Abstract
Background
The ideal population of patients for endovascular therapy (ET) in acute ischemic stroke remains undefined. Recent ET trials have moved towards selecting patients with proximal middle cerebral artery (MCA) or internal carotid artery occlusions, which will likely leave a gap in our understanding of the treatment outcomes of M2 occlusions.
Objective and methods
To examine the presentation, treatment, and outcomes of M2 compared with M1 MCA occlusions in patients undergoing ET by assessing comprehensive MRI, angiography, and clinical data.
Results
We found that M2 occlusions can lead to massive strokes defined by hypoperfused and infarcted volumes as well as death or moderate to severe disability in nearly 50% of patients at discharge. Compared with M1 occlusions, M2 occlusions achieved similar Thrombolysis in Cerebral Infarction (TICI) 2b/3 recanalization rates, with significantly less hemorrhage. M2 occlusions presented with smaller infarct and hypoperfused volumes and had smaller final infarct volumes regardless of recanalization. TICI 2b/3 recanalization of M2 occlusions was associated with smaller infarct volumes compared with TICI 0–2a recanalization, as well as less infarct expansion, in patients who received IV tissue plasminogen activator as well as those that did not. Successful reperfusion of M2 occlusions was associated with improved discharge modified Rankin scale.
Conclusions
If suitable as targets of ET, M2 occlusions should be given the same consideration as M1 occlusions.
INTRODUCTION
The ideal population of patients for endovascular therapy (ET) in acute ischemic stroke remains undefined. Recent trials with varied designs and selection criteria have failed to prove a clear benefit for this treatment over IV tissue plasminogen activator (IV tPA) or supportive care.1–3 Based on these results, some authors have called for ET to be restricted to patients treated in an investigational setting as enrollees of clinical trials.4 While these studies failed to meet their primary endpoints, post hoc analyses of their cohorts and other retrospective analyses have suggested characteristics of subgroups that may demonstrate benefit of ET over medical therapy. Building on these data, several ongoing endovascular stroke trials have been designed to capture these patients hypothesized to be the best responders.
Proximal arterial occlusion location and greater thrombus extent have become pivotal entry criteria in this new generation of trials.5,6 These features are among the easiest and earliest defined characteristics of patients with large artery strokes, and identify patients less likely to recanalize with IV tPA alone. Many of the trials currently underway are preferentially selecting patients with lesions located in the internal carotid artery (ICA) or M1 segment of the middle cerebral artery (MCA). These segments are readily visualized by non-invasive imaging and may identify a group of patients likely to benefit from ET. As a result, while lesions in the M2 segment of the MCA may be treated in clinical practice, the availability of new prospective and randomized trial data describing their presentation and treatment outcomes will be lacking, as the expected results from the upcoming trials are likely to exclude M2 occlusions.
In this study, we examine the presentation, treatment, and outcomes of M2 compared with M1 MCA occlusions in patients undergoing ET by assessing comprehensive imaging, angiography, and clinical data. To maintain consistency in a high quality dataset based on sequential MRI imaging at multiple times throughout the presentation as well as detailed angiography and clinical parameters, we have focused our efforts on data from clinical practice at a single center. If these patients are to be excluded from current trials, analyses such as these will be crucial in determining the effect of ET in this population.
METHODS
Demographic, clinical, imaging, and angiographic data were prospectively collected on a consecutive cohort of patients who received ET (intra-arterial (IA) thrombolytic therapy or mechanical thrombectomy) for acute cerebral ischemia at a single tertiary referral center from September 2004 to December 2012. Patients were included in this study if they presented with symptoms of acute cerebral ischemia within the MCA distributions, were older than 18 years, had initial MR angiography or CT angiography imaging demonstrating occlusion of the M1 or M2 segments of the MCA, and underwent conventional angiography for consideration of ET. The decision to proceed with ET at our institution is based on patient demographics, clinical history and examination, as well as imaging demonstration of large-vessel occlusion that would be amenable to ET. Patients with occlusions who were felt by the interventionalist to be inaccessible without risk of significant harm were not included in this cohort. Patients with large infarct volumes on initial imaging are generally not offered ET. Conversely, patients with substantive clinical improvement after IV tPA are observed closely but not treated with ET without recurrence of symptoms or worsening that is shown on imaging to be due to continued ischemia. As a result of these selection criteria, the resultant dataset reflects routine clinical practice while capturing advanced imaging data.
