Abstract
Background
The ability of interventions to affect declining β-cell function in screen-detected type 2 diabetes are poorly described. The Early Diabetes Intervention Program (EDIP; ClinicalTrials.gov NCT01470937) was a randomized study based on the hypothesis that improving postprandial glucose excursions with acarbose would slow the progression of fasting hyperglycemia in screen-detected type 2 diabetes. In EDIP, the effect of acarbose plus lifestyle advice on progression of fasting hyperglycemia over a 5 year period was not greater than that of placebo. However, there was an early glucose lowering effect of the trial. The objective of the current secondary analysis was to describe β-cell function changes in response to glucose-lowering.
Methods
Participants were overweight adult subjects with screen-detected type 2 diabetes. β-cell function was measured using hyperglycemic clamps and oral glucose tolerance testing. The primary outcome was the change in β-cell function from baseline to Year 1, the time point where the maximal glucose-lowering effect was seen.
Results
At baseline, participants exhibited markedly impaired first-phase insulin response. Despite significant reductions in weight, FPG, and 2-hr PG, there was no clinically significant improvement in first-phase insulin response. Late-phase insulin responses declined despite beneficial glycemic effects of interventions.
Conclusions
Insulin secretion is already severely impaired in early, screen-detected type 2 diabetes. Effective glucose-lowering intervention with acarbose was not sufficient to improve insulin secretion or halt the decline of β-cell function.
Key Terms: insulin, obesity, hyperglycemic clamp, oral glucose tolerance test, acarbose
INTRODUCTION
The pathophysiology of type 2 diabetes is characterized by decreased insulin action and progressive deterioration in insulin secretion relative to insulin sensitivity 1, 2. The Early Diabetes Intervention Program (EDIP; registered on ClinicalTrials.gov, NCT01470937) was a randomized study based on the hypothesis that improving postprandial glucose excursions with acarbose would slow the progression of screen-detected type 2 diabetes, defined as the development of frank fasting hyperglycemia, over up to 5 years of follow-up.
The study inclusion criteria included BMI ≥25 kg/m2, no history of type 2 diabetes, and no use of glucose-lowering agents. Participants were diagnosed with type 2 diabetes during study screening, defined as having a 2-hr oral glucose tolerance test (OGTT) plasma glucose (2-hr PG) value over 200 mg/dL and fasting plasma glucose (FPG) values between 100 and 139 mg/dL. The mean hemoglobin A1c (HbA1c) of this screen-detected study population at baseline was (6.34 ± 0.64%), below the current HbA1c threshold for the diagnosis of diabetes. The primary study outcome of the EDIP trial was previously published 3: The effect of acarbose plus lifestyle advice on progression of fasting hyperglycemia over a 5 year follow-up period was not greater than that of placebo plus lifestyle advice.
Here we describe in vivo β-cell function measured by OGTT and hyperglycemic clamp during the EDIP trial, and the effects of the interventions on β-cell function. In the EDIP trial, it was presumed that β-cell dysfunction was associated with the screen-detected type 2 diabetes phenotype of primarily post-prandial hyperglycemia, and the hypothesis that improved β-cell function would be associated with reduction in post-prandial hyperglycemia was pre-specified for secondary analyses. In a similar study in a Dutch population with prediabetes, there was no benefit of acarbose-related glucose lowering on insulin secretion or insulin sensitivity during a three-year treatment period 4. Here we have evaluated whether there was any improvement in β-cell functioning among EDIP participants and whether this was related to the glucose-lowering effect of study interventions. We also evaluated whether baseline anthropomorphic and metabolic parameters determined study-related changes in β-cell function in the EDIP trial.
METHODS
The study was approved by the institutional review boards of Indiana University School of Medicine and Washington University School of Medicine and all subjects provided written informed consent for the research. Inclusion and exclusion criteria, and general methods for the EDIP trial have been previously published 3. Participants were recruited from the surrounding communities using a process that included OGTT screening of asymptomatic individuals without known diabetes. Briefly, a registered dietitian counseled subjects on an appropriate diet for type 2 diabetes and subjects began either acarbose or an identical placebo based on a blinded randomization. Study drug was initiated at a dose of 25 mg once daily with the evening meal, then titrated at weekly intervals by 25 mg daily to the maximum dose of 100 mg t.i.d. with meals. Study drug was down-titrated as needed in subjects who complained of gastrointestinal side effects. Efforts were made to reach a daily dosage of at least 50 mg t.i.d..
The insulin secretion responses to enteral (OGTT) and parenteral (hyperglycemic clamp) glucose stimuli were assessed. OGTT measurements of β-cell function were performed at baseline, and at the end of years 1 and 2 in participants who had not yet met the primary outcome (FPG ≥140 mg/dL). Hyperglycemic clamp procedures were performed in a randomly assigned subset (50%) of the participants at baseline, and at the end of year 1 and year 2. We analyzed OGTT and hyperglycemic clamp data from three time points: baseline, year 1, and year 2. Maximal acarbose effectiveness for glucose-lowering was observed in the first year of the study (120 min OGTT glucose at baseline 236.5 ± 3.0 mg/dL, at Year 1 201.3±5.0, p<0.0001). Therefore, this was the optimal time point to evaluate whether glucose-lowering was associated with improved β-cell function. The primary endpoint of interest in the current analyses was change in β-cell function from baseline to year 1.
