Abstract
Background
Gender based outcome differences have been previously studied following thermal injury with a higher risk of mortality being demonstrated in females. This is opposite to what has been found following traumatic injury. Little is known regarding the mechanisms and time course of these gender outcome differences post burn injury.
Methods
A secondary analysis was performed utilizing data from a prospective observational study designed to characterize the genetic and inflammatory response following significant thermal injury (2003-2010). Clinical outcomes were compared across gender (female vs. male) and the independent risks associated with gender were determined utilizing logistic regression analysis after controlling for important confounders. Stratified analysis across age and burn severity was performed while Cox-hazard survival curves were constructed to determine the time course of any gender differences found.
Results
Over the time period of the study, 548 patients met inclusion criteria for the cohort study. Males and Females were similar age, TBSA%, inhalation injury and APACHE score. Regression analysis revealed female gender was independently associated with over a 2-fold higher mortality after controlling for important confounders. (OR 2.2, p=0.049, 95% C.I. 1.01-4.8) The higher independent mortality risk for females was exaggerated and remained significant only in PEDIATRIC patients and demonstrated a dose response relationship with increasing burn size (%TBSA). Survival analysis demonstrated early separation of female and male curves and a greater independent risk of Multiple Organ Failure was demonstrated in the PEDAITRIC cohort.
Conclusions
The current results suggest that gender based outcome differences may be different following thermal injury as compared traumatic injury and that the gender dimorphism may be exaggerated in patients with higher burn size and in those in the pediatric age group, with female gender being associated with poor outcome. These gender based mortality differences occur early and may be a result of a higher risk of organ failure and early differences in the inflammatory response following burn injury. Further investigation is required to thoroughly characterize the mechanisms responsible for these divergent outcomes.
INTRODUCTION
Burn injury represents a significant proportion of the accidental injury in the United States, with the majority of patients surviving the initial burn insult due to improvements in transport, early resuscitation, and the critical care.1,2 However, a significant number of patients that survive initially ultimately succumb to their injury or suffer significant morbidity because of the development of nosocomial infection, multisystem organ failure and sepsis.3 Studies have increasingly sought to better evaluate the risk factors for this delayed morbidity and mortality. There is increasing evidence that gender based outcome differences exist following thermal injury.4-6 Female gender has been demonstrated to be associated with worse outcome following burn injury and this gender dimorphism may be different from the response following other non-thermal injuries.7-13 A greater understanding of the mechanisms and pathways responsible for these gender based divergent outcomes following burn injury has the potential to result in novel interventions that can improve outcome and reduce the morbidity associated with this significant public health problem.
We sought to better characterize the time course and potential mechanisms responsible for the gender dimorphism after thermal injury using a multicenter cohort of severely injured burn patients for which post-injury care was relatively controlled. We hypothesized that females would have a worse outcome and that these differences would be most apparent in women of reproductive age.
