Abstract
Objective
This study had two objectives: first, to determine the degree to which experiences of victimization by peers during adolescence led to a subsequent rise in depressive symptoms; second, to identify genetic markers that predict depressive reactivity to victimization.
Method
We used a cohort sequential design to obtain a longitudinal sample of 1,475 adolescents (3,263 observations) in grades 8 to 12 (56% female; 47% Black, 46% White). Multilevel growth curve models were used to assess whether victimization predicted depressive symptoms six months later, beyond baseline trajectories for depressive symptoms. We modeled the interactive effects of peer victimization with three genetic polymorphisms (on 5-HTTLPR, DRD2 TaqIA, and BDNF Val66Met) on depressive symptoms.
Results
While victimization predicted subsequent depressive symptoms, there was substantial heterogeneity in the magnitude of the effect of victimization. Val alleles, associated with higher brain-derived neurotrophic factor (BDNF) functioning, predicted more sensitivity to victimization. Neither DRD2 TaqIA, a marker associated with dopaminergic functioning, nor 5-HTTLPR, a marker associated with serotonin activity, was associated with sensitivity to victimization.
Conclusions
The social stress of peer victimization triggers depressive symptoms most strongly in individuals who are homozygous for the Val allele on the BDNF Val/Met polymorphism. This polymorphism has been linked with sensitivity to social defeat in animal models. Future research should explore behavioral, cognitive, and emotional explanations of the effects of BDNF Val/Met on responsivity to victimization.
Keywords: DRD2TaqIA, BDNF, 5-HTTLPR, victimization, depression
Victimization by peers has been linked with depressive symptoms in school-aged children and adolescents. However, individuals differ in severity of depressive symptoms following victimization. This study examined three genetic polymorphisms to determine whether they interacted with peer victimization to predict subsequent depressive symptoms. The polymorphisms studied were: (a) the 43 base pair (bp)insertion/deletion polymorphism (5-HTTLPR; rs25531) in the promoter region of the serotonin transporter gene; (b) the glutamate-to-lysine single nucleotide polymorphism (SNP) rs1800497 (DRD2 TaqIA) associated with the dopamine D2 receptor (actually located in the adjacent ANKK1 gene); and (c) the valine-to-methionine insertion at codon 66 in the BDNF gene, rs6265 (Val66Met).
Heterogeneity in Level of Depressive Symptoms Following Peer Victimization
Peer victimization includes physical victimization (characterized by overt acts of physical aggression that may cause pain or bodily harm), verbal bullying (such as name-calling), and relational victimization (characterized by aggressive acts intended to harm an individual’s social standing or relationships; Crick, 1996). Cross-sectional studies consistently show that victimization by peers during childhood and adolescence is associated with depressive symptoms, at least for some individuals (Hawker & Boulton, 2000).Prospective studies of the effects of victimization during early adolescence have also found that victimization was associated with subsequent depressive symptoms (Copeland, Wolke, Angold, & Costello, 2013; McLaughlin, Hatzenbuehler, & Hilt, 2009; Vernberg, Abwender, Ewell, & Beery, 1992). And studies investigating the effects of peer victimization in older adolescence have found that victimization predicted subsequent depressive symptoms (Foshee, Reyes, Gottfredson, Chang, & Ennett, 2013; Prinstein, Cheah, & Guyer; 2005).
However, individuals do not have equal depressive reactions to victimization (Hankin, 2012). Gottfredson (1989) reported that some, but not all, individuals developed severe or prolonged depressive symptoms following severe victimization, and Foshee et al. (2013) detected significant inter-individual variation in depressive symptoms following psychological victimization among adolescents.
Genetic Markers Hypothesized to Predict Depressive Reactivity to Victimization
Previous studies have implicated the short-allele of the 5-HTTLPR polymorphism, the Val allele on the BDNF Val66Met polymorphism, and the A2 allele on the DRD2 TaqIA polymorphism to cognitive response and emotion regulation following stressors. One of these polymorphisms, the BDNF Val66Met polymorphism, has been implicated in depressive symptoms following social defeat with animal models that may be equivalent to peer victimization.
In studies of gene-by-environment interactions, contextual triggering occurs when “the genotype and/or context [may each] have an additive effect on the likelihood or intensity of the phenotype, but their combination significantly, additionally influences the manifestation of the phenotype,” and “social context may trigger specific genetic expressions through stress processes” (Shanahan & Hofer, 2005, p. 66). Consistent with the contextual triggering mechanism described by Shanahan and Hofer (2005), peer victimization may trigger differential expression of depressive phenotypes for polymorphisms on 5-HTTLPR, BDNF Val66Met, and DRD2 TaqIA. A stressful peer context, i.e., the experience of peer victimization, may thus trigger higher levels of depressive symptoms among those carrying alleles associated with greater risk(Shanahan & Hofer, 2005). We view peer victimization as having both a direct effect on depressive symptoms, as well as being a contextual trigger for the differential expression of depressive genotypes. Thus, whereas victimization may be associated with higher levels of depressive symptoms for adolescents in general, the effects of victimization will be strongest for those with riskier genotypes.
Serotonergic Activity
Individual differences in serotonin activity are influenced in part by genetic variation in a polymorphism residing on the serotonin transporter gene (SLC6A4, 5-HTT). The 5-HTTLPR polymorphism is in the promoter region of the serotonin transporter gene. The long (l) allele of the polymorphism results in greater transcription of the serotonin transporter gene than the short (s) allele, and therefore greater response to serotonin present in the synapse. The s allele has been associated with less transcriptional efficiency. Individuals with mores alleles are known to have more depressive symptoms (Pezawas et al., 2005).
Caspi et al. (2003) found that the 5-HTTLPR polymorphism interacted with life stress to predict higher risk for depression symptoms in a sample that spanned childhood, adolescence, and young adulthood. This finding has been contested in some meta-analyses (Munafo, Durrant, Lewis, & Flynt, 2009; Risch, Herrell, Merikangas, et al., 2009), but it has been supported in the most recent and largest meta-analysis (Karg, Burmeister, Shedden, & Sen, 2011). Further, Gyurak et al. (2012) found that, when tested experimentally, individuals with two s alleles of the serotonin transporter polymorphism were more emotionally reactive to both pleasant and aversive stimuli than individuals with a long allele.
Two studies have investigated the role of the serotonin transporter gene in the relationship between victimization and depressive symptoms. Sugden et al. (2010) found that children who were carriers of two s serotonin transporter alleles and who were bullied by their peers were at greatest risk for developing emotional problems at age 12 than those with fewer s alleles. Benjet, Thompson, and Gotlib (2010), found that 5-HTTLPR interacted with relational peer victimization to predict concurrent depressive symptoms, but only for females.
Dopaminergic Activity
Dunlop and Nemeroff (2007) and Nestler and Carlezon (2006) have described a mechanism involving dopamine systems in the etiology of depression when individuals with higher genetic risk are exposed to chronic stressors. Both groups of authors found that individuals with the highest sensitivity to reward, as measured by dopaminergic activity, were most susceptible to depression. Drawing upon animal models, Dunlop and Nemeroff (2007) suggested that chronic stressors lead to low motivation and learned helplessness, two characteristics of depression.
