Abstract
Background
Despite its importance as a public health concern, relatively little is known about the natural course of cannabis use disorders (CUDs). The primary objective of this research is to provide descriptive data on the onset, recovery, and recurrence functions of CUDs during the high-risk periods of adolescence, emerging adulthood, and young adulthood based on data from a large prospective community sample.
Methods
Probands (N = 816) from the Oregon Adolescent Depression Project (OADP) participated in four diagnostic assessments (T1 – T4) between ages 16 and 30, during which current and past CUDs were assessed.
Results
The weighted lifetime prevalence of CUDs was 19.1% with an average onset age of 18.6 years. Although gender was not significantly related to age of initial CUD onset, men were more likely to be diagnosed with a lifetime CUD. Of those diagnosed with a CUD episode, 81.8% eventually achieved recovery during the study period. Women achieved recovery significantly more quickly than men. The recurrence rate (27.7%) was relatively modest, and most likely to occur within the first 36 months following the offset of the first CUD episode. CUD recurrence was uncommon after 72 months of remission and recovery.
Conclusion
CUDs are relatively common, affecting about 1 out of 5 persons in the OADP sample prior to age 30. Eventual recovery from index CUD episodes is the norm, although about 30% of those with a CUD exhibit a generally persistent pattern of problematic use extending 7 years or longer.
Keywords: Cannabis use disorders, marijuana, natural course, onset, recovery, recurrence, gender differences
Introduction
In many countries cannabis is the most widely used illicit drug (Copeland & Swift, 2009). In the United States, cross-sectional studies suggest that adolescence and early adulthood are particularly critical developmental periods for the initiation of cannabis use and the development of cannabis use disorders (CUDs; defined as a diagnosis of cannabis abuse or dependence disorders). Findings from the 2012 Monitoring the Future Survey (Johnston et al., 2013), for example, indicated that 15% and 45% of 8th and 12th grade youth in the U.S., respectively, have used cannabis. The National Survey on Drug Use and Health (Substance Abuse and Mental Health Services Administration, 2012) documented that in 2011, 2.6 million U.S. residents aged 12 years or older initiated cannabis use, with most initiates (57.7%) younger than age 18. There were an estimated 18.1 million past year cannabis users aged 12 years or older during 2011, or about 7% of the general U.S. population. In this same year, 1.6% of the U.S. population (4.2 million persons) was estimated to have met criteria for cannabis abuse or dependence. Despite indications that rates of frequent cannabis users among U.S. adolescents are among the highest worldwide (ter Bogt et al., 2006), relatively little is known about the natural development and course of CUDs in the U.S.
Limited international and domestic longitudinal research with community samples indicate that cannabis initiation, experimentation, frequent use, and CUD emergence are most likely between the ages of 15 and 24 (Boden et al., 2006; Brook et al., 1999; Chen & Kandel, 1995; Cohen et al., 1993; Perkonigg et al., 2008; Poulton et al., 2001; Roxburgh et al., 2010). Most individuals who try cannabis, however, either cease use altogether within a short period following initiation or remain occasional users (Brook et al., 2011b; Flory et al., 2004; Lynskey et al., 2006; Perkonigg et al., 2008; Windle & Wiesner, 2004). Others, however, increase their usage with age or maintain frequent or heavy use (Brook et al., 2011b; Calabria et al., 2010; Newcomb et al., 2002; Perkonigg et al., 2008). Estimates from community-based prospective samples from Australasia and Europe suggest that 10% to 21% of adolescents are at risk for developing a CUD by early adulthood (Fergusson & Horwood, 2000; Perkonigg et al., 2008; Moffitt et al., 2010), although actual percentages are likely higher given the reluctance of some individuals to answer questions about illicit drug use (Perkonigg et al., 2008). Findings reported from different geographic regions are mixed as to whether CUD prevalence rates decline or remain stable between late adolescence and the mid-20s (Calabria et al., 2010; Newcomb et al., 2002; Perkonigg et al., 2008; Poulton et al., 2001).
