Abstract
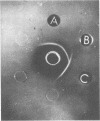
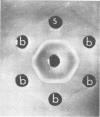
We studied 174 strains of the genus Bacillus for cross-reacting antigens to the capsular polysaccharides of groups A and C meningococcus, types I and III pneumococcus, and Haemophilus influenzae type b. Cross-reactions were detected by immunodiffusion in agarose gel by using type-specific antisera and confirmed by absorption and inhibition experiments. Of 20 Bacillus pumilis strains, six had an antigen cross-reacting with group A meningococcal polysaccharide. Other cross-reactions included one strain of B. pumilis with H. influenzae type b, one of B. cereus var. mycoides with pneumococcus type III, and one of B. alvei with both type b and SIII polysaccharides. These cross-reacting antigens are polysaccharides of vegetative cells and may be extracellular in location. Because these bacilli have antigens cross-reacting with the virulence factors of pyogenic bacteria, they may, as normal flora, be an antigenic stimulus for “natural” serum anti-capsular antibodies to the type b Haemophilus and group A meningococcus polysaccharides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. T., Wilkinson H. A., 3rd A case of meningitis and generalized Shwartzman reaction caused by Bacillus sphaericus. Johns Hopkins Med J. 1969 Jul;125(1):8–13. [PubMed] [Google Scholar]
- Curtis J. R., Wing A. J., Coleman J. C. Bacillus cereus bacteraemia. A complication of intermittent haemodialysis. Lancet. 1967 Jan 21;1(7482):136–138. doi: 10.1016/s0140-6736(67)91036-7. [DOI] [PubMed] [Google Scholar]
- FARRAR W. E., Jr Serious infections due to "non-pathogenic" organisms of the genus Bacillus. Review of their status as pathogens. Am J Med. 1963 Jan;34:134–141. doi: 10.1016/0002-9343(63)90047-0. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados O., Ewing W. H. Antigenic relationship between Escherichia coli and Neisseria meningitidis. J Infect Dis. 1970 Jul-Aug;122(1):100–103. doi: 10.1093/infdis/122.1-2.100. [DOI] [PubMed] [Google Scholar]
- Heidelberger M., Jann K., Jann B., Orskov F., Orskov I., Westphal O. Relations between structures of three K polysaccharides of Escherichia coli and cross-reactivity in antipneumococcal sera. J Bacteriol. 1968 Jun;95(6):2415–2417. doi: 10.1128/jb.95.6.2415-2417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDY M., MICHAEL J. G., WHITBY J. L. Bactericidal method for the measurement in normal serum of antibody to gramnegative bacteria. J Bacteriol. 1962 Mar;83:631–640. doi: 10.1128/jb.83.3.631-640.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffert H. L., Baptist J. N., Gidez L. I. Meningitis and bacteremia after ventriculoatrial shunt-revision: isolation of a lecithinase-producing Bacillus cereus. J Infect Dis. 1970 Dec;122(6):547–552. doi: 10.1093/infdis/122.6.547. [DOI] [PubMed] [Google Scholar]
- Pearson H. E. Human infections caused by organisms of the bacillus species. Am J Clin Pathol. 1970 Apr;53(4):506–515. doi: 10.1093/ajcp/53.4.506. [DOI] [PubMed] [Google Scholar]
- Potter M., Leon M. A. Three IgA myeloma immunoglobulins from the BALB/ mouse: precipitation with pneumococcal C polysaccharide. Science. 1968 Oct 18;162(3851):369–371. doi: 10.1126/science.162.3851.369. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]