The only study that has investigated the role of dopamine-related markers in the association between victimization and depressive symptoms in humans was conducted by Vaske, Makarios, Boisvert, Beaver, and Wright (2009). These authors found no support for an interaction between DRD2 TaqIA and violent victimization in their total sample, but they found that for African American females, the low activity A1 allele predicted greater risk for depression following victimization. This finding is in the opposite direction of what we hypothesize: we consider the A2 allele to confer risk because it is linked with more reward sensitivity. This discrepancy is reflective of a larger divide in the field: some researchers link the lower activity allele (A1)on this particular polymorphism with greater environmental sensitivity (Belsky & Beaver, 2011); others link the higher activity allele (A2) with greater environmental sensitivity (Nikolova, Ferrell, Manuck, & Hariri, 2011).
BDNF Activity
BDNF regulates the formation and plasticity of neural structures involved in emotion regulation (Elzinga, Molendijk, Oude Voshaar, et al., 2011). The most commonly examined polymorphism in the BDNF gene is the Val66Met (rs6265) polymorphism, with the Val allele considered the high activity allele (Egan, Kojima, Callicott, et al., 2003). BDNF is expressed in multiple areas of the brain, most notably in the hippocampus, the prefrontal cortex, and the nucleus accumbens (Martinowich, Manji, & Lu, 2007). BDNF has been linked to depression following a stressful event (Duman & Monteggia, 2006). According to Martinowich et al (2007), exposure to stress is linked with higher levels ofBDNF expression in the nucleus accumbens. In turn, the nucleus accumbens is associated with individual differences in reactivity to social stressors, and higher levels of BDNF in the nucleus accumbens have been linked with higher rates of depressive symptoms (Coppens, Siripornmongcolchai, Wibrand, et al, 2011).
The function of BDNF appears to differ across development stages. Coppens et al (2011) suggested that because adolescence is a period during which the neuronal circuitry corresponding to stress reactivity undergoes reorganization, BDNF plays a more prominent role in the adolescent stress response than it does in the adult stress response. Indeed, Coppens and colleagues found that socially defeated rats had higher levels of BDNF expression during adolescence, but not during adulthood.
Study Hypotheses
1. Victimization by peers will predict higher levels of depressive symptoms six months later, controlling for an individual’s latent depressive trajectory. However, we predict that there will be substantial individual variation with respect to degree of sensitivity to peer victimization.
2. In line with Shanahan and Hofer’s (2005) contextual triggering model, exposure to peer victimization will trigger subsequent depressive symptoms differently, depending on allelic polymorphisms in 5-HTTPLPR, BDNF Val66Met, and DRD2 TaqIA. Adolescents with more short alleles on the 5-HTTPLPR polymorphism, those with more Val alleles on the Val66Met polymorphism, and those carrying more A2 alleles on the DRD2 TaqIA polymorphism will be particularly vulnerable to developing higher levels of depressive symptoms following victimization experiences.
Method
Study Design, Procedures, and Sample
Data for this manuscript were from the Genes in Context Study(NICHD R01-HD057222; PI Vangie A. Foshee). Biospecimens were collected for genotyping from young adults (ages 19–25) who had participated as adolescents in a longitudinal study conducted in two rural county school systems in North Carolina (Context Linkages Study; NIDA R01-DA13459; PI Susan T. Ennett); this study examined contextual influences on adolescent health risk behaviors. Active parental consent and adolescent assent were required for adolescents to participate in the Context Linkages Study. Since all participants were over 18 when they were contacted about the Genes in Context Study, only the young adults’ assent was required for participation. All procedures for both studies were approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
The longitudinal Context Linkages Study used a cohort-sequential design with four waves of data collection in schools using self-administered questionnaires. The first wave, in Fall 2003, included students in 8th, 9th, and 10th grades. The second wave, in Spring 2004, included students in 8th, 9th, and 10th grades, the third wave, in Fall 2004, included students in 9th, 10th, and 11th grades, and the fourth wave, in Fall 2005, included participants in 10th, 11th, and 12th grades. The study population spanned mid-to-late adolescence because this is the developmental period when peer influence on mental health and behavioral outcomes increases and when heritability of depression increases (Silberg, Pickles, Rutter, et al., 1999; Spear, 2010). Response rates at each wave ranged from 73% to 77% of eligible adolescents.
Young adults who had participated as adolescents in at least one wave of data collection for the longitudinal study (N = 3,835) were later contacted via telephone and asked to collect either a saliva sample using an Oragene collection kit or a blood spot using a lancet, and return the sample to the study office by mail. Staff for the Genes in Context Study attempted to locate Context Linkages participants using contact information from when participants were in high school, social media, and publically available contact information. Respondents were given $35 for a saliva sample and $50 for a blood spot. Of the 1,519 samples received, 44 (3%)could not be genotyped, leaving a total of 1,475 samples for genotyping.
Of the 3,835 young adults who participated in the Context Linkages Study, 1,519 (40%) provided a biospecimen for genotyping; 241 (6%) agreed to provide a sample but did not, 481 (13%) refused to provide a sample, 555 (14%) could not be located, and 1,039 (27%) were located but could not be contacted after several attempts. The low participation rate is partially attributable to the long lag between administration of the school-based surveys (2003 – 2005) and the request for genetic information (2010). Individuals who provided genetic material were more likely to be female (56% as compared to 50% in the longitudinal study; p< .001), less likely to be Black (47% as compared to 51% in the longitudinal study; p = .03), more likely to be White (46% versus 41% in the longitudinal study; p< .01), and more likely to have a parent who had graduated from high school (71% versus 67% in the longitudinal study; p = .01).There were no significant differences in household structure (single parent or two-parent: 10.8% single parent in the genetic sample versus 9.4% in the longitudinal study). Table 1 displays depressive symptoms and peer victimization rates for the full Context Linkages sample and for the subset that provided genetic data. Mean differences in levels of depressive symptoms were not significant at any grade level. The genetic sample reported slightly higher rates of victimization; differences in level of victimization in the two groups reached significance at some grade levels but not others.
Table 1.