Despite the importance of CUDs as a public health concern, important gaps in knowledge remain concerning the development and course of CUDs in the general population. To address these gaps, the present research provides descriptive data on the natural course of CUDs based on data collected as part of the Oregon Adolescent Depression Project (OADP; Lewinsohn et al., 1993), a longitudinal study of a community-based cohort. Specifically, we report the first incidence and prevalence (point, period, and lifetime) of CUDs from childhood to age 30.0, which encompasses the developmental periods within which cannabis initiation, problematic use, and cessation of problematic use are most common (Chen & Kandel, 1995). We also report data on time to recovery from the index CUD episode and time to CUD recurrence. Outcomes from these analyses are expected to highlight developmental periods within which the risk for CUD onset and recurrence are especially high, as well as threshold points by which initial recovery is likely to be sustained. Because gender differences in cannabis use, abuse, and dependence often emerge during late adolescence and early adulthood, with males tending to use more frequently than females (Brook et al., 1999; Coffey et al., 2003; Griffith-Lendering et al., 2012; Kandel & Chen, 2000; Perkonigg et al., 2008; Poulton et al., 2001), we also evaluate possible gender differences in onset, recovery, and recurrence functions.
Method
Participants
Current and past DSM-defined cannabis abuse or dependence and other psychiatric diagnostic categories were assessed with OADP probands on four occasions between the ages of 16 and 30 (T1 though T4). The T1 sample (initiated between 1987 and 1989; n = 1,709; M age = 16.6, SD = 1.2) was randomly drawn from 9 high schools in 2 urban and 3 rural communities in western Oregon, and subsequently found to be representative of the regional population from which it was drawn (Lewinsohn et al., 1993). One year following T1, T2 was initiated, and 1,507 (88%) probands were reassessed.
At T3, which was initiated about 7 years after T2, a stratified sampling procedure was implemented whereby eligible participants included all persons with a positive history of a substance abuse or psychiatric diagnosis by T2 (n = 644) and a randomly selected subset of never mentally ill (NMI) probands (n = 457 of 863 persons). Of these 1,101 eligible persons, 941 (85%) completed T3. Comparisons between the T3 NMI participants who were randomly selected for further participation with unselected NMI probands revealed no significant differences with respect to T2 data. Of the 941 T3 probands, 816 (87%) participated in T4 about 6 years after T3 (59% female, 89% White, 53% married).
In our recent analysis of proband attrition across waves (Farmer et al., 2013), we compared the T4 panel to those who dropped out from the study after T1 with respect to psychiatric history (i.e., any lifetime DSM-defined disorder diagnosis) and the cumulative number of lifetime psychiatric disorders at T1. The T4 panel was not statistically different from the attrition group with respect to positive psychiatric histories (p = .96) or the cumulative number of lifetime disorders (p = .23) at T1. Similarly, when we performed an attrition analysis based exclusively on CUDs for this report, those in the attrition group, when compared to the T4 panel, did not have significantly higher rates of CUDs at T1 (8% vs. 7%, respectively; Pearson χ2 [1, n = 1299] = 0.39, p = .532). Wave-to-wave analyses, however, revealed one significant difference: discontinuation from T3 to T4 was more common among those with a history of a CUD by T3 (18% for discontinuation group vs. 12% for those who participated in T4; p = .03). Given the sample stratification procedures implemented at T3, the relatively modest attrition over successive waves, and evidence that analyses based on T3 and T4 panels produced highly similar outcomes,1 results presented in the following section are based on the T4 panel (N = 816).
Assessment of CUDs
During T1, T2, and T3, participants were interviewed with a version of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) that combined features of the Epidemiologic and Present Episode versions (Chambers et al., 1985; Orvaschel et al., 1982). Follow-up psychiatric disorder assessments at T2 and T3 also involved the joint administration of the Longitudinal Interval Follow-Up Evaluation (LIFE; Keller et al., 1987) that, in conjunction with the K-SADS, provided detailed information related to the presence and course of disorders since participation in the previous diagnostic interview. The T4 assessment included administration of the LIFE and the Structured Clinical Interview for Axis I DSM-IV Disorders–Non-Patient Edition (SCID-NP; First et al., 1994). Symptom reports related to cannabis use were evaluated in accordance with DSM-III-R diagnostic criteria (American Psychiatric Association, 1987) at T1 and T2 and DSM-IV diagnostic criteria (American Psychiatric Association, 1994) at T3 and T4.