At study initiation participants were admitted to the General Clinical Research Center for a 2-day study visit. OGTT and hyperglycemic clamp studies were done in the fasting state on separate days. OGTTs were performed in all participants using a standard 75-g glucose load with blood samples collected for measurement of plasma glucose and insulin at −10, 0, 30, 60, and 120 minutes. Glucose concentrations were determined using a glucose oxidase method (YSI, Yellow Springs, OH). Insulin was measured by radioimmunoassay (Linco Research). HbA1C was measured by immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN).
For hyperglycemic clamp studies, fasting samples for plasma glucose and insulin were obtained at −10 and 0 min. A priming glucose bolus and then a maintenance glucose infusion, calculated by modification of the DeFronzo and Andres method 5, was infused to rapidly bring the plasma glucose to 200 mg/dL, then adjusted according to bedside glucose measurements every 5 minutes to maintain plasma glucose at this level for 4 hours. Samples for insulin were obtained every 2 minutes for the first 10 minutes of glucose infusion, at 15 and 30 minutes, and then at 30 minute intervals for the remainder of the 4-hour study.
Calculations
For both procedures, fasting insulin values were the average of the two baseline values. The OGTT-derived measures of β-cell function were the insulinogenic index (IGI) [(30 minute − fasting insulin [μU/mL]) ÷ (30 minute − fasting glucose [mg/dL])] reflecting OGTT early-phase insulin response; and the insulin area under the curve (AUC; calculated using the trapezoidal rule) reflecting OGTT late-phase insulin response. The hyperglycemic clamp-derived insulin secretion measures included clamp first-phase insulin (mean of values between +2 and +10 minutes), and clamp second-phase insulin (mean insulin concentrations during the final hour of the clamp).
These measures of β-cell function exhibited the expected hyperbolic inverse relationships with measures of insulin sensitivity (not shown). Insulin sensitivity parameters used were inverse fasting insulin for OGTT-derived β-cell function parameters, and clamp-derived ISI for clamp-derived β-cell function parameters (calculation below). A disposition index was calculated to adjust insulin secretion for insulin sensitivity measures from the OGTT (oral disposition index, oDI) and hyperglycemic clamp (clamp disposition index, cDI) 6. The oDI was calculated as (IGI x inverse fasting insulin) * 100. An insulin sensitivity index (ISI) was derived from the steady state of the hyperglycemic clamp as (mean glucose-space adjusted glucose disposition rate ÷ mean insulin concentration during the last h of the clamp) ×1007. The cDI was calculated as first-phase insulin × ISI. Results for late or second-phase insulin secretion were presented as unadjusted values (Table 1, baseline correlations) or with statistical adjustment for the appropriate insulin sensitivity parameter (inverse fasting insulin for OGTT data and inverse ISI for clamp data; Tables 2–4) due to collinearity with the measures of insulin sensitivity that precluded similar derivations of disposition indices.
Table 1.
Baseline Characteristics of the Study Population and OGTT and Clamp Measures of β-Cell Function According to Treatment Group
| Acarbose n=109 |
Placebo n=110 |
p value | |
|---|---|---|---|
| Gender | |||
| Male | 36 (33.0) | 38 (34.6) | 0.74 |
| Female | 73 (67.0) | 72 (65.4) | |
| Race | |||
| White | 84 (77.1) | 87 (79.1) | 1.00 |
| Other Race | 25 (22.9) | 23 (20.9) | |
| Age (years) | 53.7 ± 11.0 | 53.7 ± 11.7 | 0.98 |
| BMI (kg/m2) | 35.1 ± 7.2 | 35.2 ± 7.1 | 0.83 |
| HbA1c | 6.4 ± 0.6 | 6.3 ± 0.6 | 0.84 |
| OGTT measures of insulin secretion | n=104 | n=100 | |
| IGI | 0.609 ± 0.726 | 0.508 ±0.534 | 0.26 |
| Insulin AUC (μU/mL*min) | 8619 ± 4712 | 9699 ± 7188 | 0.21 |
| oDI | 2.90 ± 2.50 | 3.08 ± 2.81 | 0.63 |
| Clamp measures of insulin secretion | n=47 | n=48 | |
| 1st-phase insulin (μU/mL) | 26.5 ± 17.6 | 30.8 ± 28.2 | 0.39 |
| 2nd-phase insulin (μU/mL) | 61.5 ± 39.2 | 83.3 ± 97.5 | 0.16 |
| cDI | 143 ± 55 | 157 ± 139 | 0.55 |
Data are n (%) or means ± SD.
Table 2.