Routine clinical care included MRI before angiography if not contraindicated. Follow-up imaging was then performed 24 h after arrival and before discharge. Diffusion-weighted (DWI) and perfusion-weighted imaging (PWI) lesion volume measurements were performed by one of the authors blinded to the clinical information using a computer-assisted volumetric analysis program (Olea Medical, La Ciotat, France). Diffusion was measured at three values of b (b=0, 500, 1000 s/mm2), and average apparent diffusion coefficient (ADC) maps were generated. DWI volumes were quantified from analysis of isotropic b1000 images and ADC maps with a threshold of ADC <600. Final infarct measurements were gathered from the final MRI performed before discharge. PWI volumes were determined with Tmax of ≥6 s.7 Determination of arterial occlusion site (M1 vs M2 MCA) was made by review of angiographic images according to a recommended standard definition of a “division occlusion beyond the bifurcation of M1.”8 Hemorrhagic transformation was categorized as hemorrhagic infarctions (HI) or parenchymal hematomas (PH), as previously described.9 Patients were excluded if tandem vessel occlusions (ie, ICA +MCA) were identified. In all cases, angiography was performed subsequent to the initial DWI and PWI studies.
Univariate comparisons between categorical variables were made using Fisher’s exact test and the χ2 test, and between continuous variables using the Mann–Whitney U test. Shift analysis of the ordinal modified Rankin scale (mRS) variables was performed using the Van Elteren test according to previously published techniques.10 All statistical analyses were carried out using commercially available software (Prism V.5.0a, GraphPad Software, La Jolla, California, USA and SAS V.9.4, SAS Institute Inc, Cary, North Carolina, USA). A value of p<0.05 was considered statistically significant.
This study was approved by the institutional review board of the local institution and was conducted in compliance with the Health Information Portability and Accountability Act.
RESULTS
A total of 115 patients undergoing angiography for MCA occlusions were identified, 61 with M1 occlusions and 54 with M2 occlusions. Two patients with M2 occlusions demonstrated recanalization after IV tPA and underwent diagnostic angiography, but no ET was performed. Demographic, treatment, and angiographic outcome measures for the cohort who underwent ET are shown in table 1. A prior history of diabetes was more common in patients who presented with M1 occlusions. There were no differences in age, sex, baseline function, or other aspects of the past medical history. There was a trend towards lower National Institutes of Health Stroke Scale (NIHSS) scores in the group of patients with an M2 occlusion (16 vs 11, M1 vs M2 p=0.1). More patients with M2 occlusions received IV tPA (36% vs 60%, M1 vs M2, p<0.05). Door to groin puncture was faster for M1 (121 vs 150 min, M1 vs M2 p=0.05). Compared with M1 occlusions, M2 occlusions were more likely to be treated with IA tPA or the Solitaire device, and less likely to be treated with the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) device. Final Thrombolysis in Cerebral Infarction (TICI) scores were similar for both sets of patients.8
Table 1.
Patient demographics
| Demographics | M1 | M2 | p Value |
|---|---|---|---|
| Number | 61 | 52 | |
| Age (median and IQR) | 70 (53–81) | 72 (57–83) | 0.47 |
| Female | 40 (66) | 28 (54) | 0.25 |
| History of HTN | 36 (59) | 32 (62) | 0.85 |
| History of diabetes | 15 (25) | 1 (2) | <0.001 |
| History of HLD | 19 (31) | 12 (23) | 0.4 |
| Prior stroke or TIA | 7 (11) | 10 (19) | 0.3 |
| History of AF | 21 (34) | 23 (44) | 0.34 |
| Baseline mRS | 0.21 | ||
| 0–1 | 61 (100) | 50 (96) | |
| 2 | 0 (0) | 2 (4) | |
| NIHSS (median and IQR) | 16 (11–20) | 11 (9–19) | 0.1 |
| Left hemisphere | 30 (49) | 29 (56) | 0.57 |
| IV tPA | 22 (36) | 31 (60) | <0.05 |
| Door to groin puncture (min) | 121 (90–173) | 150 (112–182) | 0.05 |
| Endovascular treatments used | |||
| IA tPA | 3 (5) | 33 (63) | <0.001 |
| MERCI | 54 (89) | 10 (19) | <0.001 |
| Angioplasty | 6 (10) | 1 (2) | 0.07 |
| Solitaire | 1 (2) | 6 (12) | <0.001 |
| Mechanical disruption | 1 (2) | 3 (6) | 1 |
| Penumbra | 7 (11) | 4 (8) | 0.53 |
| TICI 2b/3 | 28 (46) | 23 (44) | 0.31 |
Results are shown as number (%) unless stated otherwise.