Comparison of Depressive Symptoms and Peer Victimization Rates for Full Sample versus the Genetic Sub-Sample, by Grade Level
| Depressive Symptoms -- M (SD) | Peer Victimization -- Proportion | |||||
|---|---|---|---|---|---|---|
| Grade | Full Sample | Genetic Sub- Sample |
p-valuea | Full Sample | Genetic Sub- Sample |
p-valueb |
| 8 | 1.41 (1.22) | 1.51 (1.22) | .95 | .27 | .33 | <.01 |
| 8.5 | 1.44 (1.23) | 1.48 (1.28) | .74 | .26 | .29 | .09 |
| 9 | 1.41 (1.18) | 1.45 (1.20) | .82 | .17 | .22 | <.001 |
| 9.5 | 1.47 (1.22) | 1.55 (1.29) | .89 | .20 | .23 | .08 |
| 10 | 1.42 (1.15) | 1.44 (1.17) | .71 | .17 | .19 | .04 |
| 10.5 | 1.47 (1.17) | 1.54 (1.18) | .86 | .20 | .22 | .18 |
| 11 | 1.35 (1.13) | 1.39 (1.15) | .82 | .13 | .15 | .06 |
Note. Genetic sub-sample N=1,475; Full sample N=3,979. Depressive symptoms represent the average level of endorsement of symptoms, on a scale from 0 to 4.
Results of t-tests comparing mean level of depressive symptoms for the full sample and the genetic sample are reported for each grade level.
Results of z-tests for proportions comparing rates of victimization in the full sample and in the genetic sample are reported for each grade level.
Based on 2000 U.S. Census data, residents of the two study counties tended to be more disadvantaged than residents of North Carolina (N.C.) and the U.S. in terms of median income (study counties: $40,494; N.C.: $46,335; U.S.: $50,046), percent in poverty (study counties: 16%; N.C.: 12%; U.S. 12%), and percentage of adults without a high school diploma (study counties: 29%; N.C. 20%; U.S. 22%). The study counties also had a larger percentage of Blacks in the population (38%) than N.C. (22%) or the U.S. (12%).
Genotyping and Genetic Marker Coding
DNA was extracted from samples at the University of North Carolina Biospecimen Processing Facility, and the DNA was sent to the Institute of Behavioral Genetics at the University of Colorado, Boulder, for genotyping. Genotyping of 5-HTTLPR was performed as described in Whisman, Richardson, and Smolen (2011). The SNPs in DRD2 and BDNF were assayed using the Applied Biosystems (Foster City, CA) Open Array® System (a low volume Taqman® method) using reagents and protocols supplied by the company.
Measures
Victimization
Our victimization measure was based on the premise that peer victimization occurs within the context of social networks (Faris & Ennett, 2012).At each wave, participants identified up to five names of peers who were mean to them or had picked on them during the past 3 months. Participants then indicated whether each of the nominated peers had:(a) made fun of them or called them names to their face;(b) said bad things about them behind their back to get others not to be friends with them; or (c) physically attacked them in any way (hitting, shoving, tripping).Victimization was coded such that a value of 1 indicated that participants had experienced at least one of the three indicators of victimization at a given time and 0 indicated that they had not. In the fall of 8th grade, 33% of participants reported any type of victimization. Reports of victimization decreased across grade levels, reaching a low in 12th grade when 15% of participants reported any victimization. The prevalence of peer victimization was somewhat lower than that in a national sample: Nansel, Overpeck, Pilla, et al.(2001) found that 41% of 8th graders, 36% of 9th graders, and 28% of 10th graders reported being bullied. However, the bullying measure used by Nansel and colleagues was more general (i.e., bullying was defined as a group of students saying or doing nasty or unpleasant things, or as repeated teasing). The Nansel et al. study also used a more open-ended time frame (i.e., ‘the current term’), whereas the current study emphasized victimization that had occurred within the past three months. The current findings were consistent with other findings that victimization peaked in early adolescence or middle school and decreased from there on (Pellegrini 2002; Pepler, Craig, Connolly, et al., 2006).
Depressive Symptoms
Five items from Angold, Costello, and Messer’s (1995) Short Mood and Feelings Questionnaire were used to assess feelings of depression in the longitudinal study. Validity of the Short Mood and Feelings Questionnaire (SMFQ) was established in a community sample of children and adolescents aged 8–16 (Thapar & McGuffin, 1998). The scale showed a sensitivity of .75 and a specificity of .74 with DSM-III-R depression diagnosis when compared with other, related diagnoses. We selected five items from the SMFQ based on psychometric work with a sample of 500 adolescents in a pilot study. Participants were asked the extent to which they agreed or disagreed with the following statements as descriptors of how they had felt in the past three months: “I got mad easily,” “I was tired a lot,” “I hated myself,” “I was a bad person,” and “I did everything wrong.” Responses ranged from 0 (strongly disagree) to 4 (strongly agree). A mean of the five items was computed for each individual at each data collection point. As shown in Table 1, mean depressive scores remained relatively constant across grade levels, with a high of 1.54 (SD = 1.182) in the spring of grade 10 and a low of 1.39 (SD = 1.15) in the fall of grade 11. Alpha reliabilities ranged from .83 to .87 across the four waves. The depression scale was standardized (M = 0, SD = 1) for data analysis to facilitate the interpretation of regression coefficients.
Genetic Markers
To limit assumptions regarding allelic dominance, information on each of the two alleles of each marker was combined additively (i.e., scores reflect the proportion of alleles associated with elevated risk).
5-HTTLPR was genotyped following the strategy of Caspi et al. (2003). Individuals homozygous for short alleles were given a value of 1, those homozygous for long alleles were given a value of 0, and those with one short and one long allele were given a value of .5. In all, 14% of the participants had two low activity alleles, 42% had one low activity allele, and 44% had no low activity alleles.
BDNF Val66Met was coded such that individuals homozygous for the Val allele were given a score of 1, those homozygous for the Met allele were given a score of 0, and heterozygotes were given a score of .5. Higher values indicated higher activity. In all, 79% of participants had two Val alleles, 19% had one Val allele, and 2% had two Met alleles.
Individuals homozygous for DRD2 TaqIA A2 (high activity) alleles were scored 1 and those homozygous for low activity A1 alleles were scored 0. Heterozygous individuals were given a score of .5. In all, 9% of participants carried two low activity alleles, 38% carried one low activity alleles, and 53% carried two high activity alleles.
Results
Data Analysis Overview
To take advantage of the cohort sequential design of this study, grade-level of the adolescent was used as the primary metric for time rather than wave of assessment (Biesanz, Deeb-Sossa, Papadakis, Bollen, & Curran, 2004).1 After the data were organized by grade, 3,263 repeated measures were available for the eight discrete time points: grade 8 fall (n=305 observations), grade 8 spring (n=282 observations), grade 9 fall (n=602 observations), grade 9 spring (n=280 observations), grade 10 fall (n= 816 observations), grade 10 spring (n = 262 observations), grade 11 fall (n=499 observations) and grade 12 fall (n=217 observations). More information was available for some grade levels because multiple cohorts passed through these developmental stages during the study (e.g., one cohort was assessed in the fall of 8th grade; three cohorts were assessed in the fall of 10th grade).