Univariate Determinants of Change in β-Cell Function at Year 1
| Early-Phase OGTT Insulin Secretion Δ oDI | Late-Phase OGTT Insulin Secretion Δ AUCins (adjusted) | First-Phase Clamp Insulin Secretion Δ cDI | Second-Phase Clamp Insulin Secretion Δ Phase-2 Insulin (adjusted) | |||||
|---|---|---|---|---|---|---|---|---|
| Std beta | p value | Std beta | p value | Std beta | p value | Std beta | p value | |
| OGTT IGI baseline | −0.656 | <0.001 | ||||||
| OGTT AUCins baseline | −0.643 | <0.001 | ||||||
| Clamp Phase-1 Insulin baseline | −0.206 | 0.136 | ||||||
| Clamp Phase-2 Insulin baseline | −0.670 | <0.001 | ||||||
| Treatment Group | 0.078 | 0.385 | 0.115 | 0.206 | −0.127 | 0.361 | 0.135 | 0.313 |
| Sex | 0.039 | 0.666 | −0.111 | 0.124 | −0.099 | 0.475 | 0.132 | 0.322 |
| Age (yr) | 0.103 | 0.253 | 0.076 | 0.404 | 0.095 | 0.493 | 0.152 | 0.255 |
| Weight (kg) | −0.178 | 0.049 | −0.111 | 0.223 | 0.291 | 0.033 | −0.083 | 0.535 |
| ΔWeight | −0.083 | 0.360 | 0.139 | 0.126 | 0.135 | 0.333 | −0.040 | 0.765 |
| HbA1c | −0.206 | 0.024 | 0.115 | 0.211 | 0.105 | 0.451 | 0.239 | 0.071 |
| ΔHbA1c | −0.046 | 0.621 | −0.053 | 0.574 | 0.089 | 0,532 | −0.256 | 0.054 |
| FPG (mmol/L) | −0.142 | 0.114 | 0.034 | 0.708 | 0.150 | 0.278 | −0.166 | 0.202 |
| ΔFPG (mmol/L) | 0.058 | 0.519 | 0.016 | 0.864 | −0.182 | 0.191 | −0.157 | 0.244 |
| 2-hr PG (mmol/L) | −0.015 | 0.865 | 0.105 | 0.248 | −0.121 | 0.382 | −0.032 | 0.812 |
| Δ2-hr PG (mmol/L) | 0.033 | 0.718 | 0.062 | 0.499 | −0.125 | 0.371 | −0.133 | 0.325 |
| Fasting Insulin (pmol−1) | −0.034 | 0.708 | 0.302 | <0.001 | ||||
| ΔFasting Insulin (pmol−1) | 0.087 | 0.335 | −0.173 | 0.056 | ||||
| ISI | −0.015 | 0.917 | 0.247 | 0.061 | ||||
| ΔISI | −0.221 | 0.116 | −0.330 | 0.011 | ||||
Statistical analysis
Parallel analyses were performed for early and late phase insulin secretion parameters derived from the OGTT, and for first- and second-phase insulin secretion parameters derived from the hyperglycemic clamp. Over the course of the study, study participants in both groups had changes in weight, FPG, and 2-hr PG concentrations. Also, we examined weight, FPG, 2-hr PG, sex, and age as potential determinants of baseline β-cell function and of treatment-induced changes in β-cell function.
Many of the parameters evaluated had right-skewed distributions. Baseline correlation analyses were therefore performed using nonparametric testing (Spearman’s rho). Regression modeling was performed on untransformed variables. The primary outcome of interest was the change in each parameter of β-cell function, calculated as the year 1 value minus baseline. Univariate models were constructed against each of these change variables, followed by multivariable models. First the full set of parameters of interest was entered into the model, and then a forward stepwise procedure was applied to identify the parameters with the strongest effects on change in β-cell function. Effect sizes for these models were expressed as standardized beta coefficients (effect size per SD of the independent variable) to facilitate comparisons of magnitude of effect. SPSS software was used to perform all statistical analysis (Version 20, IBM). Two-sided p values <0.05 were considered statistically significant.
RESULTS
Baseline demographic and metabolic characteristics of the study participants are shown in Table 1. There were no significant differences in OGTT- or clamp-derived measures of insulin secretion between the treatment groups at baseline (Table 1).
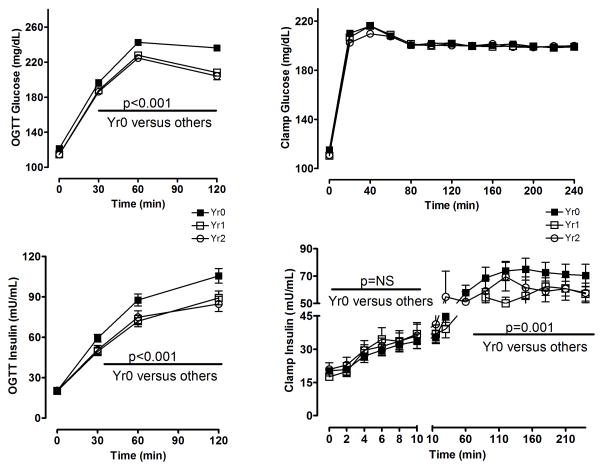
Glucose and insulin excursions in response to OGTT and clamp testing are shown in Figure 1. OGTT and clamp measurements of β-cell function were performed at the end of years 1 and 2, only in patients who had not yet met the primary outcome (FPG ≥ 140 mg/dL). Therefore, subjects included in this analysis either had glucose lowering or failed to rise above this threshold. In both acarbose and placebo treatment groups OGTT FPG, 2-hr PG, and stimulated insulin values were significantly reduced from baseline at years 1 and 2. The reduction in FPG was significantly greater in acarbose versus placebo (−8.6 mg/dL versus −3.4 mg/dL, p=0.041, Year 1). The reduction in 2-hr PG was not significantly different between treatment groups (−26.5 mg/dL acarbose, −20.2 mg/dL placebo, p=0.12, Year 1). Pooled data from both treatment groups are provided in Figure 1, presenting OGTT and clamp data from baseline, Year 1 and Year 2. Clamp insulin excursion, measured under circumstances where the glycemic load is held constant, did not change significantly in either treatment group after 1 year of study intervention (Figure 1, bottom right panel). As is evident in that panel, first-phase insulin production measured during the clamp was markedly impaired at baseline (with essentially no peak and a notably delayed maximal response) and did not improve after 1 year of study intervention.