METHODS
Data were obtained from the Inflammation and the Host Response to Injury Large Scale Collaborative Program (www.gluegrant.org), supported by the National Institute of General Medical Sciences (NIGMS), which was a multicenter prospective cohort study designed to characterize the genomic and proteomic response following burn injury.14 Burn patients admitted to one of six institutions (one pediatric center and 5 adult centers) over an 8 year period (2003-2010) were included in the current analysis. Inclusion criteria for the overall cohort study included: burn size ≥ 20% TBSA (> 40% TBSA for children) that required surgical treatment and arrival to an enrolling burn center within 96 hours of injury. Exclusion criteria consisted of: age > 90 years, chemical or deep electrical burns, significant associated traumatic injuries (ISS >24) preexisting severe cardiac dysfunction (< 20% ejection fraction), glucocorticoid treatment, malignancy and prior bilateral lower-extremity amputations.14,15 Clinical data were entered and stored in TrialDb, a web-based data collection platform, by trained research nurses.16 Integrity of the data was maintained through ongoing curation and external data review by an independent chart abstractor. Standard operating procedures were developed and implemented across all participating centers to minimize variation in post-burn care including: goal directed resuscitation, glycemic control, burn wound management, perioperative antibiotic prophylaxis, nutrition and enteral feeding, management of central venous catheters and blood stream infections, acute lung injury, ventilator associated pneumonia, diagnosis of inhalation injury and venous thromboembolism prophylaxis.15
Outcomes of primary interest were in-hospital mortality, nosocomial infection (NI) and the development of Multiple Organ Failure (MOF). While patients were admitted to the ICU, Denver post injury multiple organ failure scores were determined daily for pulmonary, renal, hepatic and cardiac organ systems.17 The diagnosis of MOF required a maximum Denver post injury multiple organ failure score > 3 beyond 48 hours from burn injury.18 All nosocomial infectious complications were monitored for, recorded (infection type, culture specimen source, and bacteriology) with the diagnosis of NI requiring specific clinical criteria along with positive culture evidence. Diagnosis of a ventilator associated pneumonia required a quantitative culture threshold of ≥ 104 CFU/ml for bronchoalveolar lavage specimens. Diagnosis of catheter-related blood stream infections required positive peripheral cultures with the identical organism obtained from either a postivie semiquantitative culture (>15 CFU/segment), or positive quantitative culture (>103 CFU/segment) from a catheter segment specimen.
For the current secondary analysis and due to the spectrum of age and burn severity across the enrolled patients, the cohort was also further stratified by age of the patient (PEDIATRIC 1-14 years, YOUNG 15-50 years, and OLD > 50 years) and burn size (LOW-≥20% and ≤ 40% TBSA, MODERATE- >40% and ≤ 60% TBSA, HIGH- >60% TBSA) Demographics, burn injury characteristics, resuscitation requirements, and outcomes were compared across gender (Females vs. Males) in univariate analysis and across the age stratified groups. Multivariate logistic regression analysis was then utilized to determine the independent outcome risk differences across gender (Female vs. Male) after controlling for important confounders for the overall cohort and when stratified by age and burn size. Finally, to characterize the time course of any gender based outcome differences, Cox-hazard regression analysis using the same model covariates was employed. Covariates used in the final regression models in addition to gender, included age, burn mechanism, burn size (TBSA%), presenting base deficit, APACHE II score, presence of inhalation injury (yes/no), Glasgow Coma Scale (GCS), body mass index, and presence of pre-existing comorbidities (yes/no).
Data analysis was conducted using SPSS version 20 (Chicago, IL). For univariate analyses Chi-square tests were used to compare categorical variables, and Mann-Whitney tests were used to compare continuous variables. Continuous data are presented as median (interquartile range [IQR]) or mean ± standard deviation unless noted. A p-value of ≤ 0.05 was considered significant. The institutional review board of each participating center approved the cohort study, while the institutional review board at the University of Pittsburgh Medical Center approved this current secondary analysis.
RESULTS
Over the time period of the study, 548 patients met inclusion criteria and constituted the study cohort. Mean burn %TBSA for the cohort was 48±20% with an overall mortality of 15%. Over 26% of patients suffered > 60% TBSA and an additional 33% having between 40% and 60% TBSA. Just over 46% of patients suffered from inhalation injury and the incidence of multiple organ failure, nosocomial infection was 27%, 69%, respectively. The age of the cohort spanned pediatric ages thru the elderly population. (Figure 1.) The study cohort was 72% male. Univariate analysis was performed across gender for the entire cohort and males and females were found to be similar in demographics, burn size, shock severity, preexisting comorbidities and hospital and ICU requirements. Females did have a significantly lower nadir Mean Arterial Pressure over the first 24 hours post-injury and a trend towards lower 24 hour crystalloid resuscitation and more commonly suffered scald burns as compared to flame or flash burn mechanism (Table.1) This unadjusted comparison across gender also revealed no clinical differences in the incidence of multiple organ failure or infectious complications but a higher mortality was found for females which did not reach statistical significance.
Figure 1.