Preliminary Analyses
Ball, Arseneault, Taylor, Maughan, Caspi, and Moffitt (2008) found that childhood victimization was substantially heritable (73% of the variation in victimization was accounted for by genetic factors, in their analysis of twin data), and Beaver, Mancini, DeLisi, and Vaughn (2011) implicated 5-HTTLPR as a predictor of victimization. Therefore, to rule out gene-environment correlations as a confound, we first assessed the degree to which participant genotypes correlated with their reports of victimization (Plomin, DeFries, & Loehlin, 1977). To compute gene-environment correlations, victimization reports were averaged across all waves for each participant. Average self-reported victimization was not significantly correlated with any of the polymorphisms investigated (r=.05 for 5-HTTLPR, r=−.04 for Val66Met, and r=.03 for DRD2 TaqIA).
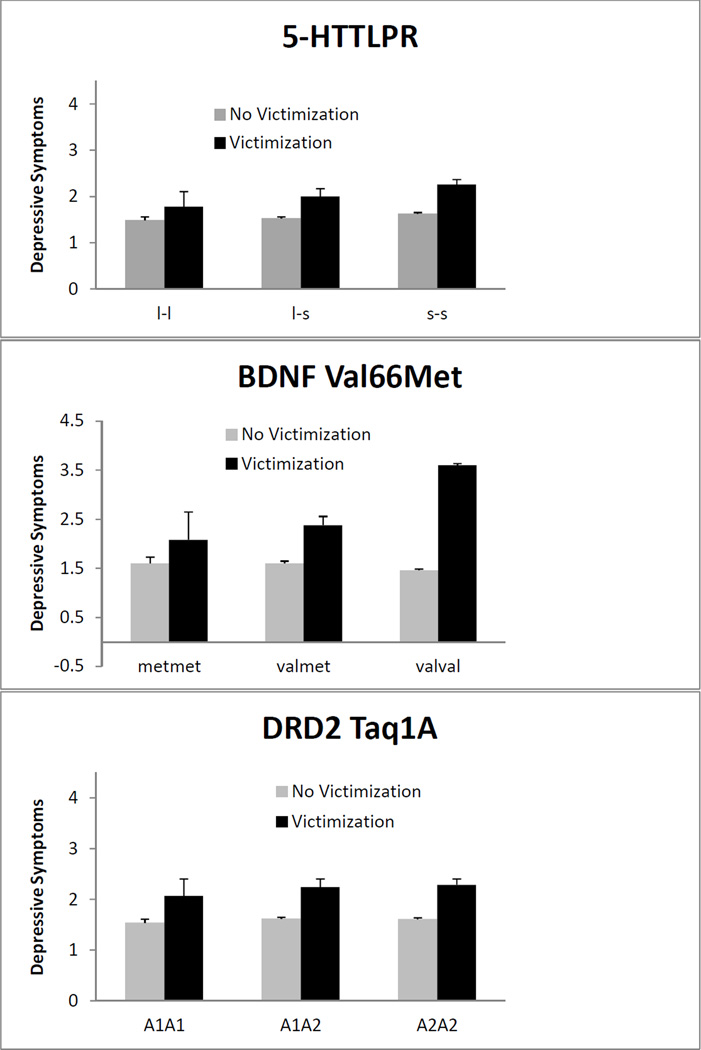
Figure 1 shows mean levels of depressive symptoms among individuals who reported experiencing victimization six months earlier, and among individuals who did not report experiencing victimization six months earlier, as a function of their genetic profiles (homozygous for the ‘risk’ allele, homozygous for the ‘non-risk’ allele, or heterozygous). In all cases, there was a clear trend toward higher mean levels of depressive symptoms among individuals who had experienced victimization six months earlier. There was no apparent effect of genotype on depressive symptoms for either 5-HTTLPR or for DRD2 TaqIA. However, individuals who were homozygous for the Val allele on the BDNF Val66Met marker appeared to have higher levels of depressive symptoms than individuals who were homozygous for the Met allele or who were heterozygous, but only when they were exposed to peer victimization six months earlier.
Figure 1.
Mean levels of depressive symptoms six months following experiences of victimization (black) or no victimization (grey), by genotype. Bars represent the upper-tail of the 95% confidence interval.
We used multilevel growth curve models to predict depressive symptoms from peer victimization that had occurred six months earlier, genetic information, and the interaction between the two. The main effect of lagged victimization and the three genetic markers were tested before testing the interaction between lagged victimization and the genetic markers. A random effect of victimization was included to test and account for individual heterogeneity in the effect of victimization on the development of depressive symptoms. Race/ethnicity and gender were included in the model, and interactions between these demographic variables and time were tested to determine whether change in depressive symptoms over time differed across demographic groups. We also tested for a cohort and cohort-by-age effect on depressive symptoms. These effects were not significant so were omitted from subsequent models.
SAS Proc Mixed (Version 9.3) was used to estimate all mixed models. Before adding predictors of interest, we established an unconditional growth model for change in depressive symptoms between eighth and twelfth grade. Grade was centered at eighth grade so that the intercept was interpretable as the average level of depressive symptoms for students in eighth grade (this value was zero because the depressive symptoms measure was standardized). There was no systematic increase or decrease in depressive symptoms with respect to grade (B=.00, SE = .02, p=.81, 95% CI [−.03,.04]). However, there were significant random effects for both the intercept and the slope for the grade effect. That is, there was variability in baseline levels of depressive symptoms (Var(B)=.66, SE=.07, p<.001), and some individuals had systematically increasing depressive scores whereas others had systematically decreasing depressive scores (Var(B)=.02, SE=.01, p=.04).
No evidence of moderation of the victimization effect by gender or race/ethnicity was found. Males had significantly lower depressive scores than females(B = −.21, SE = .06, p< .01, 95% CI[−.29, −.09]), and White people had significantly higher depressive scores than others (B = .15, SE = .06, p< .01, 95% CI[.04,.24]). Because the depressive symptom scale was standardized, these effects are interpretable as the standardized effect of being male or being White on expected depressive symptoms. All subsequent models built upon this baseline model.
Hypothesis 1: Peer victimization is related to higher levels of subsequent depressive symptoms, controlling for baseline depressive trajectories. There is substantial heterogeneity with respect to the magnitude of the effect of victimization on depressive symptoms.
Reports of victimization by peers six months earlier were included as predictors of depressive symptoms. Because the effects were estimated in the context of a growth model, underlying depressive trajectories were controlled for. Thus, estimated effects of lagged victimization represent time-varying perturbations from one’s pre-existing depressive trajectory that are due to victimization that occurred six months earlier. The lagged victimization scores were grand mean centered (Enders & Tofighi, 2007). Quadratic effects for victimization were tested and found to be non-significant. Victimization did not interact with grade. Results are reported in the left-hand side of Tables 2, Tables 3, and 4 (they are repeated for ease of comparison with models that are described subsequently). The most substantively interesting parameter estimates have been bolded in all tables. The fixed effect of victimization on subsequent (standardized) depressive symptoms was significant and moderate. However, as predicted, there was substantial inter-individual heterogeneity with respect to the degree to which victimization was associated with subsequent depressive symptoms.
Table 2.