DSM-III-R and DSM-IV hierarchically arrange substance use disorders into abuse and dependence categories, whereby dependence takes precedence over abuse when criteria for both conditions are satisfied. This hierarchical taxonomic approach has been challenged by data that fail to support the cannabis abuse/dependence distinction as operationalized in DSM (Beseler & Hasin, 2010; Blanco et al., 2007; Hartman et al., 2008; Langenbucher et al., 2004). This hierarchical organization has also been discontinued in DSM-5 (American Psychiatric Association, 2013) in favor of a single “use disorder” category. Consequently, for the analyses described below, we combine cannabis abuse and dependence diagnoses into a single category (cannabis use disorders, or CUDs) to indicate problematic cannabis use that has resulted in a symptomatic presentation coupled with significant impairment in functioning that rises to the threshold of diagnosis and, consequently, warrants clinical attention.
All interviews were recorded and randomly selected for reliability assessments by a second interviewer. Interrater reliability was indexed by kappa (κ). Diagnostic agreement among raters for CUD diagnoses since the previous interview was good to excellent (κs: T1 = .72, T2 = .93, T3 = .83, T4 = .82).
CUD recovery and recurrence
Definitions of recovery and recurrence in the present research are informed by previous conceptualizations of these concepts (Chung & Maisto, 2006; Frank et al. 1991), LIFE interview naming conventions (Keller et al., 1987), and by guidelines provided in DSM-IV (American Psychiatric Association, 1994). Given our emphasis on the natural course of disorders rather than symptoms, and the elimination of the abuse/dependence distinction with respect to CUDs in DSM-5 (American Psychiatric Association, 2013), we applied the following definitions regardless as to whether the index episode was cannabis abuse or cannabis dependence. Remission as used here refers to offset of an initial CUD episode lasting at least 1 full month but less than 12 months during which the individual no longer meets diagnostic criteria for the index CUD episode but may continue to use cannabis at subthreshold levels. The re-emergence of an index CUD episode during the remission period is regarded as a continuation of the index episode (i.e., a relapse). The resolution of the index episode, defined as a period of uninterrupted remission lasting at least 12 months, is regarded as a recovery from the index episode. Recovery is only achieved after a 12-month period following the sustained offset of the index CUD episode, during which there is no relapse of the index episode. A recurrence is regarded as a new CUD episode after a period of recovery.
Statistical analyses
Because of the unequal stratified sampling strategy implemented at T3, weighting procedures were used to estimate prevalence rates, incidence rates, and odds ratios (ORs). Time-to-event analyses were implemented using SUDAAN statistical software, and standard errors were estimated using the Taylor series linearization method to appropriately account for the unequal stratified sampling procedure implemented at T3. In the analyses that follow, rate, ratio, and proportion values are based on weighted data.
Potential gender moderation of the time-to-event functions was tested using Cox proportional hazards (PH) models. An assumption of Cox PH models is the absence of a significant time–by–predictor interaction. Consistent with recommendations (Singer & Willett, 1991), we initially included a time-by-gender interaction term in the model. Subsequent findings indicated that in no instance was the interaction term statistically significant. Given that this assumption of the proportional hazards model was met, the interaction term was removed and the models rerun, with data from these analyses reported. Hazard ratio (HR) estimates, which index differences in onset curves as a function of gender, were calculated along with 95% confidence intervals. Cumulative hazard functions were used to describe CUD onset, recovery, and recurrence functions in the presence of censorship (i.e., participants who do not experience a CUD onset, recovery or recurrence during the observation period). Time-to-event was measured in months. In instances where the cumulative hazard functions exceeded 0.5, the median survival time was reported to facilitate data interpretation. The demarcation of age ranges in the reporting of first incidence and period prevalence rates was based on a developmental framework outlined by Arnett (2007). Within this framework, developmental periods analyzed were childhood through emerging adolescence (childhood to age 13.9), adolescence (14.0 to 17.9), adolescence transitioning to emerging adulthood (18.0 to 24.9), and emerging adulthood transitioning into young adulthood (25.0 to 30.0).