AF, atrial fibrillation; HLD, hyperlipidemia; HTN, hypertension; IA, intra-arterial; IQR, interquartile range; MERCI, Mechanical Embolus Removal in Cerebral Ischemia; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack; TICI, Thrombolysis in Cerebral Infarction; tPA, tissue plasminogen activator.
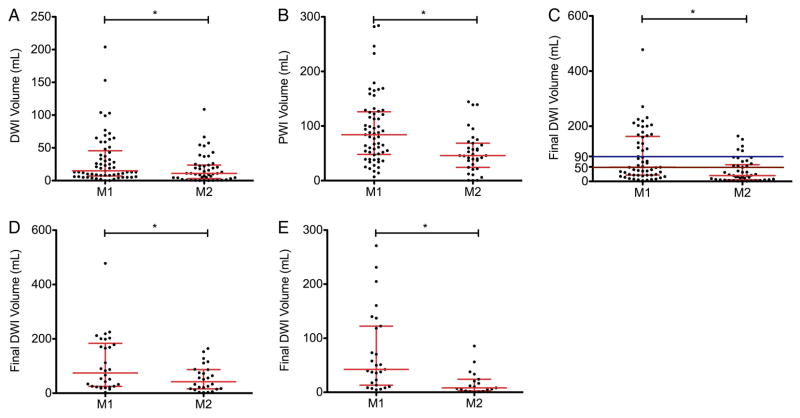
Ninety-seven per cent of all patients had MRI available for analysis. As shown in figure 1, patients with M2 occlusions presented with smaller infarct sizes as determined by DWI volume on initial MRI compared with patients with M1 occlusions. M2 patients also presented with smaller hypoperfused volumes, as determined by PWI. Final infarct volumes, regardless of revascularization status, were decreased in the M2 group, as shown in figure 1C. After stratifying for reperfusion, this relationship was still maintained, for patients with TICI 0–2a reperfusion (figure 1D) and TICI 2b/3 reperfusion (figure 1E).
Figure 1.
Initial and final infarct and hypoperfused volumes for patients presenting with M1 and M2 occlusions. Compared with patients who presented with M1 occlusions, patients with M2 occlusions presented with (A) smaller infarcts (15 vs 11 mL, p<0.05) and (B) smaller hypoperfused volumes (84 vs 46 mL, p<0.001). Compared with patients who presented with M1 occlusions, patients with M2 occlusions had (C) smaller final infarcts volumes (52 vs 22 mL, p<0.001). For the subsets of patients who achieved (D) Thrombolysis in Cerebral Infarction (TICI) 0–2a or (E) TICI 2b/3 reperfusion, final infarct volumes were also smaller in M2 than M1 occlusions (74 vs 51 mL, p<0.05 and 42 vs 8 mL, p<0.01). Each point represents an individual patient, with the median and IQR shown in red. Colored horizontal lines indicate final infarct volumes of 90 mL (blue) and 50 mL (brown). *Indicates p<0.05. DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging.
Final infarct volumes of ≥50 mL have been shown to be the most accurate for distinguishing between good and poor clinical outcome, and final infarct volumes of 90 mL are very specific for poor clinical outcome.11 Fewer patients with M2 occlusions had final infarcts ≥50 mL (54% vs 33%, p<0.05) and ≥90 mL (37% vs 11%, p<0.01).
Rates of hemorrhagic conversion were lower in the group with M2 occlusions. The total number of hemorrhages in the M2 group was lower (56% vs 21%, M1 vs M2 p<0.001), as was the number of HI type 2 (23% vs 0%, M1 vs M2 p<0.001). Rates of HI type 1 (10% vs 4%, M1 vs M2 p=0.46), PH type 1 (12% vs 6%, M1 vs M2 p=0.51) and PH type 2 (12% vs 10%, M1 vs M2 p=1.0) were similar. Within the group of patients with M2 occlusions, attempting ET but not achieving TICI 2b/3 reperfusion was not associated with an increased rate of hemorrhage (27% vs 16%, TICI 0–2a vs TICI 2b/3 p=0.49).