Figure 1.
Glucose and insulin excursions in response to OGTT and hyperglycemic clamp testing at baseline (solid squares), after 1 year of treatment (open squares), and after 2 years of treatment (open circles). Change in OGTT FPG, 2-hr PG, and 2-hr insulin values from baseline to year 1, p<0.001.
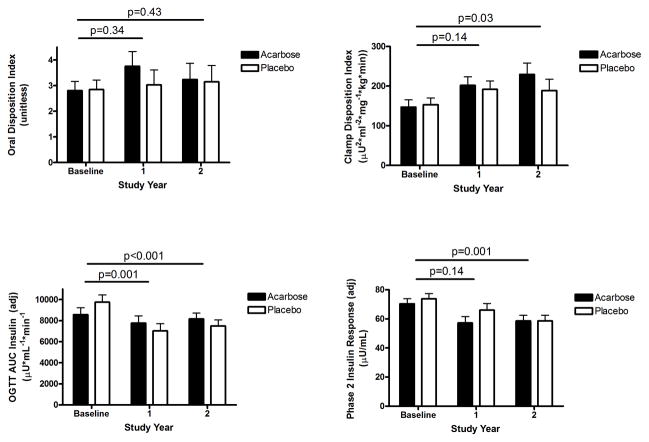
OGTT early-phase insulin secretion and clamp-derived first-phase insulin secretion adjusted for insulin sensitivity (oDI and cDI) at baseline and follow-up are shown in Figure 2. There were no treatment group differences in oDI or cDI measures between the treatment groups at baseline (not shown). There was a modest increase in oDI with acarbose at Year 1, not significantly different from baseline (Figure 2 upper left panel). There was no difference in oDI change over time between treatment groups. The cDI was significantly increased with time on treatment (Figure 2 upper right panel), but the change over time was not significantly different between the acarbose and placebo groups. Further, the absolute values for these first-phase responses remained very low..
Figure 2.
Measures of first-phase insulin secretion adjusted for insulin sensitivity (oDI and cDI) and second-phase insulin secretion (AUCins and phase 2 insulin) by treatment group during treatment. P values indicate comparisons across 1 or 2 years of intervention, as indicated by the horizontal bars; in all instances there was no significant treatment-specific effect.
OGTT late-phase insulin secretion and clamp-derived second-phase insulin secretion at baseline and follow-up are also shown in Figure 2 (lower panels). Both the OGTT late-phase insulin (AUC) and clamp second-phase insulin responses decreased over time despite decreasing FPG and 2-hr PG values. There were no significant differences in change over time between the acarbose group and the placebo group (not shown).
Study interventions had significant effects on weight. Baseline BMI did not differ between treatment group (Table 1). In placebo-treated subjects, baseline weight was 98 ± 21 kg, and at Year 1 95± 21 (p=0.001 versus baseline) and Year 2 94± 16 (p=0.01 versus baseline). In acarbose-treated subjects, baseline weight was 97± 20 kg, and at Year 1 93± 20 (p<0.001 versus baseline) and Year 2 93± 20 (p<0.001 versus baseline). There were no significant differences in weight between treatment groups at any of these time points.
Spearman correlations of phenotypic characteristics of the subjects with OGTT and clamp-derived measures of β-cell function at baseline are available in the Appendix (Appendix Table 1). Higher baseline OGTT early-phase insulin (oDI) and late-phase insulin (AUC) values (i.e. better β-cell function) were associated with female sex (oDI r=0.197, p=0.005; AUC r=0.150, p=0.033), lower baseline FPG (oDI r=−0.239, p=0.001), lower 2-hr PG (oDI r=−0.220, p=0.002; AUC r=−0.206, p=0.003), and lower baseline HbA1c (oDI r=−0.168, p=0.018). Higher baseline OGTT late-phase insulin (AUC) was also associated with higher baseline weight (r=0.194, p=0.006). Similarly, higher baseline first-phase clamp insulin secretion (cDI) values were associated with lower baseline FPG (r=−0.321, p=0.002), lower 2-hr PG (r=−0.267, p=0.012), and lower baseline HbA1c (r=−0.397, p<0.001). Higher baseline clamp second-phase insulin was associated with higher baseline weight (r=0.320, p=0.001), lower age (r=−0.220, p=0.032), lower FPG (r=−0.228, p=0.025) and lower 2-hr PG (r=−0.303, p=0.003).
There were 121 participants with FPG < 126 mg/dL at baseline. Of these, 67 were assigned to placebo (mean ± SD FPG 113 ± 10 mg/dL) and 54 were assigned to acarbose (FPG 110 ± 10 mg/dL, p = 0.023). There was no difference between those who were treated with acarbose or placebo in FPG lowering at the end of Year 1 (−4.0 ± 13 versus −0.5 ± 15 mg/dL, p=0.228). There was also no difference in mean change at Year 1 in the OGTT IGI (0.03 ± 0.73 versus −0.22 ± 0.73, p=0.166), OGTT disposition index (0.67 ± 3.28 versus 0.62 ± 6.18, p=0.972), OGTT insulin AUC (−1059 ± 4467 versus −2461 ± 5286, p=0.245), clamp phase 1 insulin (−9.1 ± 16.5 versus 4.4 ± 32.6, p=0.213), clamp disposition index (15.3 ± 66.3 versus 20.0 ± 209.8, p=0.942), or clamp phase 2 insulin (−18.8 ± 30.5 versus −18.9 ± 70.0, p=0.996).