Results
Prevalence rates, incidence rates, and age of onset for index CUD episodes
Prevalence and incidence rates
The weighted lifetime prevalence of CUD from childhood to age 30.0 in the OADP sample was 19.1%. Men (22.5% of T4 male proband sample) were more likely than women (16.4% of T4 female proband sample) to be diagnosed with a lifetime CUD (Likelihood Ratio [LR] χ2 [1, n = 816] = 4.74, p = .030, OR [CI95] = 1.48 [1.04, 2.09]).
Weighted first incidence and period prevalence rates, presented in Table 1, highlight age ranges during which CUD risk is greatest. Findings presented in this table highlight the significance of ages 14.0 to 24.9 as a period of exceptional risk for initial CUD onset. This risk, however, is substantially diminished after age 25. Ages 18.0 to 24.9 additionally correspond to a period where the prevalence of CUDs reaches its peak.
Table 1.
Natural Course of Cannabis Use Disorders from Childhood to Age 30: Gender Comparisons
| Female Probands % [CI95] | Male Probands % [CI95] | OR [CI95] | |
|---|---|---|---|
| Lifetime prevalence | 16.4 [13.1, 19.7] | 22.5 [18.2, 26.8] | 1.48 [1.04, 2.09] |
| First incidence | |||
| 0.0 to 13.9 years | 1.9 [0.7, 3.1] | 2.4 [0.8, 4.0] | 1.29 [0.50, 3.36] |
| 14.0 to 17.9 years | 6.0 [3.8, 8.2] | 7.6 [4.9, 10.3] | 1.30 [0.75, 2.25] |
| 18.0 to 24.9 years | 7.1 [4.7, 9.5] | 11.6 [8.3, 14.9] | 1.71 [1.06, 2.77] |
| 25.0 to 30.0 years | 1.4 [0.2, 2.6] | 0.8 [0.0, 1.8] | 0.56 [0.14, 2.26] |
| Period prevalence | |||
| 14.0 to 17.9 years | 7.2 [4.8, 9.6] | 9.9 [6.8, 13.0] | 1.40 [0.85, 2.30] |
| 18.0 to 24.9 years | 11.4 [8.5, 14.3] | 19.0 [14.9, 23.1] | 1.83 [1.24, 2.71] |
| 25.0 to 30.0 years | 5.7 [3.5, 7.9] | 11.9 [8.6, 15.2] | 2.25 [1.35, 3.74] |
| Point prevalence | |||
| T1 (~ age 16) | 1.1 [0.1, 2.1] | 2.0 [0.6, 3.4] | 1.84 [0.58, 5.83] |
| T2 (~ age 17) | 0.6 [0.0, 1.4] | 1.8 [0.4, 3.2] | 2.90 [0.72, 11.72] |
| T3 (~ age 24) | 2.1 [0.7, 3.5] | 6.7 [4.2, 9.2] | 3.42 [1.59, 7.36] |
| T4 (~ age 30) | 2.8 [1.2, 4.4] | 7.3 [4.6, 10.0] | 2.68 [1.36, 5.29] |
| Recovery rates | 86.5 [78.7, 94.3] | 77.4 [68.4, 86.4] | 0.54 [0.23, 1.25] |
| Recurrence rates for those who recovered | 25.4 [14.8, 36.0] | 30.0 [18.6, 41.4] | 1.26 [0.57, 2.74] |
Note. CI95 = 95% confidence interval. OR = Odds ratio. Bolded ORs are statistically significant. All summary statistics account for sample weighting.