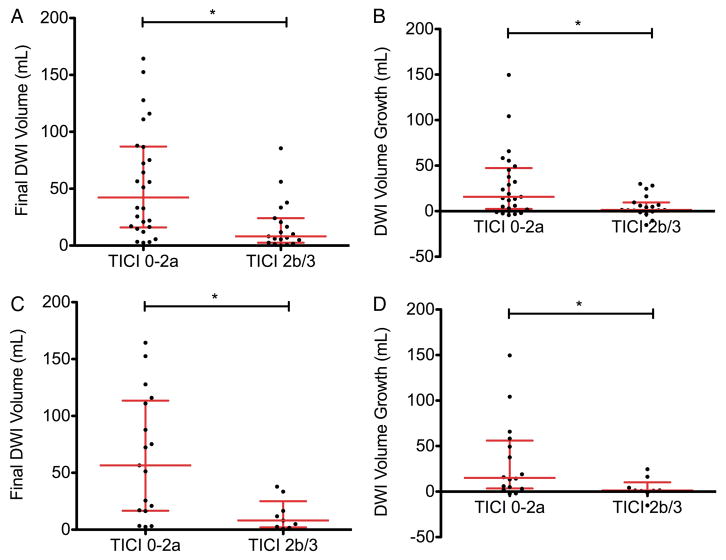
Of all patients who were found to have M2 occlusions on non-invasive vascular imaging who received IV tPA and went on to angiography, 94% (31/33) were found to have persistent occlusions. In the remaining two patients who did not have vessel occlusions, diagnostic angiography was performed without additional intervention. Patients with M2 occlusions who achieved TICI 2b/3 reperfusion had decreased final infarct volumes, and less infarct expansion from their initial stroke (figure 2A, B). In the subset of patients who received IV tPA, this relationship was maintained: TICI 2b/3 reperfusion was associated with smaller final infarct volumes, and less infarct expansion (figure 2C, D).
Figure 2.
Final infarct volumes and infarct expansion in patients with M2 occlusions by reperfusion status and receipt of IV tissue plasminogen activator (tPA). In the cohort of all M2 patients, Thrombolysis in Cerebral Infarction (TICI) 2b/3 reperfusion was associated with (A) smaller final infarct volumes (51 vs 8 mL, p<0.05) and (B) less infarct expansion (19 vs 1 mL, p<0.01). In the subset of patients who received IV tPA, TICI 2b/3 reperfusion was also associated with (C) smaller final infarct volumes (64 vs 8 mL, p<0.01) and (D) less infarct expansion (17 vs 1 mL, p<0.05). Each point represents an individual patient, with the median and IQR in red. *Indicates p<0.05. DWI, diffusion-weighted imaging.
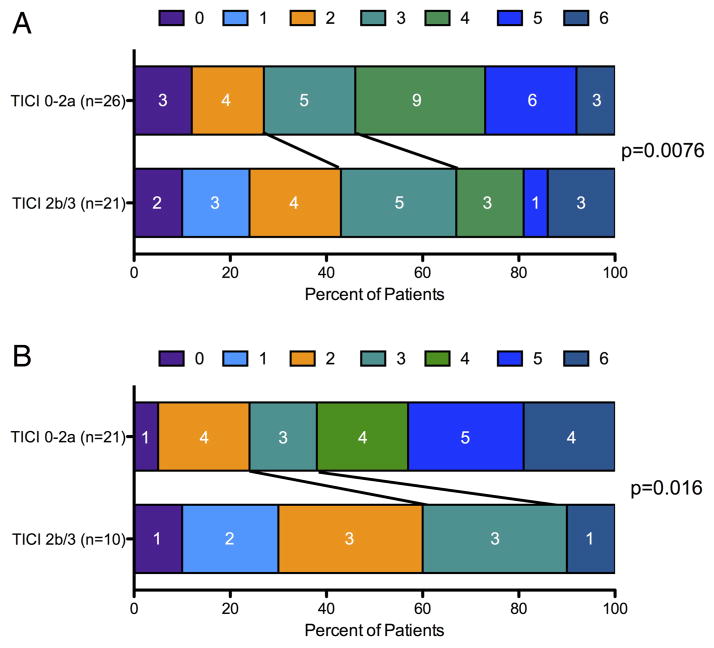
Clinical outcomes in patients with M2 occlusions included significant morbidity and mortality. Rates of discharge mRS 4–6 were similar between patients with M1 and M2 occlusions (64% vs 49%, M1 vs M2 p=0.13). Outcomes in patients with M2 occlusions treated with ET were improved in the cohort who achieved TICI 2b/3 reperfusion, as shown in figure 3A. In the subset of these patients who received tPA, achieving TICI 2b/3 reperfusion with ET was also associated with improved outcomes at discharge (figure 3B).