Genetic Moderation of Depressive Reactivity to Victimization: 5-HTTLPR
| VictimizationOnly | Additive Model | Interaction Model | ||||
|---|---|---|---|---|---|---|
| B (SE) | p-value | B (SE) | p-value | B (SE) | p-value | |
| Fixed Effects | ||||||
| Intercept | .01 (.05) | .91 | −.02 (.05) | .76 | −.02 (.06) | .78 |
| Grade | .01 (.02) | .67 | .01 (.02) | .67 | .01 (.02) | .67 |
| White | .13 (.05) | .01 | .12 (.05) | .01 | .12 (.05) | .01 |
| Male | −.18 (.05) | <.001 | −.18 (.05) | <.001 | −.18 (.05) | <.001 |
| Victimization | .29 (.08) | <.001 | .28 (.08) | <.001 | .25 (.12) | .05 |
| 5-HTTLPR | .08 (.07) | .27 | .07 (.07) | .31 | ||
| Victimization*5-HTTLPR | .09 (.23) | .69 | ||||
| Random Effects | ||||||
| Intercept Variance | .65 (.07) | <.001 | .65 (.07) | <.001 | .65 (007) | <.001 |
| Grade Variance | .03 (.01) | .02 | .03 (.01) | .02 | .03 (.01) | .02 |
| Victimization Variance | .47 (.13) | <.01 | .35 (.13) | <.01 | .36 (.13) | <.01 |
| Residual Variance | .35 (.13) | <.01 | .47 (.02) | <.001 | .47 (.02) | .02 |
| Model Fit | ||||||
| AIC | 8228.8 | 8155.8 | 8171.6 | |||
| BIC | 8264.2 | 8221.4 | 8206.9 | |||
Note. 5-HTTLPR is scored additively with each ‘s’ alleles = .5 and each ‘l’ allele = .5(for a total score between 0–1). Grade is centered at 8th grade, depressive symptoms are standardized, andvictimization is scored as present or absent. AIC = Akaike Information Criterion (lower is better). BIC = Bayesian Information Criterion (lower is better). Substantively interesting parameter estimates are bolded.
Table 3.
Genetic Moderation of Depressive Reactivity to Victimization: BDNF Val66Met
| VictimizationOnly | Additive Model | Interaction Model | ||||
|---|---|---|---|---|---|---|
| B (SE) | p-value | B (SE) | p-value | B (SE) | p-value | |
| Fixed Effects | ||||||
| Intercept | .01 (.05) | .91 | .02 (.05) | .74 | .02 (.05) | .66 |
| Grade | .01 (.02) | .67 | .01 (.02) | .70 | .01 (.02) | .74 |
| White | .13 (.05) | .01 | .17 (.05) | <.01 | .17 (.05) | <.01 |
| Male | −.18 (.05) | <.001 | −.19 (.05) | <.001 | −.19 (.05) | <.001 |
| Victimization | .29 (.08) | <.001 | .27 (.08) | <.01 | .14 (.10) | .14 |
| BNDF Val66Met | −.13 (.11) | .25 | −.17 (.11) | .13 | ||
| Victimization* BNDF Val66Met | .75 (.31) | .02 | ||||
| Random Effects | ||||||
| Intercept Variance | .65 (.07) | <.001 | .65 (.07) | <.002 | .66 (.07) | <.001 |
| Grade Variance | .03 (.01) | .02 | .03 (.01) | .03 | .03 (.01) | .03 |
| Victimization Variance | .47 (.13) | <.01 | .39 (.13) | <.01 | .36 (.13) | <.01 |
| Residual Variance | .35 (.13) | <.01 | .46 (.02) | <.001 | .46 (.01) | <.001 |
| Model Fit | ||||||
| AIC | 8228.8 | 7926.6 | 7935.2 | |||
| BIC | 8264.2 | 7991.9 | 7970.3 | |||
Note. BDNF is scored additively with each Met allele = .5 and each Val allele = 0 (for a total score between 0–1). Grade is centered at 8th grade, depressive symptoms are standardized, andvictimization is scored as present or absent. AIC = Akaike Information Criterion (lower is better). BIC = Bayesian Information Criterion (lower is better). Substantively interesting parameter estimates are bolded.
Table 4.
Genetic Moderation of Depressive Reactivity to Victimization: DRD2 TaqIA
| VictimizationOnly | Additive Model | Interaction Model | ||||
|---|---|---|---|---|---|---|
| B (SE) | p-value | B (SE) | p-value | B (SE) | p-value | |
| Fixed Effects | ||||||
| Intercept | .01 (.05) | .91 | −.06 (.08) | .41 | −.06 (.07) | .44 |
| Grade | .01 (.02) | .67 | .01 (.02) | .67 | .01 (.02) | .68 |
| White | .13 (.05) | .01 | .13 (.05) | .01 | .13 (.05) | .01 |
| Male | −.18 (.05) | <.001 | −.20 (.05) | <.001 | −.20 (.05) | <.001 |
| Victimization | .29 (.08) | <.001 | .27 (.08) | <.001 | .19 (.22) | .37 |
| DRD2 TaqIA | .10 (.08) | .19 | .10 (.08) | .22 | ||
| Victimization* DRD2 TaqIA | .10 (.26) | .68 | ||||
| Random Effects | ||||||
| Intercept Variance | .65 (.07) | <.001 | .66 (.07) | <.001 | .66 (.07) | <.001 |
| Grade Variance | .03 (.01) | .02 | .03 (.01) | .01 | .03 (.01) | .01 |
| Victimization Variance | .47 (.13) | <.01 | .36 (.13) | <.01 | .37 (.13) | <.01 |
| Residual Variance | .35 (.13) | <.01 | .47 (.02) | <.001 | .47 (.01) | .01 |
| Model Fit | ||||||
| AIC | 8228.8 | 8103.4 | 8118.9 | |||
| BIC | 8264.2 | 8168.9 | 8154.1 | |||
Note. DRD2 TaqIA is scored additively with each A2 allele = .5 and each A1 allele = 0 (for a total score between 0–1). Grade is centered at 8th grade, depressive symptoms are standardized, andvictimization is scored as present or absent. AIC = Akaike Information Criterion (lower is better). BIC = Bayesian Information Criterion (lower is better). Substantively interesting parameter estimates are bolded.
Hypothesis 2: Exposure to peer victimization will trigger subsequent depressive symptoms differently, depending on allelic polymorphisms in 5-HTTPLPR, BDNF Val66Met, and DRD2 TaqIA. Adolescents with more short alleles on the 5-HTTPLPR polymorphism, those with more Val alleles on the Val66Met polymorphism, and those carrying more A2 alleles on the DRD2 TaqIA polymorphism will be particularly vulnerable to developing higher levels of depressive symptoms following victimization experiences.
First, additive models containing only the main effects of each genetic marker were estimated. Results for the 5-HTTLPR models are shown in the center of Table 2, results for the BDNF Val66Met models are shown in the center of Table 3, and results for the DRD2 TaqIA models are shown in the center of Table 4. Nested likelihood ratio tests were used for formal model comparisons (not shown); Bayesian Information Criterion and Akaike Information Criterion indices of fit are shown in the tables.