Table 1 also includes ORs to illustrate how first incidence and prevalence rates for CUDs differ by gender. First incidence rates significantly differed by gender within the 18.0 to 24.9 period only (11.6% of males compared to 7.1% of females; LR χ2 [1, n = 816] = 4.84, p = .024, OR [CI95] = 1.71 [1.06, 2.77]). Period prevalence rates also significantly differed by gender within the 18.0 to 24.9 developmental period (19.0% of males compared to 11.4% of females; LR χ2 [1, n = 816] = 9.34, p = .002, OR [CI95] = 1.83 [1.24, 2.71]), and within the 25.0 to 30.0 period as well (11.9% of males compared to 5.7% of females; LR χ2 [1, n = 816] = 10.14, p = .001, OR [CI95] = 2.25 [1.35, 3.74]). Similarly, point prevalence rates significantly differed by gender at T3 (6.7% of males compared to 2.1% of females; LR χ2 [1, n = 816] = 11.09, p < .001, OR [CI95] = 3.42 [1.59, 7.36] and T4 (7.3% of males compared to 2.8% of females; LR χ2 [1, n = 816] = 8.66, p = .003, OR [CI95] = 2.68 [1.36, 5.29]).
Time to CUD onset
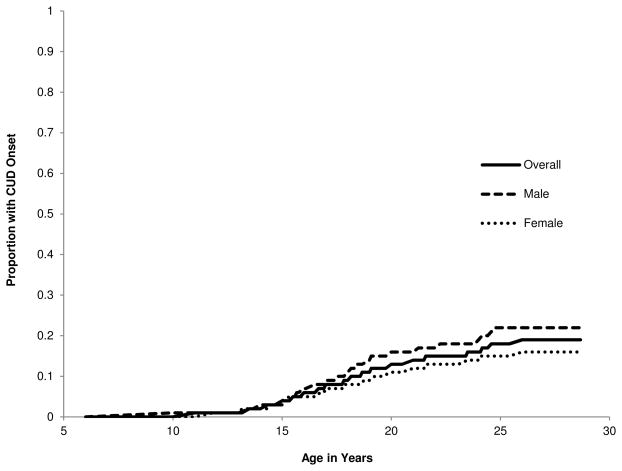
For those with a lifetime CUD diagnosis, the average age of onset for the first episode was 18.6 years (SD = 4.2), which did not differ by gender (observed Ms: males = 18.6, SD = 4.1; females = 18.7, SD = 4.3; t[153] = 0.13, p = .901). Cumulative hazard functions for CUD onset for the combined sample and separately by gender are presented in Fig. 1. The cumulative hazard functions significantly differed by gender (HR [CI95] = 1.42, [1.03, 1.95], p = .033).
Fig. 1.
Cumulative Hazard Functions for CUD Onset by Age in Years
Recovery following the index CUD episode
Rates of recovery
Among the persons with a lifetime CUD, 81.8% experienced recovery from the index CUD episode by age 30. Rates of recovery did not differ by gender (77.4% of males and 86.5% of females recovered; LR χ2 [1, n = 155] = 2.17, p = .141, OR [CI95] = 0.54 [0.23, 1.25]).
Time to recovery
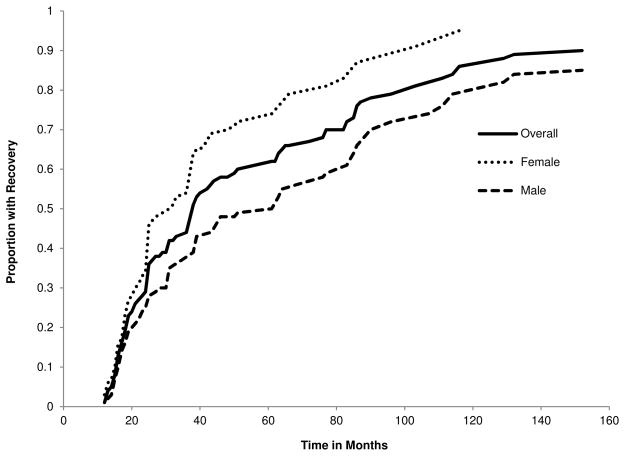
Among those who recovered from the index CUD episode, the mean duration of the index CUD episode was 32.5 months (SD = 35.6), which significantly differed with respect to gender (males = 41.2 months, SD = 42.7; females = 24.2 months, SD = 24.8; t[125] = −2.77, p = .006). Time to recovery is based on the full duration of the index CUD episode plus a 12-month period of sustained remission following episode offset, during which CUD symptomatology do not again rise to the level of diagnosis. If, for example, an individual met CUD criteria for 14 consecutive months and did not again meet criteria for a CUD in the 12 months following the offset of the index episode, the time to recovery would be 26 months. Participants that did not experience 12-months of sustained remission for their index CUD episode prior to the T4 diagnostic interview were right censored from the survival analysis. Cumulative hazard functions for recovery from a CUD episode for the complete subsample with a lifetime diagnosis and separately by gender are presented in Fig. 2. Hazard rates were estimated for recovery in 1-month intervals commencing with the onset of the first CUD episode. The cumulative hazard functions that assessed time to recovery from the initial CUD episode significantly differed by gender (HR [CI95] = 0.57, [0.39, 0.82], p = .003). The median recovery time (i.e., survival time) was 61 months for males and 31 months for females.