Figure 3.
Clinical outcomes for patients with M2 occlusions and the subset that received IV tissue plasminogen activator (tPA). Successful recanalization with endovascular therapy was associated with improved modified Rankin scale outcomes in (A) all patients with M2 occlusions and (B) the subset who received IV tPA. TICI, Thrombolysis in Cerebral Infarction.
DISCUSSION
In this analysis of consecutive patients considered for endovascular intervention, patients with M2 compared with M1 occlusions tended to have fewer neurologic deficits at presentation and had smaller pretreatment lesion volumes of ischemic injury and perfusion deficit. Nonetheless, patients with M2 MCA occlusions were at substantial risk of adverse outcomes. Half of the patients with M2 MCA occlusions were discharged with moderately severe disability or death; a rate that is comparable to that of patients with M1 occlusions. In response to ET, patients with M2 occlusions demonstrated clear improvement in imaging and clinical outcomes with successful reperfusion, which was often not accomplished by IV tPA alone.
TICI 2/b3 recanalization rates were similar for M1 and M2 occlusions, though door to groin puncture times were slightly longer in the M2 group, probably reflecting more deliberative decision-making before bringing patients with distal lesions for intervention. As expected, treatment modalities differed for the two occlusion locations, with distal occlusions more likely to be treated with IA tPA. Patients with M2 occlusions presented with smaller infarct volumes, smaller hypoperfused volumes, and ultimately had less hemorrhage and smaller final infarct volumes regardless of reperfusion status. Rates of poor clinical outcome on discharge were comparable, suggesting that categorizing patients based solely on M1/M2 distinctions does not fully capture the severity of their stroke. The inefficiency of this determination is particularly poignant given the varied definitions of what constitutes an M1 vs M2 artery as well as the considerable anatomic variation from patient to patient.12
Among patients with M2 occlusions, successful reperfusion was associated with smaller final infarct volumes, less infarct expansion, and improved clinical outcomes. Furthermore, among the subgroup of patients with M2 occlusions for whom IV tPA failed, successful rescue reperfusion was associated with smaller strokes and improved clinical outcomes. Given our findings that revascularization of M2 occlusions in patients ineligible for IV tPA, or for whom this treatment has failed, leads to improved outcomes, perhaps these patients should be treated with the same vigor as those with M1 occlusions.