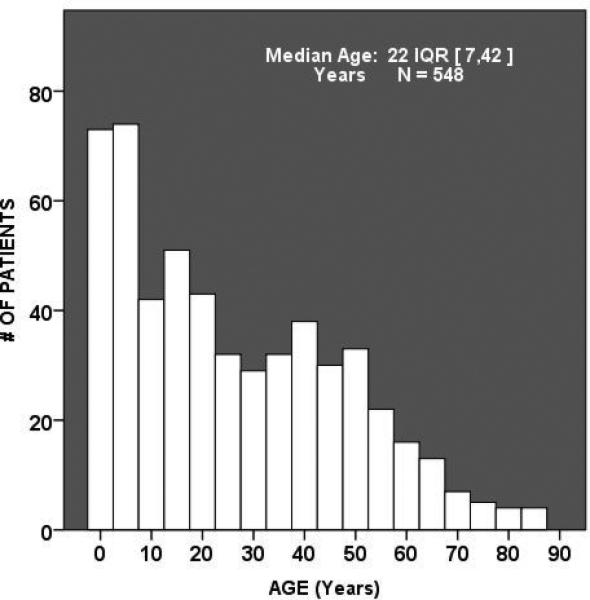
Histogram demonstrating age of study cohort
Table 1.
Demographic and burn injury characteristic comparison across gender.
| Females (n=155) | Males (n=393) | p-value | |
|---|---|---|---|
| Age | 24.7±23 | 26.7±20 | 0.073 |
| Burn Size (TBSA%) | 48.0±19 | 48.8±20 | 0.686 |
| Burn Mechanism | |||
| Flame | 77.4% | 81.2% | |
| Flash | 1.3% | 5.9% | 0.010* |
| Scald | 18.1% | 9.2% | |
| Other | 3.2% | 3.8% | |
| Inhalational Injury (%) | 46.1% | 47.1% | 0.826 |
| Body Mass Index (kg/m2) | 24.2±9 | 24.0±7 | 0.347 |
| Presenting Base Deficit (meq/L) | −6.3±5 | −5.5±5 | 0.141 |
| Lowest GCS (first 24hrs) | 9.8±5 | 10.2±5 | 0.386 |
| Lowest MAP (first 24hrs, mmHg) | 66±15 | 69±17 | 0.034* |
| APACHE II score | 10.2±5 | 9.8±5 | 0.418 |
| First 24hr crystalloid (Liters) | 11.9±9 | 14.2±10 | 0.064 |
| 2nd Degree Burns (%) | 18.1% | 19.8% | 0.228 |
| 3rd Degree Burns (%) | 40.4% | 38.3% | 0.329 |
| Pre-Admission Comorbidities (%) | 58.1% | 52.9% | 0.277 |
| Pre-Admission Medications (%) | 65.8 % | 70.4% | 0.294 |
| Hospital Length of Stay (days) | 37.7±33 | 40.1±35 | 0.379 |
| ICU Days | 322±32 | 34.6±35 | 0.371 |
| Unadjusted Mortality (%) | 18.7% | 13.3% | 0.106 |
| Unadjusted Rate of MOF (%) | 27.3% | 27.1% | 0.969 |
| Unadjusted Rate of NI (%) | 71.4% | 69.1% | 0.599 |
Comparison of burn injury characteristics across the different stratified age groups (PEDIATRIC 1-14 years, YOUNG 15-50 Years, OLD > 50 years) demonstrated greater burn size and a higher percentage of 3rd degree burns, lower APACHE scores, a worse presenting base deficit and, as expected, a lower volume of resuscitation in the first 24 hours in the PEDIATRIC group. Importantly, despite higher burn severity, PEDIATRIC patients had a significantly lower mortality rate. (Table 2.)
Table 2.