Univariate determinants of the change in measures of OGTT- and clamp-derived insulin secretion at Year 1 are shown in Table 2. These responses exhibited a mean increase in values. Change in OGTT early-phase insulin secretion (oDI) was inversely related to baseline OGTT IGI, HbA1c, and weight. Change in first-phase clamp insulin secretion (cDI) was directly related to higher baseline weight; this is opposite to the observed relationship between oDI and lower weight. Late phase/second-phase responses exhibited a mean decrease in values. Change in OGTT late-phase insulin (AUC) was inversely related to baseline insulin AUC, and directly related to baseline fasting insulin. Change in clamp second-phase insulin was inversely related to baseline second-phase insulin and change in insulin sensitivity. Pertinent to the main hypothesis being tested, the univariate relationships between change in glucose and change in β-cell function measures was directionally positive (direct association) for early phase measures, and directionally negative (inverse association) for late phase measures, but did not achieve statistical significance for any of these relationships.
Parameters that exhibited the strongest effects in determining change in insulin secretion when evaluated concurrently using multivariable analysis, unadjusted for insulin sensitivity are shown in Table 3 (Year 1 versus baseline). In these analyses neither the baseline fasting glucose values nor the change in fasting or 2-hr post-challenge glucose proved significantly associated with change in β-cell function. Baseline β-cell function measures were highly significant determinants of change for all measures tested, such that lower baseline measures were associated with greater decline in insulin secretion. Higher baseline weight was the only determinant of change in clamp first-phase insulin, and change in weight was inversely related to change in OGTT late-phase insulin (AUC). Insulin sensitivity (ISI) and change in ISI were directly associated with change in clamp second-phase insulin response.
Table 3.
Multivariate Determinants of Change in β Cell Function at the End of Year 1
| Early-Phase OGTT Insulin Secretion Δ IGI | Late-Phase OGTT Insulin Secretion Δ AUCins | First-Phase Clamp & Insulin Secretion Delta; Phase-1 Insulin | Second-Phase Clamp Insulin Secretion Δ Phase-2 Insulin | |||||
|---|---|---|---|---|---|---|---|---|
| Std β | p value | Std β | p value | Std β | p value | Std β | p value | |
| Model r2 (p value) | 0.494 | <0.001 | 0.417 | <0.001 | 0.143 | 0.117 | 0.596 | <0.001 |
| OGTT IGI baseline | −0.743 | <0.001* | ||||||
| OGTT AUCins baseline | −0.725 | <0.001* | ||||||
| Clamp Phase-1 Insulin baseline | −0.373 | 0.025* | ||||||
| Clamp Phase-2 Insulin baseline | −0.831 | <0.001* | ||||||
| Treatment Group | 0.032 | 0.634 | 0.088 | 0.227 | −0.073 | 0.647 | 0.083 | 0.407 |
| Sex | 0.083 | 0.286 | 0.078 | 0.345 | 0.141 | 0.382 | 0.162 | 0.121 |
| Age (yr) | 0.111 | 0.121 | 0.048 | 0.538 | 0.174 | 0.309 | 0.129 | 0.228 |
| Weight (kg) | 0.035 | 0.667 | 0.037 | 0.673 | 0.411 | 0.036* | 0.224 | 0.073 |
| ΔWeight | −0.002 | 0.998 | 0.211 | 0.021 | 0.244 | 0.120 | 0.149 | 0.142 |
| FPG (mmol/L) | −0.044 | 0.579 | 0.055 | 0.529 | −0.063 | 0.738 | −0.075 | 0.519 |
| ΔFPG (mmol/L) | 0.040 | 0.636 | −0.002 | 0.984 | −0.203 | 0.255 | −0.156 | 0.190 |
| 2-hr PG (mmol/L) | −0.058 | 0.493 | 0.045 | 0.629 | −0.155 | 0.385 | −0.153 | 0.191 |
| Δ2-hr PG (mmol/L) | −0.009 | 0.925 | −0.082 | 0.487 | −0.116 | 0.513 | −0.116 | 0.344 |
| Fasting Insulin (pmol−1) | −0.215 | 0.005* | −0.135 | 0.150 | ||||
| ΔFasting Insulin (pmol−1) | 0.049 | 0.487 | −0.202 | 0.010* | ||||
| ISI | −0.147 | 0.460 | −0.282 | 0.034* | ||||
| ΔISI | −0.229 | 0.189 | −0.361 | 0.002* | ||||
Results from a simultaneous analysis are presented. To avoid collinearity with glucose measures, HbA1c parameters were not included in these models.
Parameters that remain significant in stepwise multivariable analyses, all p<0.01.