5-HTTLPR
The additive model fit the data better than the victimization-only or the interaction model. This suggests that inclusion of the 5-HTTLPR allele produced a significant benefit in the prediction of depressive symptoms. However, the main effect of genotype on depressive symptoms was not large enough to reach statistical significance. These results are consistent with the lack of an observable pattern in Figure 1.
BDNF Val66Met
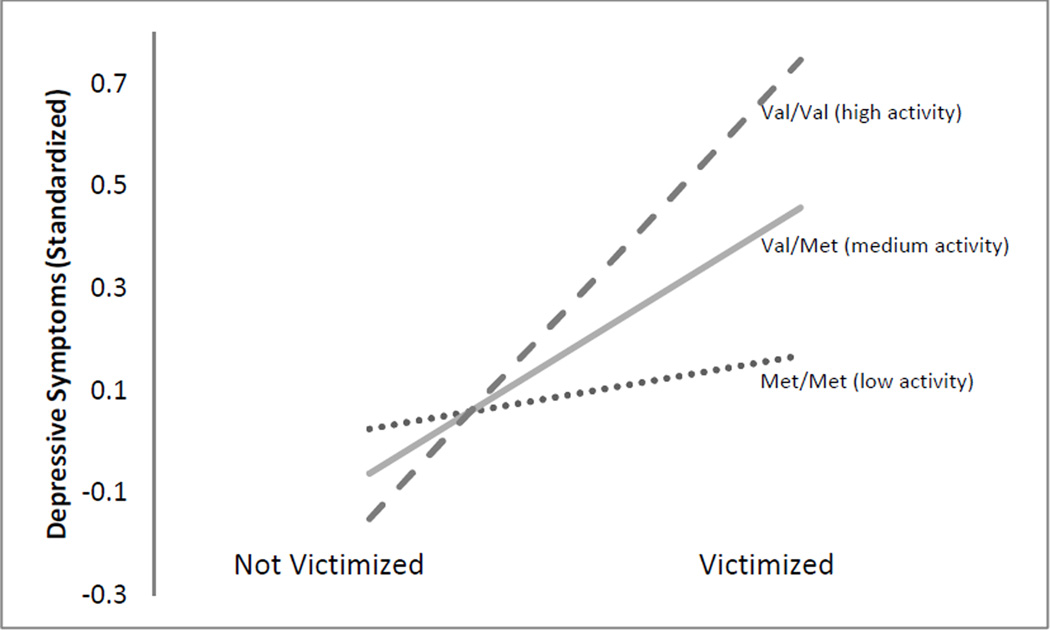
The additive model provided a significantly better fit to the data than the victimization-only model, and the interactive model fit significantly better than the additive model or the victimization-only model. This finding is consistent with the pattern of results observable in Figure 1. The interaction of victimization and BDNF Val66Met was statistically significant, and this interaction remained significant after applying the False Discovery Rate correction for multiple comparisons (Thissen, Steinberg, & Kuang, 2002). As Figure 2 shows, there was little difference in the level of depressive symptoms between carriers of the MetMet, ValMet, and ValVal alleles when victimization had not occurred in the past six months. When victimization had occurred, however, the differences between these groups were striking. Carriers of two Val alleles (79% of the sample) tended to have much higher depressive symptom scores than heterozygotes or individuals homozygous for the Met allele. The simple intercepts and slopes for each genotype are as follows: individuals homozygous for the Val allele who had not experienced victimization had an expected standardized depressive symptom score of −.15. For these individuals, experiencing victimization was linked with .89 standard deviation (SD) more symptoms (the simple slope; a large effect; SE = .27, p = .001). Individuals with one Met allele and one Val allele had an expected standardized symptom score of −.06 when not exposed to victimization, with .52 SD more expected symptoms following exposure to victimization (a moderate effect; SE= .13, p < .001). Finally, individuals who were homozygous for the Met allele had an expected standardized symptom score of .02 when not exposed to victimization, with an expected value of .14 SD more symptoms following exposure to victimization (a small effect; SE = .10, p= .15).
Figure 2.
Simple slopes for the effect of victimization on subsequent depressive symptoms. 79% of the sample had the high activity allelic combination, 19% had the medium activity allelic combination, and 2% of the sample had the low activity allelic combination.
DRD2 TaqIA
As with 5-HTTLPR, the additive model containing genetic information fit the data significantly better than the victimization-only model, but the main effect of the DRD2 TaqIA marker was not significant. The additive model provided a better fit to the data than the interaction model. These results are consistent with the descriptive statistics shown in Figure 1.
Discussion
We expected that exposure to victimization would be prospectively associated with depressive symptoms six months later and that there would be substantial individual heterogeneity in the magnitude of the association between victimization and subsequent depressive symptoms. We also expected that peer victimization would trigger the differential expression of genetic polymorphisms in 5-HTTLPR, BDNF Val66Met, and DRD2 Taq IA to predict depressive symptoms. Like previous studies, we found that victimization did predict subsequent depressive symptoms beyond one’s baseline expected depressive trajectory, and some individuals are more sensitive to this exposure than others.
Contrary to our expectations, neither 5-HTTLPR polymorphism nor the DRD2 Taq IA polymorphism interacted significantly with exposure to peer victimization to trigger depressive symptoms. However, as expected, we found that the Val allele on BDNF Val66Met was related to greater depressive reactivity in response to peer victimization and the Met allele was associated with less reactivity.
Because peer victimization leads to different mental health outcomes for adolescents depending on their genetic make-up, victimization may be considered a contextual trigger for development of depressive symptoms for individual with susceptible genotypes(Shanahan & Hofer, 2005). Because most people are homozygous for the Val allele (e.g., 79% of participants were homozygous for the Val allele), and because the Met allele resulted in fewer depressive symptoms following the peer victimization, the Met allele is protective for adolescents who encounter peer victimization. This finding is supported by findings from an animal model in which mice whose BDNF functioning had been knocked out did not display aversion to social situations following social aggression, while mice with functioning BDNF genes did display aversion (Berton, McClung, DiLeone, et al., 2006).
The lack of an observed interaction between 5-HTTLPR and peer victimization differs from findings of Sugden et al (2010), who reported that children who were carriers of two short alleles on the 5-HTTLPR polymorphism and who were exposed to victimization were at greater risk for developing emotional problems in early adolescence. However, Sugden and colleagues used a younger sample, and it may be that developmental stage moderates the effect of this polymorphism. We consider this to be a possibility because a meta-analysis by Uher and McGuffin (2010) found that adolescents were less sensitive to the 5-HTTLPR polymorphism than younger children, although this was only true for males. Nevertheless, a post-hoc analysis of our data failed to find a three-way interaction between gender, victimization, and the 5-HTTLPR polymorphism. Another explanation for the difference in study results may be the different operationalization of victimization. Sugden and colleagues conducted in-home interviews with children and characterized victimization as something that happened chronically; our measure of victimization assessed similar types of behaviors, but we relied on survey reports and did not measure or model persistent victimization. It may be that 5-HTTLPR is moderated only by the effect of chronic peer victimization.