Burn characteristics compared across age groups (PEDIATRIC 1-14 years, YOUNG 15-50 years, OLD > 50).
| PEDIATRIC (n=210) | YOUNG (n=254) | OLD (n=84) | p-value | |
|---|---|---|---|---|
| Burn Size (TBSA%) | 58.3±17 | 43.8±19 | 38.7±17 | <0.001 |
| 3rd Degree Burns (%) | 47.8±24 | 34.2±21 | 29.4±18 | <0.001 |
| APACHE II score | 15.5±9 | 19.2±9 | 23.1±8 | <0.001 |
| Presenting Base Deficit (meq/L) | −6.5±−9 | −5.3±5 | −4.5±4 | 0.003 |
| First 24hr crystalloid (Liters) | 4.4±3 | 16.9±10 | 15.4±8 | <0.001 |
| Mortality (%) | 8.1% | 13.0% | 36.9% | <0.001 |
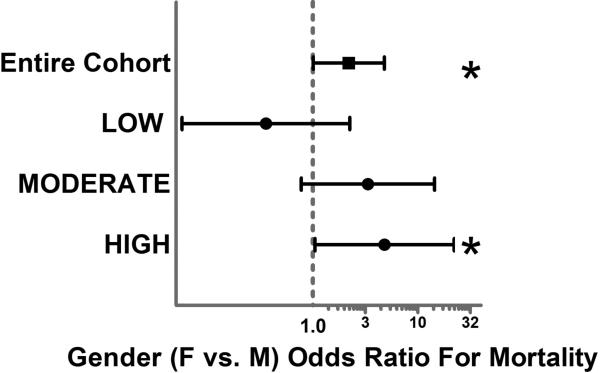
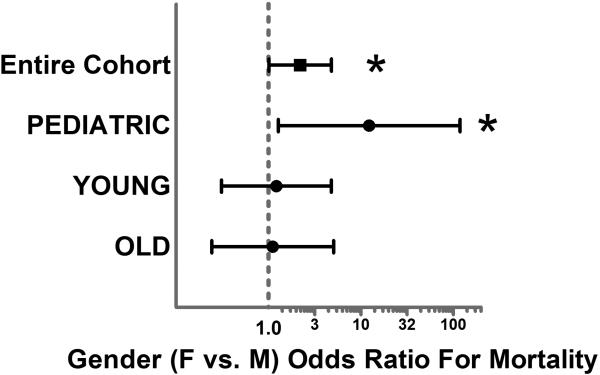
Logistic regression was first utilized to determine the independent risks of poor outcome associated with gender for the overall cohort. Our regression model was an excellent predictor of mortality with an area under the curve (AUC) of 0.90 via receiver operating characteristic. Logistic regression analysis revealed that female gender was independently associated with over a 2-fold higher risk of mortality (OR 2.2, p=0.049, 95% C.I. 1.01-4.8). When the regression model was stratified by burn size (LOW-20% and ≤ 40%, MODERATE- >40% and ≤ 60% TBSA, HIGH- >60% TBSA), a dose response relationship was demonstrated and female gender was again associated with a significantly exaggerated (over 4-fold greater) independent risk of mortality in the HIGH burn size group. (Figure 2.) Interestingly, when the cohort was stratified by burn depth (% of 3rd degree burns) no significant dose response relationship was found. When the regression model was stratified by age (PEDIATRIC 1-14 years, YOUNG 15-50 Years, OLD > 50 years) the significantly higher mortality risk for females remained significant and exaggerated only in the PEDIATRIC group (OR 12.3, p=0.030, 95% C.I. 1.2-118.5). (Figure 3.) To determine if these gender based outcome differences varied across burn centers in the cohort we tested for interaction between gender and burn center site. This interaction term was not significant (p=0.89) suggesting these gender based outcome differences were consistent across all burn centers.
Figure 2.
Forest-Plot depicting the independent gender (female vs. male) odds ratio for mortality stratified by burn size (LOW-20% and ≤ 40%, MODERATE- >40% and ≤ 60% TBSA, HIGH- >60% TBSA).
Figure 3.
Forest-Plot depicting the independent gender (female vs. male) odds ratio for mortality stratified by age group (PEDIATRIC 1-14 years, YOUNG 15-50 Years, OLD > 50 years).