DISCUSSION
We have characterized in vivo β-cell function measured by OGTT and hyperglycemic clamp in a population with early, screen-detected type 2 diabetes, and evaluated the effects of a glucose-lowering intervention on β-cell function. In aggregate, our findings indicate that β-cell function is profoundly impaired in screen-detected type 2 diabetes, with essentially absent first-phase insulin response to acute hyperglycemia during the hyperglycemic clamp. Contrary to our hypothesis, there was no effect of acarbose to specifically improve β-cell function compared to placebo; This was not due to the study-related changes seen in the placebo group but rather to a true lack of relationship between changes in glucose and measures of β-cell function; even in univariate analyses there was no relationship of change in glucose with change in β-cell function. In fact, the study-related effects on glucose in the placebo group magnify our ability to comment on the hypothesized beneficial effects of reducing glucose. Significant glucose reductions were associated with 1) no improvement in the poor clamp first-phase insulin response, 2) no improvement in OGTT early-phase insulin response adjusted for insulin sensitivity (oDI), and 3) statistically significant but physiologically unimportant increase in clamp first-phase insulin adjusted for insulin sensitivity (cDI). Further, the OGTT late-phase and clamp second-phase insulin responses declined over time despite glucose-lowering effects of the interventions. In univariate analyses, decreases in weight and insulin resistance influenced β-cell function, resulting in decreases in OGTT late-phase insulin secretion as the demand for insulin lessened with decreasing FPG and 2-hr PG. In multivariable analyses adjusting for changes in weight and insulin resistance the relationship between changes in glucose and changes in insulin secretion was no longer statistically significant. This implies that weight and insulin sensitivity are important determinants of changes in β-cell function. Overall these observations argue that, contrary to our hypothesis, treatment-related changes in glucose did not promote improvements in β-cell function in EDIP; however, we did observed modest changes that were due to non-glucose effects of randomized therapies.
Others have previously shown that the first-phase β-cell response is blunted in type 2 diabetes and prediabetes 8–12, and we have further extended these observations. The Whitehall II prospective occupational cohort study has shown significant differences in measures of glycemia and markers of insulin sensitivity and secretion (fasting HOMA values) among normoglycemic individuals who went on to develop diabetes as compared with those who did not develop diabetes 13. Differences in glucose and insulin values, as well as trajectories of change in these values, are noted several years prior to the diagnosis of diabetes. These published findings further support our data.
Although the EDIP study was initiated in an era when dysglycemic states were defined differently than currently defined, it is clear that the study population, identified with FPG between 100 and 139 mg/dL and 2-hr PG > 200 mg/dL, already had significant β-cell impairment. Moreover, it has been observed that individuals with both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) rather than one or the other are at least twice as likely to progress to diabetes, with annualized incidence rates of progression of ~4–6% for IGT, ~6–9% for IFG, and ~15–19% for IGT + IFG 14. We now know, from the US Diabetes Prevention Program trial among others, that prevention of progression to diabetes is dependent on baseline glucose values and on the achievement of normal glucose tolerance 15. Hence, the patient population selected for study in EDIP had more profound β-cell dysfunction than was widely appreciated at the time, and therefore was evidently less likely to demonstrate a therapeutic effect either by acarbose or lifestyle modification. This study leaves unanswered the question whether glucose lowering alone is sufficient to reverse β-cell dysfunction in prediabetic populations with isolated IFG or isolated IGT, which inherently differ in their degree of β-cell dysfunction and perhaps in the pathophysiologic changes that underlie progressive metabolic dysfunction 16, 17.
The results of EDIP differ from the STOP-NIDDM study, a multi-centered trial of acarbose versus placebo conducted outside the U.S, which found acarbose to be superior to placebo for delaying the progression of dysglycemia after 3 months of therapy 18. The study population in STOP-NIDDM was prediabetic at baseline as evidenced by lower mean FPG (~112 mg/dL) and 2-hr PG (~167 mg/dL). They also had lower BMI (~31 kg/m2). STOP-NIDDM did not similarly measure β-cell function. However, these results and those from successful diabetes prevention programs using other treatment modalities suggest that populations with prediabetes do have a capacity to improve β-cell function, in contrast to the population with screen-detected diabetes studied in EDIP.
The 3-year Dutch Acarbose Intervention Study in Persons with Impaired Glucose Tolerance (DAISI) also investigated the effect of acarbose in persons with impaired glucose tolerance on glycemia and hyperglycemic clamp measures of β-cell function 4. The study population in the DAISI had a mean FPG of ~118 mg/dL, 2-hr PG of ~172 mg/dL, an even lower mean BMI than the STOP-NIDDM trial (~29 kg/m2). After 3 years, acarbose was associated with lower mean 2-h PG (−21 mg/dL, 95% CI: −37; −3), no difference in FPG, and an absolute risk reduction for diabetes of 6%. However, there was no measured treatment effect on insulin clamp-derived measures of insulin secretion or insulin sensitivity. This study used a lower dose of acarbose (50 mg three times daily). It is conceivable that a higher dose of acarbose in a population such as this could have further delayed the progression of dysglycemia with the potential for an improvement in β-cell function.
Although this particular glucose-lowering therapy (acarbose) was not sufficient to promote improvement in β-cell function in our population with screen-detected diabetes there is evidence that early pharmacological intervention with agents that target incretin effects on β-cell functioning can potentially prevent or delay progression of diabetes. Acute improvements in functional β-cell capacity during treatment with dipeptidyl peptidase inhibitors, and glucagon-like peptide 1 (GLP-1) agonists have been demonstrated 19–21. Intensive insulin therapy in patients with newly diagnosed type 2 diabetes has been shown to be superior to oral hypoglycemic agents for achieving normoglycemia 22, and has been shown to have a durable effect to improve endogenous insulin secretion after therapy is stopped 22, 23. This suggests that it is imperative to identify if interventions earlier in the course of dysglycemia or prediabetes can further prevent or delay these progressive changes. It should be noted that in EDIP, despite having no benefit on measures of β-cell function, there was some improvement in glycemic measures with acarbose, and little progression in the placebo group likely related to dietary counseling 3. This highlights the benefit of participation in a clinical trial and dietary counseling, which is often overlooked, in patients with prediabetes and type 2 diabetes.