M2 occlusions have been examined in subgroup analyses of several large endovascular stroke trials. These more distal lesions have been shown to lead to better clinical outcomes than proximal ones, regardless of revascularization status.13–15 Our M2 cohort’s median NIHSS of 11 was slightly lower than those of several of these studies, which had median values ranging from 12 to 19.16–18 Our TICI 2b/3 recanalization rates are similar to those of previous series that used similar treatment modalities.3,16–18 The majority of M2 patients in our cohort were treated with IA tPA. This treatment was shown in the subset of M2 occlusions in the PROACT II study to improve revascularization rates, though improvement in clinical outcome was not seen.17 The MERCI device was also effective at revascularization of M2 vessels in the MERCI and Multi-MERCI trials, and required fewer passes than more proximal lesions.18 In spite of narrower diameters, the complication rates in these distal vessels were no different from those in the proximal ones.18
Currently active trials including the SWIFT PRIME and the THERAPY trials have moved towards excluding patients with more distal occlusions.5,6 The inclusion criteria for SWIFT PRIME mandates TICI 0–1 flow in the intracranial ICA, M1 segment of the MCA, or carotid terminus. The THERAPY trial requires a clot length of ≥8 mm, which would probably exclude most distal occlusions. One rationale for these decisions draws upon data that more proximal ICA and M1 occlusions are unlikely to dissolve with IV tPA alone.19 Further, the Interventional Management of Stroke III trial found that at 24 h, reperfusion rates of M2 occlusions were as high as 75% with IV tPA alone. In addition, post hoc analyses from this trial suggested that ET produced greater clinical benefit than IV tPA in occlusions of the most proximal intracranial occlusions, the ICA/MCA‘T/L’ lesions.3
Our findings suggest that M2 occlusions may also benefit from successful revascularization with ET, in patients who are ineligible for IV tPA and in those who received IV tPA but failed to recanalize. While recanalization rates at 24 h with medical treatment may be higher in this group than in those with carotid T lesions, it is the recanalization in the first few hours after stroke that is probably the most meaningful, and those who fail to do so rapidly are more likely to do poorly.20 Although some of the patients who underwent ET might have recanalized spontaneously or with IV tPA had we waited longer, the likelihood of a good outcome would be significantly less.
Thus, our cohort was limited to patients who underwent angiography for consideration of ET and therefore excluded patients who improved after tPA, probably secondary to successful recanalization. There remain, however, a significant number of patients with M2 occlusions who do not improve after IV tPA and benefit from additional treatment. Of note, while the M2 cohort in the Interventional Management of Stroke III trial did show high recanalization rates at 24 h with IV tPA alone, it also showed significant benefit from ET. Although a limited sample size, and one that did not account for the success of recanalization, patients with M2 occlusions alone were found to have an improved 90-day mRS by shift analysis in the endovascular compared with the IV tPA group.3
One of the limitations to this study is the use of now outdated devices in ET attempts. The TICI 2b/3 rates recorded for these patients are significantly lower than those attained with newer devices. Details of a cohort of patients treated with more modern stentrevier devices were recently published and patients were noted to have TICI 2b/3 recanalization rates of nearly 80%. Successful recanalization was associated with a doubling of the rate of favorable outcomes.21 In our cohort, although higher recanalization rates would have been desirable, the lower technical success rate did provide a larger comparator sample of patients with no reperfusion. As rates of TICI 2b/3 reperfusion continue to improve, understanding the outcome of patients considered for ET without successful recanalization may become more difficult. However, because these patients were not randomized, the interpretation of these data may be complicated by confounding variables such as collateral status and other factors that influence recanalization rates.22 In addition, as noted above, our cohort reflected routine clinical practice and thus does not include patients with M2 occlusions that were felt to be inaccessible or unsafe to access. Thus the interpretation of our findings cannot be generalized to all M2 occlusions, but rather is limited to those who can be treated with ET.
Further, we do not make a comparison between patients with M2 occlusions who received IV tPA alone versus ET. To evaluate the benefit of recanalization in M2 occlusions we compare patients who received ET but did not achieve TICI 2b/3 versus those that did. It is possible that patients who undergo ET without adequate reperfusion do worse than patients without any intervention, as the procedure itself may lead to morbidity. To evaluate this possibility, we compared hemorrhage rates of patients who achieved recanalization and those who did not, and found no difference. Because these data are not randomized, the patients with M2 occlusions who received IV tPA but did not undergo ET in our cohort would be defined by characteristics that would make them poor endovascular candidates. Thus, this comparison would have to be made in a prospective randomized setting.
In this study, we find that M2 occlusions can lead to significant morbidity and mortality, and treatment with IV tPA may not be sufficient for all patients with these occlusions. Revascularization with endovascular techniques led to decreased stroke volumes, diminished infarct expansion, and improved clinical outcomes. Compared with M1 lesions, M2 occlusions present with smaller infarct volumes and develop less hemorrhage after ET. In patients with M2 occlusions for whom IV tPA did not result in immediate clinical benefit, successful ET resulted in improved imaging and clinical outcomes.
CONCLUSION
M2 occlusions can lead to substantial morbidity and mortality. Successful endovascular revascularization, among patients for whom tPA fails or who are ineligible for such treatment, leads to reduced infarct growth and improved clinical outcomes. These findings suggest that patients with M2 occlusions are an important candidate population for endovascular intervention.