Our 5-HTTLPR findings also contrast with those of Benjet and colleagues (2010), who found that females with two short alleles had higher concurrent depression scores when exposed to relational victimization than females with a long allele who were exposed to relational victimization. This suggests that the specific type or severity of victimization may play a role in triggering genetic heterogeneity of depressive symptoms following victimization experiences.
The finding that the DRD2 Taq IA allele did not significantly interact with the effect of victimization on subsequent depressive symptoms is not entirely surprising. There is some debate about whether the A1 allele or the A2 allele confers risk. Whereas some researchers have linked the lower activity allele (A1) with more environmental sensitivity (Belsky & Beaver, 2011), others have linked the higher activity allele (A2) with more sensitivity (Nikolova, Ferrell, Manuck, & Hariri, 2011). Our finding of no moderation suggests that both hypotheses may be correct-- there may be complex opposing pathways such that each allele provides some protection and some risk. Additionally, supporting literature shows that individuals with higher levels of reward sensitivity are at risk for depressive symptoms under chronic stress, whereas our measure of victimization did not specify level of chronicity.
Limitations and Future Directions
Although attrition was relatively low across study waves in the initial Context Linkages Study, a substantial proportion of participants did not provide biospecimens when we re-contacted them several years later. Those who were re-contacted were less transient and of higher average socio-economic status, and the disproportionate exclusion of higher-risk individuals may have limited our power to detect significant effects due to restriction of range on victimization and depressive symptoms. However, attrition analysis showed that the individuals who were re-contacted reported higher rates of peer victimization, thereby increasing power to detect effects. Furthermore, we have no reason to think that the association between victimization and subsequent depressive symptoms would differ for those providing genetic data and those not providing genetic data, nor do we have reason to suspect that genetic markers would behave differently for these groups.
Heterogeneity in an adolescent’s response to peer victimization cannot be fully explained by a few genetic polymorphisms. We identified three genetic markers that are representative of a much more complex interplay among many polymorphisms (Plomin, Haworth, & Davis, 2009). The practice of creating genetic composite scores has been shown to explain a higher proportion of variance in phenotypes than the approach of relying on a single polymorphism for prediction (e.g., Nikolova et al., 2011; Stice, Yokum, Burger, Epstein, & Smolen, 2012).2 Therefore, future research should identify multiple polymorphisms that are mechanistically involved in the mesolimbic dopamine pathway and determine whether multiple polymorphisms and the interactions among these polymorphisms more strongly predict reactivity to victimization than a single polymorphism alone.
Our findings indicate that the Val allele on BDNF Val66Met promotes depressive symptoms when triggered by victimization. One mechanism through which this may occur is by inducing an aversion to social settings (based on the animal models mentioned above). Future research should test the cognitive and emotional explanations for this genetic marker’s effects on depressive symptoms. Other studies have identified a subset of the cognitive and emotional characteristics that explain some of the heterogeneity in depressive reactivity to victimization. For instance, Rudolph, Troop-Gordon, and Granger (2011) found that victimization predicted higher levels of rumination and depressive symptoms in children with heightened cortisol activation, a physiological marker of the biological stress response. Cortisol levels and rumination following peer victimization may be endopheno types in the causal network leading from high BDNF functioning to depressive symptoms (in the presence of peer victimization).
Finally, because studies have identified different sequelae to different forms of victimization (e.g., physical versus relational; Sinclair, Cole, Duke wich, et al., 2012; Storch, Masla-Warner, Crisp, & Klein, 2005), it would be beneficial for future studies to test genetic moderation of these forms of victimization separately.
Conclusion
Despite its limitations, this study has several strengths. Perhaps most important was that the data were collected longitudinally across an important developmental time span (Hankin, 2012). The use of a longitudinal, cohort sequential design enabled us to measure the prospective association between peer victimization experiences and later depressive symptoms, while controlling for individual differences in baseline depressive trajectories.
We found significant heterogeneity in the degree to which individuals exposed to victimization experienced greater depressive symptoms. A portion of this heterogeneity was accounted for by variation in BDNF activity, with high levels of activity being associated with higher levels of sensitivity.
It is neither practical nor desirable to identify the adolescents most likely to be adversely affected by victimization on the basis of genetic markers. However, it would be beneficial to use the evidence from this study to inform research on mediating mechanisms that have implications for treatment for adolescents who experience heightened levels of depressive symptoms following peer victimization. Those who suffer from high levels of depressive symptoms after such experiences may benefit from interventions to attenuate the deleterious cognitive, emotional, and behavioral effects of the neural reorganization and dysregulation that arise throughout the brain reward system (which involves a high degree of signaling from BDNF) following exposure to social stressors. Specifically, individuals who appear to be particularly sensitive to peer victimization might be trained and encouraged to maintain pro-social friendship bonds to ameliorate the risk for depressive symptoms that occur with social isolation.
Acknowledgments
Genes in Context/Linkage Studies were funded by National Institutes of Health (NICHD R01-HD057222, PI Vangie A. Foshee; NIDA R01-DA13459, PI Susan T. Ennett). This report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data were re-structured because grade level is a more meaningful metric of developmental context than wave in the study or age, especially when considering peer victimization. We first confirmed that there were no significant cohort effects.
We chose to examine the isolated effects of three candidate polymorphisms because we hypothesized a unique effect of each, and also because some allelic combinations would be too rare to study precisely in our study population (e.g., we did not wish to further divide the subset of individuals homozygous for the Met allele according to their genotypes on the DRD2 Taq1A or 5-HTTLPR polymorphisms).
Contributor Information
Nisha C. Gottfredson, Email: gottfredson@unc.edu, University of North Carolina, Chapel Hill, Center for Developmental Science, 100 East Franklin Street, CB#8115, Chapel Hill, NC, 27599.
Vangie A. Foshee, Email: foshee@email.unc.edu, University of North Carolina, Chapel Hill, Center for Developmental Science, 100 East Franklin Street, CB#8115, Chapel Hill, NC, 27599.
Susan T. Ennett, Email: sennett@email.unc.edu, University of North Carolina, Chapel Hill, Center for Developmental Science, 100 East Franklin Street, CB#8115, Chapel Hill, NC, 27599.
Brett Haberstick, Email: Brett.Haberstick@Colorado.edu, University of Colorado, Boulder, Boulder, CO.
Andrew Smolen, Email: Andrew.Smolen@Colorado.EDU, University of Colorado, Boulder, Boulder, CO.