Cox hazard regression analysis was then utilized to further assess the timing of the mortality outcome differences across gender in the different age groups while controlling for important confounders. After the incorporation of time into the model, PEDIATRIC (age 1-14 years) female patients continued to demonstrate over a 10-fold higher independent risk of mortality (HR 10.4, p=0.005, 95% C.I. 2.0-53) with the survival curves demonstrating an early separation soon after admission. (Figure 4.) No clinical or significant differences were found for those patients in the YOUNG or OLD age groups.
Figure 4.
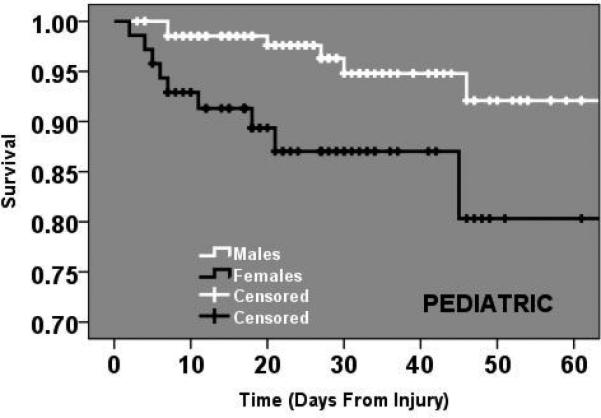
Cox-hazard survival curves comparing male and female PEDIATRIC patients.
Finally, to characterize the potential mechanisms responsible for these gender based mortality risk differences, both the development of multiple organ failure (MOF) and nosocomial infection (NI) were also analyzed using the same logistic regression models for the overall cohort and for the stratified groups. No significant independent differences across gender were found for the development of nosocomial infection, overall or in the stratified groups. With MOF as the model outcome variable, in PEDIATRIC patients with MODERATE or HIGH burn size (> 40% TBSA), female gender was significantly associated with over a 4-fold greater independent risk of MOF (OR 4.1, p=0.027, 95%C.I. 1.2-14.3)
DISCUSSION
Insight into those pre-injury factors that can provide a beneficial or protective effect has the potential to promote novel interventions or mechanistic understanding that results in improved outcomes following injury. An increasing pool of both basic science and clinical literature has documented outcome differences attributable to gender (female vs. male) following traumatic and thermal injury.4,5,7,8,19-25 Interestingly, the majority of this literature suggests that female gender may be protective following traumatic injury and associated with detrimental outcome following burn injury, relative to their similarly injured and burned male counterparts. Both sex hormones and genetic differences have been postulated to be responsible such outcome differences, with the specific mechanisms responsible remaining controversial and incompletely characterized clinically.4,7,8,23,26 The results of the current study suggest that the gender dimorphism following significant burn injury may be most exaggerated in patients with higher burn size (TBSA%) and in those in the pediatric age group, with female gender being associated with poor outcome. These gender based mortality differences occur early and may be a result of a higher risk of organ failure and early differences in the inflammatory response following burn injury.
These results add to prior literature which have also demonstrated worse outcome for females following burn injury and provides further insight into the mechanisms that may be responsible. Previous large retrospective studies following burn injury in adults have demonstrated a similar higher mortality risk for females. The age groups where these gender differences were strongest were within the 10 years and 70 years age groups with smaller retrospective studies suggesting a more narrow age range (30-60 years).4-6 Prior evaluation of gender based outcome differences following pediatric burn injury has been less consistent with some suggesting female pediatric burn patients have an attenuated inflammatory and metabolic response leading to shorter hospital utilization, while others have demonstrated no gender based divergent outcomes.27-29 Basic science literature both in traumatic and thermal injury has provided a strong argument for a sex hormome mechanism, however, translation of these mechanistic results into the clinical arena have yet to materialize.
