Scholar]
- 10.Rogers JG, Bostic RR, Tong KB, et al. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail 2012;5:10-6. [DOI] [PubMed] [Google Scholar]
- 11.Neyt M, Van den Bruel A, Smit Y, et al. Cost-effectiveness of continuous-flow left ventricular assist devices. Int J Technol Assess Health Care 2013;29:254-60. [DOI] [PubMed] [Google Scholar]
- 12.Samson D.Special report: cost-effectiveness of left-ventricular assist devices as destination therapy for end-stage heart failure. Technol Eval Cent Assess Program Exec Summ 2004;19:1. [PubMed] [Google Scholar]
- 13.Oz MC, Gelijns AC, Miller L, et al. Left ventricular assist devices as permanent heart failure therapy - The price of progress. Ann Surg 2003;238:577-83; discussion 583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messori A. Methods for meta-analysis: reconstructing individual survival times through the analysis of Kaplan-Meier graphs. eBMJ 2008.
- 15.Coyle LA, Ising MS, Gallagher C, et al. Destination therapy: one-year outcomes in patients with a body mass index greater than 30. Artif Organs 2010;34:93-7. [DOI] [PubMed] [Google Scholar]
- 16.Moskowitz AJ, Weinberg AD, Oz MC, et al. Quality of life with an implanted left ventricular assist device. Ann Thorac Surg 1997;64:1764-9. [DOI] [PubMed] [Google Scholar]
- 17.Kirkels JH, de Jonge N, Lahpor JR. Assist devices in the new decade: from technical developments to political decisions. Eur J Heart Fail 2010;12:217-8. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers C, Denollet J, de Jonge N, et al. Patient-reported outcomes in left ventricular assist device therapy: a systematic review and recommendations for clinical research and practice. Circ Heart Fail 2011;4:714-23. [DOI] [PubMed] [Google Scholar]
- 19.Pruijsten RV, Lok SI, Kirkels HH, et al. Functional and haemodynamic recovery after implantation of continuous-flow left ventricular assist devices in comparison with pulsatile left ventricular assist devices in patients with end-stage heart failure. Eur J Heart Fail 2012;14:319-25. [DOI] [PubMed] [Google Scholar]
- 20.Endpoints used for relative effectiveness assessment of pharmaceuticals: health-related quality of life and utility measures. EUnetHTA—European network for Health Technology Assessment, 2013.