References
- Angold A, Costello EJ, Messer SC. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–249. [Google Scholar]
- Ball HA, Arseneault L, Taylor A, Maughan B, Caspi A, Moffitt TE. Genetic and environmental influences on victims, bullies and bully-victims in childhood. The Journal of Child Psychology and Psychiatry. 2008;49:104–112. doi: 10.1111/j.1469-7610.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Mancini C, DeLisi M, Vaughn MG. Resiliency to victimization: The role of genetic factors. Journal of Interpersonal Violence. 2011;26:873–898. doi: 10.1177/0886260510365860. [DOI] [PubMed] [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. The Journal of Child Psychology and Psychiatry. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. Journal of Child Psychology and Psychiatry. 2010;51:173–179. doi: 10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnana V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, Curran PJ. The role of coding time in estimating and interpreting growth curve models. Psychological Methods. 2004;9:30–52. doi: 10.1037/1082-989X.9.1.30. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harringon H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Angold AA, Costello JE. Adult psychiatric outcomes of bullying and being bullied by peers in childhood and adolescence. JAMA Psychiatry. 2013;70:419–426. doi: 10.1001/jamapsychiatry.2013.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens CM, Siripornmongcolchai T, Wibrand K, Alme MN, Buwalda B, de Boer SF, Koolhaas JM, Bramham CR. Social defeat during adolescence and adulthood differentially induce BDNF-regulated immediate early genes. Frontiers in Behavioral Neuroscience. 2011;5:1–8. doi: 10.3389/fnbeh.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick NR. The role of overt aggression, non-physical aggression, and prosocial behavior in the prediction of children’s future social adjustment. Child Development. 1996;67:2317–2327. [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. http://dx.doi.org/10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in pathophysiology of depression. Archives of General Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Egan M, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF Val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. http://dx.doi.org/10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Molendijk ML, Oude Voshaar RC, Bus BAA, Prickaerts J, Spinhoven P, Pennix BJWH. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology. 2011;214:319–328. doi: 10.1007/s00213-010-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Mehtods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Faris R, Ennett S. Adolescent aggression: The role of peer group status motives, peer aggression, and group characteristics. Social Networks. 2012;34:371–378. doi: 10.1016/j.socnet.2010.06.003. http://dx.doi.org/10.1016/j.socnet.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foshee VA, Reyes HLM, Gottfredson NC, Change L, Ennett ST. A longitudinal examination of psychological, behavioral, academic, and relationship consequences of dating abuse victimization among a primarily rural sample of adolescents. Journal of Adolescent Health. 2013;53:723–729. doi: 10.1016/j.jadohealth.2013.06.016. http://dx.doi.org/10.1016/j.jadohealth.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson GD. The experiences of violent and serious victimization. In: Weiner NA, Wolfgang ME, editors. Pathways to Criminal Violence. Newbury Park, CA: 1989. pp. 202–232. [Google Scholar]
- Gyurak A, Haase CM, Sze J, Goodkind MS, Coppola G, Lane J, Miller BL, Levenson RW. The effect of the serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotional reactivity. Emotion. 2012 doi: 10.1037/a0029616. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Future directions in vulnerability to depression among youth: Integrating risk factors and processes across multiple levels of analysis. Journal of Clinical Child & Adolescent Psychology. 2012;41:695–718. doi: 10.1080/15374416.2012.711708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker DSJ, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: A meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry. 2000;41:441–455. [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Hilt LM. Emotion dysregulation as a mechanism linking peer victimization to depressive symptoms in adolescents. Journal of Consulting and Clinical Psychology. 2009;77:894–904. doi: 10.1037/a0015760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flynt J. Gene x environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. http://dx.doi.org/10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Overpeck M, Pilla RS, June Ruan W, Simons-Morton B, Scheidt P. Bullying behaviors among US youth: Pevalence and association with psychological adjustment. Journal of the American Medical Association. 2001;285:2094–2100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. http://dx.doi.org/10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36(9):1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini AD. Bullying, victimization, and sexual harassment during the transition to middle school. Educational Psychologist. 2002;37:151–163. [Google Scholar]
- Pepler DJ, Craig WM, Connolly JA, Yuile A, McMaster L, Jiang D. A developmental perspective on bullying. Aggressive Behavior. 2006;32:376–384. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nature Review Genetics. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Cheah CL, Guyer AE. Peer Victimization, Cue Interpretation, and Depressive Symptoms: Preliminary Concurrent and Longitudinal Findings for Children and Adolescents. Journal Of Clinical Child And Adolescent Psychology. 2005;34(1):11–24. doi: 10.1207/s15374424jccp3401_2. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA: Journal Of The American Medical Association. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology. 2011;214:209–210. doi: 10.1007/s00213-010-1879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: Retrospect and prospect. The Journals of Gerontology. 2005;60B:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Archives of General Psychiatry. 1999;56:225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Sinclair KR, Cole DA, Dukewich T, Felton J, Weitlauf AS, Maxwell MA, Tilghman-Osborne C, Jacky A. Impact of physical and non-physical peer victimization on depressive cognitions in children and adolescents. Journal of Clinical Child & Adolescent Psychology. 2012;41:570–583. doi: 10.1080/15374416.2012.704841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. New York: W. W. Norton & Co., Inc; 2010. [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuit responsivity. The Journal of Neuroscience. 2012;18:10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Masia-Warner C, Crisp H, Klein RG. Peer Victimization and Social Anxiety in Adolescence: A Prospective Study. Aggressive Behavior. 2005;31(5):437–452. [Google Scholar]
- Sugden K, Arseneault L, Harrington H, Moffitt TE, Williams B, Caspi A. Serotonin transporter gene moderates the development of emotional problems among children following victimization. Journal Of The American Academy Of Child & Adolescent Psychiatry. 2010;49(8):830–840. doi: 10.1016/j.jaac.2010.01.024. http://dx.doi.org/10.1016/j.jaac.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. Validity of the shortened Mood and Feelings Questionnaire in a community sample of children and adolescents: A preliminary research note. Psychiatry Research. 1998;81:259–268. doi: 10.1016/s0165-1781(98)00073-0. http://dx.doi.org/10.1016/S0165-1781(98)00073-0. [DOI] [PubMed] [Google Scholar]
- Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg Procedure for controlling the false positive rate in multiple comparisons. Journal of Educational and Behavioral Statistics. 2002;27:77–83. [Google Scholar]
- Uher R, McGUffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Vaske J, Makarios M, Boisvert D, Beaver KM, Wright J. The interaction of DRD2 and violent victimization on depression: An analysis by gender and race. Journal Of Affective Disorders. 2009;112(1–3):120–125. doi: 10.1016/j.jad.2008.03.027. http://dx.doi.org/10.1016/j.jad.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Vernberg EM, Abwender DA, Ewell KK, Beery SH. Social anxiety and peer relationships in early adolescence: A prospective analysis. Journal of Clinical Child Psychology. 1992;21:189–196. [Google Scholar]
- Whisman MA, Richardson ED, Smolen A. Behavioral inhibition and triallelic genotyping of the serotonin transporter promoter (5-HTTLPR) polymorphism. Journal of Research in Personality. 2011;45:706–709. http://dx.doi.org/10.1016/j.jrp.2011.08.009. [Google Scholar]