The current results differ from prior studies by demonstrating a robust gender dimorphism primarily in the pediatric age group when the effect of sex hormones should be primarily absent. Additionally, secondary to the robust nature of the dataset utilized for the study, this pediatric gender dimorphism for mortality was demonstrated to be most prominent early following burn injury and resulted in part from a higher independent risk of multiple organ failure in females. The question remains why the current study differs from prior literature. The dataset was a prospective cohort with attempts to control post burn care using standard operating procedures. It may be that the homogeneity of the care allowed these differences to be demonstrated in a relatively small cohort of patients. The results also demonstrated a dose-dependent effect in the strength of the gender dimorphism with burn size (TBSA%). The greater risk of multiple organ failure in females and the early separation of the survival curves suggest that differences in the early inflammatory response may be responsible. It may be that the competence of the immune response in the pediatric group may in part play a role and result in these gender based outcome difference following burn injury.
This study has several limitations. The current study is a secondary analysis of the Host Response to Injury burn cohort and was not designed to address the specific questions in this analysis. Although the current dataset was collected prospectively and standardized protocols were in place, there is likely variation in care practices across burn centers that could impact our results. We attempted to control for important differences in burn injury severity and other risk factors for poor outcome and investigated any potential bias across different burn centers. Despite our regression models having high predictive characteristics, there remains the possibility that we were unable to control for important confounders. Enrolling centers for the cohort study included multiple adult burn centers and a pediatric burn center. The immune response following burn injury across different age groups may be variable and may bias the current results. Importantly, the inclusion for the cohort study for pediatric patients was a burn size greater than 40%. Although we attempted to adjust for this fact in the age stratified analysis, this different inclusion criterion may be responsible in part for the results found in this group. The relatively small sample size of the cohort, as compared to prior literature, and the spectrum of ages may also limit the applicability of the current conclusions formulated. Finally, despite attempts at controlling confounders in the model, there were differences in burn injury mechanism across the gender groups overall which may play a role in our results and conclusions. Importantly, when we focused our comparison on burn mechanism in the pediatric age group alone, these differences in burn mechanism did not reach statistical significance (p=0.136).
In conclusion, the current results suggest that gender based outcome differences may be different following thermal injury as compared traumatic injury and that the gender dimorphism may be exaggerated in patients with higher burn size and in those in the pediatric age group, with female gender being associated with poor outcome. These gender based mortality differences occur early and may be a result of a higher risk of organ failure and early differences in the inflammatory response following burn injury. Further investigation is required to thoroughly characterize the mechanisms responsible for these divergent outcomes so that the morbidity and mortality associated with burn injury can ultimately be reduced.
Acknowledgments
Funding/Support: NIH NIGMS U54 GM062119-1 and NIH NIGMS K23GM093032-1.
Footnotes
This paper was presented as an oral presentation at the annual meeting of the American Burn Association, April 24, 2013.
REFERENCES
- 1.Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996 Mar-Apr;17(2):95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.LaBorde P. Burn epidemiology: the patient, the nation, the statistics, and the data resources. Crit Care Nurs Clin North Am. 2004 Mar;16(1):13–25. doi: 10.1016/j.ccell.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Cumming J, Purdue GF, Hunt JL, O'Keefe GE. Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. J Trauma. 2001 Mar;50(3):510–515. doi: 10.1097/00005373-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 4.McGwin G, Jr., George RL, Cross JM, Reiff DA, Chaudry IH, Rue LW., 3rd. Gender differences in mortality following burn injury. Shock. 2002 Oct;18(4):311–315. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe GE, Hunt JL, Purdue GF. An evaluation of risk factors for mortality after burn trauma and the identification of gender-dependent differences in outcomes. J Am Coll Surg. 2001 Feb;192(2):153–160. doi: 10.1016/s1072-7515(00)00785-7. [DOI] [PubMed] [Google Scholar]