Over the course of the study, there was a modest increase in clamp first-phase insulin secretion adjusted for insulin sensitivity (cDI), and a decrease in OGTT late-phase insulin secretion. Increase in OGTT early-phase insulin (oDI) was associated with lower weight and lower HbA1c at baseline. Conversely, increase in the clamp-derived cDI was related to higher baseline weight, which may imply that weight loss during the study was important for increasing the acute insulin response to IV glucose. Changes in OGTT late-phase insulin (AUC) were primarily determined by baseline insulin values (lower baseline OGTT insulin AUC, higher baseline fasting insulin) and insulin sensitivity. Together these results are consistent with previous studies indicating that weight reduction, associated with improving insulin sensitivity, favorably affects β-cell function 24, 25. It is notable that these effects were evident while glucose lowering per se was not related to changes in β-cell function in this population with screen-detected type 2 diabetes.
Our study had some limitations. Individuals who failed therapy during the study, defined as having a FPG ≥140 mg/dL, met the study end-point and were no longer followed on a yearly basis; so follow-up data presented here represents those whose diabetes did not significantly progress within the interval study period. The results of this study may only apply directly to adults with screen-detected type 2 diabetes detected via OGTT. The data were obtained from a clinical trial intervention using lifestyle recommendations and a single pharmaceutical agent (acarbose) that resulted in moderate reductions in glycemia. It should be noted that others have demonstrated glucose-lowering with acarbose to have a similar effect on HbA1c as metformin in patients with newly diagnosed type 2 diabetes 26. While these results may not be generalizable to populations being treated with more aggressive glucose reduction, they can likely be generalized to interventions that reduce glycemia overall without concurrent systemic effects of the agent to improve insulin sensitivity. The study interventions included lifestyle recommendations for both treatment groups, and produced significant improvements in metabolic parameters including FPG and 2-hr PG in the placebo group, minimizing the treatment benefits that could be attributed to acarbose. Although this biased against finding an acarbose-specific effect, this phenomenon functionally increased our power to evaluate the contributions of changes in glucose to changes in β-cell function, strengthening our conclusion that glucose lowering effects alone are not sufficient to produce recovery of β-cell function in adults with very early, screen-detected diabetes.
Despite these limitations, our study has important strengths. These include the combined use of OGTT and hyperglycemic clamp tests to evaluate β-cell function over several years of follow-up in a population with earlier diabetes than is typically found in clinical practice or clinical trials. The hyperglycemic clamp has been considered the established gold-standard procedure for measuring β-cell function, allowing for precise and reliable measurements of first- and second-phase glucose-stimulated insulin secretion. The OGTT delivers enteral glucose, stimulating incretin effects on β-cell function, while the hyperglycemic clamp singly addresses parenteral glucose-stimulated insulin secretion. Incretin effects on β-cell function are important factors in the regulation of β-cell function and impairments in the incretin system may contribute to progression of the diabetic state. Few studies have utilized these combined measures prospectively during treatment protocols, and further study to assess the utility of these measures (singly or in combination) in trials of β-cell preservation is warranted.
We conclude that individuals with early screen-detected diabetes already have marked β-cell dysfunction and that effective glucose-lowering was not sufficient to significantly improve β-cell function in this population. Consideration must be given to intervene earlier in the process of transition to diabetes, i.e. in the prediabetic stage, to restore or preserve β-cell function. Implementation of this recommendation will require randomized controlled studies of treatments that include adequate analysis of pertinent biologic measures in the prediabetes stage of the disease.
Acknowledgments
This work was supported by an investigator-initiated grant from Bayer with additional support from the National Institutes of Health (grants P60 DK20542, P60 DK20579, GCRC M01RR00750, and M01RR00036). Data analysis and manuscript preparation and presentation were performed completely independently from the study sponsor. The study was registered on a public accessible database (www.clinicaltrials.gov; NCT01470937).
We gratefully acknowledge the contributions of our study participants, and those of the study staff and CRC staff at the participating institutions.
Appendix Table 1.