Acknowledgments
Funding Funding for this manuscript includes grant support from NIH/NINDS K24NS072272, R13NS082049.
Footnotes
Contributors SAS was responsible for substantial contributions to the conception and design of the work, drafting and critically revising it and its final approval. DSL was responsible for the conception of the work, revising the article, and for its final approval. BY, SS, LKA, DK, NRG, RJ, ST, GD, JLS, and FV were responsible for revising the article and for its final approval. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval Institutional review board of the University of California, Los Angeles.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement The data discussed in this article are taken from our institutional cohort and are available to collaborators with institutional review board and Health Insurance Portability and Accountability Act approval.
Competing interests DSL reports consulting fees from Stryker, Covidien, Zoll (modest). ST reports consulting fees from Penumbra, Covidien, Stryker, and Reverse Medical. RJ reports consulting fees from Covidien and Stryker. GD reports consulting agreements with Asahi Medical and Sequent Medical. He is also a proctor for the Pipeline device (Covidien). The University of California, Regents receive funding for the services of JLS as a scientific consultant regarding trial design and conduct to Covidien, CoAxia, Stryker, BrainsGate, Genervon, and Grifols. JLS has served as an unpaid site investigator in multicenter trials run by Lundbeck and Covidien, for which the UC Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled. The University of California has patent rights in retrieval devices for stroke.
References
- 1.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SC, Hauser SL. The dangers of clinical conviction: an “M&M” of endovascular therapies for stroke. Ann Neurol. 2013;73:A5–6. doi: 10.1002/ana.23942. [DOI] [PubMed] [Google Scholar]
- 5.Solitaire™ FR as Primary Treatment for Acute Ischemic Stroke (SWIFT PRIME) http://clinicaltrials.gov/ct2/show/NCT01657461.
- 6.Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY) http://clinicaltrials.gov/show/NCT01429350.
- 7.Olivot J-M, Mlynash M, Thijs VN, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–75. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 9.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Savitz SI, Lew R, Bluhmki E, et al. Shift analysis versus dichotomization of the modified Rankin scale outcome scores in the NINDS and ECASS-II trials. Stroke. 2007;38:3205–12. doi: 10.1161/STROKEAHA.107.489351. [DOI] [PubMed] [Google Scholar]
- 11.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43:1323–30. doi: 10.1161/STROKEAHA.111.639401. [DOI] [PubMed] [Google Scholar]
- 12.Grand W, Hopkins LN. Vasculature of the brain and cranial base: variations in clinical anatomy. 1. Thieme Medical Publishers, Inc; 1998. [Google Scholar]
- 13.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. Am J Neuroradiol. 2008;29:582–7. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35:904–11. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 15.IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127–35. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 16.Galimanis A, Jung S, Mono ML, et al. Endovascular therapy of 623 patients with anterior circulation stroke. Stroke. 2012;43:1052–7. doi: 10.1161/STROKEAHA.111.639112. [DOI] [PubMed] [Google Scholar]
- 17.Rahme R, Abruzzo TA, Martin RH, et al. Is intra-arterial thrombolysis beneficial for M2 occlusions? Subgroup analysis of the PROACT-II trial. Stroke. 2013;44:240–2. doi: 10.1161/STROKEAHA.112.671495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z-S, Loh Y, Walker G, et al. Clinical outcomes in middle cerebral artery trunk occlusions versus secondary division occlusions after mechanical thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI trials. Stroke. 2010;41:953–60. doi: 10.1161/STROKEAHA.109.571943. [DOI] [PubMed] [Google Scholar]
- 19.Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–7. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 20.Mazighi M, Chaudhry SA, Ribo M, et al. Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013;127:1980–5. doi: 10.1161/CIRCULATIONAHA.112.000311. [DOI] [PubMed] [Google Scholar]
- 21.Flores A, Tomasello A, Cardona P, et al. Endovascular treatment for M2 occlusions in the era of stentrievers: a descriptive multicenter experience. J NeuroInterv Surg. 2014 doi: 10.1136/neurintsurg-2014-011100. Published Online First: 27 February 2014. http://dx.doi.org/10.1136/neurintsurg-2014-011100. [DOI] [PubMed]
- 22.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–9. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]