- 6.Kerby JD, McGwin G, Jr., George RL, Cross JA, Chaudry IH, Rue LW., 3rd. Sex differences in mortality after burn injury: results of analysis of the National Burn Repository of the American Burn Association. J Burn Care Res. 2006 Jul-Aug;27(4):452–456. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- 7.Sperry JL, Nathens AB, Frankel HL, et al. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008 Jun;36(6):1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- 8.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008 Mar;83(3):499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 9.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. The Journal of trauma. 2000 May;48(5):932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Napolitano LM, Greco ME, Rodriguez A, Kufera JA, West RS, Scalea TM. Gender differences in adverse outcomes after blunt trauma. The Journal of trauma. 2001 Feb;50(2):274–280. doi: 10.1097/00005373-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Harbrecht BG, Peitzman AB, Rivera L, et al. Contribution of age and gender to outcome of blunt splenic injury in adults: multicenter study of the eastern association for the surgery of trauma. The Journal of trauma. 2001 Nov;51(5):887–895. doi: 10.1097/00005373-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 12.George RL, McGwin G, Jr., Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003 Jan;19(1):28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 13.George RL, McGwin G, Jr., Metzger J, Chaudry IH, Rue LW., 3rd. The association between gender and mortality among trauma patients as modified by age. The Journal of trauma. 2003 Mar;54(3):464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 14.Klein MB, Silver G, Gamelli RL, et al. Inflammation and the host response to injury: an overview of the multicenter study of the genomic and proteomic response to burn injury. J Burn Care Res. 2006 Jul-Aug;27(4):448–451. doi: 10.1097/01.BCR.0000227477.33877.E6. [DOI] [PubMed] [Google Scholar]
- 15.Silver GM, Klein MB, Herndon DN, et al. Standard operating procedures for the clinical management of patients enrolled in a prospective study of Inflammation and the Host Response to Thermal Injury. J Burn Care Res. 2007 Mar-Apr;28(2):222–230. doi: 10.1097/BCR.0B013E318031AA44. [DOI] [PubMed] [Google Scholar]
- 16.Brandt CA, Deshpande AM, Lu C, et al. TrialDB: A web-based Clinical Study Data Management System. AMIA ... Annual Symposium proceedings / AMIA Symposium. AMIA Symposium. 2003:794. [PMC free article] [PubMed] [Google Scholar]
- 17.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009 May;31(5):438–447. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciesla DJ, Moore EE, Johnson JL, et al. Multiple organ dysfunction during resuscitation is not postinjury multiple organ failure. Arch Surg. 2004 Jun;139(6):590–594. doi: 10.1001/archsurg.139.6.590. discussion 594-595. [DOI] [PubMed] [Google Scholar]
- 19.Gregory MS, Faunce DE, Duffner LA, Kovacs EJ. Gender difference in cell-mediated immunity after thermal injury is mediated, in part, by elevated levels of interleukin-6. J Leukoc Biol. 2000 Mar;67(3):319–326. [PubMed] [Google Scholar]
- 20.Gregory MS, Duffner LA, Faunce DE, Kovacs EJ. Estrogen mediates the sex difference in post-burn immunosuppression. J Endocrinol. 2000 Feb;164(2):129–138. doi: 10.1677/joe.0.1640129. [DOI] [PubMed] [Google Scholar]
- 21.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997 Jan;25(1):106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Knoferl MW, Jarrar D, Angele MK, et al. 17 beta-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol. 2001 Oct;281(4):C1131–1138. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 23.Jarrar D, Wang P, Knoferl MW, et al. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery. 2000 Aug;128(2):246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 24.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H1015–1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 25.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000 Aug;14(2):81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 26.Chaudry IH, Samy TS, Schwacha MG, Wang P, Rue LW, 3rd, Bland KI. Endocrine targets in experimental shock. The Journal of trauma. 2003 May;54(5 Suppl):S118–125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]
- 27.Jeschke MG, Mlcak RP, Finnerty CC, et al. Gender differences in pediatric burn patients: does it make a difference? Ann Surg. 2008 Jul;248(1):126–136. doi: 10.1097/SLA.0b013e318176c4b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mlcak RP, Jeschke MG, Barrow RE, Herndon DN. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006 Jul;244(1):121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrow RE, Wolfe RR, Dasu MR, Barrow LN, Herndon DN. The use of beta-adrenergic blockade in preventing trauma-induced hepatomegaly. Ann Surg. 2006 Jan;243(1):115–120. doi: 10.1097/01.sla.0000193834.07413.91. [DOI] [PMC free article] [PubMed] [Google Scholar]