Fig. 2.
Cumulative Hazard Functions for CUD Recovery by Time since Disorder Onset
Recurrence following the first CUD episode
Recurrence rates following a period of recovery
Of the participants who recovered from their index CUD episode, 27.7% developed another CUD episode before age 30. Recurrence rates were not significantly different between male and female probands (30.0% of males, 25.4% of females; LR χ2 [1, n = 127] = 0.33, p = .564, OR [CI95] = 1.26 [0.57, 2.74]).
Time to recurrence
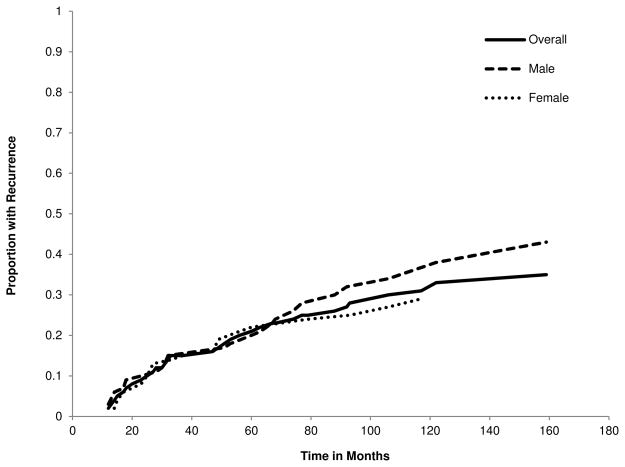
Among those with a second CUD, the mean time to recurrence was 46.1 months (SD = 35.6), which did not significantly differ with respect to gender (males = 51.9 months, SD = 39.0; females = 40.1 months, SD = 31.6; t[35] = −1.00, p = .326). Cumulative hazard functions for recurrence for the complete subsample with CUD recovery and separately by gender are presented in Fig. 3. Hazard rates were estimated for recurrence in 1-month intervals, with recurrence defined as a second CUD episode occurring after a period of at least 12 months of remission from the index CUD episode. The cumulative hazard functions that assessed time to recurrence did not differ by gender (HR [CI95] = 1.21, [0.66 – 2.22], p = .539). Data presented in Fig. 3 indicate that the highest rates of recurrence occurred within 24 months after recovery. CUD recurrence for females is rare after the 60th consecutive month since offset of the initial episode. For males, however, there is no clear recovery threshold evident within the interval surveyed. Overall, there is little support from Fig. 3 that a disorder-free period of 12 months is an optimal threshold for denoting recovery from CUDs.
Fig. 3.
Cumulative Hazard Functions for CUD Recurrence by Time since Initial Disorder Offset
Discussion
Although prevalence of cannabis initiation and frequency of use have been well-documented in adolescent and young adult samples (e.g., Johnston et al., 2013), comparatively little is known about the course of prolonged cannabis use that rises to the threshold of a CUD diagnosis. Previous research indicates that the progression from cannabis experimentation or use to CUD is comparatively rare (Chen et al., 2005; Wittchen et al., 2007). In the current study, the weighted lifetime prevalence of a CUD from childhood to age 30 was 19.1%. Consistent with findings from other prospective community samples (Coffey et al., 2003; Perkonigg et al., 2008), men were 1.5 times more likely than women to be diagnosed with a lifetime CUD. Findings further indicate that initial CUD incidence and period prevalence rates peaked between the ages of 18 and 25 years for both men and women, and declined sharply thereafter. Point prevalence rates, however, were highest at T4 (~ age 30), and mostly influenced by individuals who exhibited a more chronic course.