Spearman correlations of phenotypic characteristics with OGTT and hyperglycemic clamp measures of β cell function
| Early-Phase OGTT Insulin Secretion oDI | Late-Phase OGTT Insulin Secretion OGTT AUCins | First-Phase Clamp Insulin Secretion cDI | Second-Phase Clamp Insulin Secretion Clamp Phase-2 Insulin | |||||
|---|---|---|---|---|---|---|---|---|
| (n=204) | p value | (n=202) | p value | (n=87) | p value | (n=94) | p value | |
| Sex (F=1, M=0) | 0.197 | 0.005 | 0.150 | 0.033 | 0.166 | 0.124 | 0.122 | 0.236 |
| Age | 0.052 | 0.461 | 0.032 | 0.650 | −0.026 | 0.810 | −0.220 | 0.032 |
| Weight | −0.070 | 0.320 | 0.194 | 0.006 | −0.279 | 0.009 | 0.320 | 0.001 |
| Fasting Glucose | −0.239 | 0.001 | −0.050 | 0.483 | −0.321 | 0.002 | −0.228 | 0.025 |
| OGTT 2-hr Glucose | −0.220 | 0.002 | −0.206 | 0.003 | −0.267 | 0.012 | −0.303 | 0.003 |
| HbA1c | −0.168 | 0.018 | −0.083 | 0.249 | −0.397 | <0.001 | −0.176 | 0.088 |
| Fasting Insulin−1 | 0.106 | 0.132 | −0.720 | <0.001 | 0.157 | 0.155 | −0.599 | <0.001 |
| Triglycerides | −0.059 | 0.406 | 0.073 | 0.307 | 0.024 | 0.828 | 0.021 | 0.839 |
| Total cholesterol | 0.023 | 0.739 | 0.029 | 0.687 | 0.064 | 0.560 | −0.131 | 0.101 |
| HDL cholesterol | 0.111 | 0.115 | −0.176 | 0.012 | 0.125 | 0.253 | −0.265 | 0.010 |
| LDL cholesterol | 0.045 | 0.524 | 0.031 | 0.665 | 0.038 | 0.732 | −0.124 | 0.234 |
| Non-HDL cholesterol | 0.010 | 0.887 | 0.077 | 0.280 | 0.032 | 0.774 | −0.068 | 0.518 |
Footnotes
Disclosures: The authors have nothing further to disclose.
References
- 1.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 2.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. The Journal of clinical investigation. 2006;116(7):1802–12. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkman MS, Shankar RR, Shankar S, Shen C, Brizendine E, Baron A, et al. Treating postprandial hyperglycemia does not appear to delay progression of early type 2 diabetes: the Early Diabetes Intervention Program. Diabetes care. 2006;29(9):2095–101. doi: 10.2337/dc06-0061. [DOI] [PubMed] [Google Scholar]
- 4.Nijpels G, Boorsma W, Dekker JM, Kostense PJ, Bouter LM, Heine RJ. A study of the effects of acarbose on glucose metabolism in patients predisposed to developing diabetes: the Dutch acarbose intervention study in persons with impaired glucose tolerance (DAISI) Diabetes/metabolism research and reviews. 2008;24(8):611–6. doi: 10.1002/dmrr.839. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology. 1979;237(3):E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SE, Porte D., Jr Islet dysfunction in non-insulin-dependent diabetes mellitus. The American journal of medicine. 1988;85(5A):4–8. doi: 10.1016/0002-9343(88)90392-0. [DOI] [PubMed] [Google Scholar]
- 7.Mitrakou A, Vuorinen-Markkola H, Raptis G, Toft I, Mokan M, Strumph P, et al. Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemia clamp. The Journal of clinical endocrinology and metabolism. 1992;75(2):379–82. doi: 10.1210/jcem.75.2.1639939. [DOI] [PubMed] [Google Scholar]
- 8.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes care. 2010;33(10):2225–31. doi: 10.2337/dc10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbein SC, Wegner K, Kahn SE. Reduced beta-cell compensation to the insulin resistance associated with obesity in members of caucasian familial type 2 diabetic kindreds. Diabetes care. 2000;23(2):221–7. doi: 10.2337/diacare.23.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Kahn SE, Montgomery B, Howell W, Ligueros-Saylan M, Hsu CH, Devineni D, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86(12):5824–9. doi: 10.1210/jcem.86.12.8105. [DOI] [PubMed] [Google Scholar]
- 11.Giannini C, Weiss R, Cali A, Bonadonna R, Santoro N, Pierpont B, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61(3):606–14. doi: 10.2337/db11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanat M, Winnier D, Norton L, Arar N, Jenkinson C, Defronzo RA, et al. The relationship between {beta}-cell function and glycated hemoglobin: results from the veterans administration genetic epidemiology study. Diabetes care. 2011;34(4):1006–10. doi: 10.2337/dc10-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes research and clinical practice. 2007;78(3):305–12. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu R, Barosa C, Jones J, Dube S, Carter R, Basu A, et al. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. The Journal of clinical endocrinology and metabolism. 2013;98(3):E409–17. doi: 10.1210/jc.2012-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–49. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 18.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 19.Bunck MC, Corner A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, et al. Effects of exenatide on measures of beta-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes care. 2011;34(9):2041–7. doi: 10.2337/dc11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derosa G, Franzetti IG, Querci F, Carbone A, Ciccarelli L, Piccinni MN, et al. Exenatide plus metformin compared with metformin alone on beta-cell function in patients with Type 2 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2012;29(12):1515–23. doi: 10.1111/j.1464-5491.2012.03699.x. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes care. 2011;34 (Suppl 2):S202–9. doi: 10.2337/dc11-s221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 23.Harrison LB, Adams-Huet B, Raskin P, Lingvay I. beta-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes care. 2012;35(7):1406–12. doi: 10.2337/dc11-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes care. 2009;32 (Suppl 2):S151–6. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber AJ. Incretin effects on beta-cell function, replication, and mass: the human perspective. Diabetes care. 2011;34 (Suppl 2):S258–63. doi: 10.2337/dc11-s230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, et al. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, noninferiority randomised trial. The Lancet Diabetes & Endocrinology. 2013 doi: 10.1016/S2213-8587(13)70021-4. Early Online Publication. [DOI] [PubMed] [Google Scholar]