Although cannabis use appears to be quite stable during the adolescent, emerging adulthood, and young adulthood developmental periods (Perkonigg et al., 2008), data presented here indicate that there are moderate rates of both cessation and persistence of CUDs across these same periods. Whereas 54% of those with an index CUD episode fully recovered without a subsequent recurrence by age 30, the remaining 46% never recovered, remitted less than 12 months prior to the end of the study, or recovered only to experience a subsequent recurrence. When lifetime CUD rates for female and male probands (16.4% and 22.5%, respectively) are compared with CUD point prevalence rates at T4 (~ age 30; 2.8% and 7.3%, respectively), however, it is apparent that a majority of individuals, especially women, who develop CUDs during the developmental periods studied recovered by age 30. Rather than a chronic and relapsing condition, CUDs for many appear to be developmentally limited (see also Flory et al., 2004; Lynskey et al., 2006; Windle & Wiesner, 2004).
Although total recovery rates did not significantly differ between men and women, time to CUD recovery was significantly more rapid for women than men. In DSM-5 (American Psychiatric Association, 2013), the “recovery” course specifier is not used. Instead, the interval following CUD offset is specified as “early remission” or “sustained remission,” with the main distinction being the timeframe within which CUD-defining criteria are absent after disorder offset (> 3 months but < 12 months versus ≥ 12 months, respectively). Sustained remission in DSM-5 is analogous the concept of recovery used in the present research, with the main difference being the emphasis placed on the absence of all CUD-defining criteria except craving (DSM-5) versus the absence of a symptom presentation that rises to the threshold of diagnosis (present study). Remission and recovery functions in the current research did not reveal abrupt discontinuities or sudden shifts in hazard functions over time; therefore, at the CUD disorder-level of specification, a continuum of behavior change processes rather than distinct recovery stages also appears to be evident. Future refinements in the terminology used to describe the course of CUDs should jointly consider not only disorder thresholds and time since disorder offset, but also the degree of subthreshold symptomatology evident during the remission and recovery periods, the frequency or quantity of cannabis use, and the associated level of functional impairment (cf. Rush et al., 2006).
Rates of recurrence did not significantly differ by gender, and were relatively uncommon following the offset of the index CUD episode, occurring in slightly more than one-quarter of participants with CUD who recovered. Recurrences, when observed, were most likely to occur within 36 months following offset of the first CUD episode, and were relatively rare after 60 months. Based on their comprehensive review of the natural recovery literature, Sobell and colleagues (Sobell et al., 2000) recommended a period of at least 5 years as the minimal threshold for a recovery designation given accumulated findings which indicate that recovery processes usually stabilize by this time and that subsequent recurrence is uncommon. Findings from the present research are consistent with this recommendation.
Although the incidence and point prevalence of CUDs peak during the emerging adulthood period, developmental pathways for CUDs and other forms of substance abuse likely emerge long before problematic cannabis use begins (Clark, 2004; Zucker et al., 2008). Furthermore, significantly lower lifetime prevalence rates of CUDs for women compared to men, coupled with significantly quicker times to recovery among women, suggest possible gender-related risk mechanisms associated with CUD onset and offset. Cannabis use and CUDs are heritable (Agrawal & Lynskey, 2006), and associated with latent liabilities for externalizing disorders (which include attention-deficit hyperactivity disorder, oppositional defiant disorder, conduct disorder, adult antisocial behavior, and other substance use disorders; see Farmer et al., 2009, and Krueger & Markon, 2006). Externalizing disorders and associated liabilities are also more commonly observed among males (e.g., Hicks et al., 2007; Kessler et al., 2005) and are heritable (Hicks et al., 2004; Young et al., 2000). The extent to which transmitted risk factors are specific to CUDs versus broad temperamental factors inclusive of CUDs, or the extent to which these mechanisms are gender-related, requires additional study.
There are a few noteworthy study limitations that must be considered in conjunction with findings from this research. First, the ethnic diversity of the OADP sample, although representative of the ethnic distribution of western Oregon, is limited. A majority of the sample (89%) was Caucasian. Some studies (e.g., Brook et al., 2011a) suggest that rates of CUDs might vary considerably as a joint function of race and gender. Second, this research was conducted with a community sample of western Oregon youth. Cannabis is probably more readily available in the Pacific Northwest region compared to other U.S. regions, and cannabis availability has been associated with an increased risk for cannabis initiation and abuse (Gillespie et al., 2009). The lifetime prevalence rates of CUDs reported here, however, are generally consistent with findings reported in international prospective samples (Fergusson & Horwood, 2000; Moffitt et al., 2010; Perkonigg et al., 2008). Third, there was sample attrition across assessment waves, and it is possible that this attrition may have biased some findings. Analyses to determine whether distributions of CUDs at T1 differed between the attrition group and the reference sample revealed no evidence of selective bias based on adolescent CUD history. Wave-to-wave attrition analyses, however, indicated a significantly higher rate of attrition between T3 and T4 for those with a CUD history by T3. Parallel analyses to those reported here were conducted with the T3 panel, and few differences were noted in the findings observed (see Footnote 1). Fourth, data collection and diagnostic coding procedures adopted for this study precluded us from estimating CUD course transition rates (i.e., recovery and recurrence rates) for time intervals less than those specified (e.g., rate comparisons when recovery was defined as an uninterrupted remission lasting at least 6 months versus 12 months). Cumulative hazard functions (Figs. 2 and 3), however, provide information about the implications for rate data when recovery and recurrence transition points are extended beyond this study’s definitional parameters.
To illuminate possible mechanisms that underlie CUD onset, maintenance, recovery, and recurrence processes, future research might examine the predictive value of proximal and distal factors associated with each of these events. Additionally, as suggested by limited longitudinal research (Brook et al., 2011b; Flory et al., 2004; Kandel & Chen, 2000; Windle & Wiesner, 2004, Wittchen et al., 2009), individuals who currently or historically met criteria for a CUD might be quite heterogeneous along a number of important dimensions. To clarify the heterogeneity among those with CUDs, future research might attempt to identify distinct developmental trajectories based on patterns of cannabis use or abuse over time, and evaluate the extent to which the resultant trajectories overlap with those associated with other forms of substance use (e.g., alcohol use, abuse of other illicit drugs). Although each substance appears to have a unique developmental trajectory (Rohde & Andrews, 2006), it might be that the distinctiveness of trajectories associated with different substances is diminished among more problematic users.
Acknowledgments
Grant support. National Institutes of Health grants MH40501, MH50522, and DA12951 to Peter M. Lewinsohn and DA032659 to Richard F. Farmer and John R. Seeley supported this research.
Footnotes
To evaluate if differential rates of attrition between T3 and T4 for those with a lifetime CUD by T3 had an effect on the conclusions reached in the present research, we repeated the analyses presented in the Results section with the T3 panel (n = 941). Only modest differences were observed between samples, and in only two instances did non-significant findings for the T4 panel emerge as significant in the T3 panel. These exceptions were noted in gender comparisons for the first incidence and period prevalence rates for ages 14.0 through 17.9. When rates based on T3 and T4 panel data were compared for this age interval, male probands demonstrated higher CUD first incidence and period prevalence rates in the T3 panel compared to the T4 panel (9.3% versus 7.6% for first incidence, 11.5% versus 9.9% for period prevalence, respectively). These higher rates for males resulted in statistically significant odds ratio comparisons between female and male probands for this age interval when based on T3 panel data (first incidence: OR [CI95] = 1.70 [1.04, 2.80]; period prevalence: OR [CI95] = 1.71 [1.09, 2.68], with males having higher rates of lifetime CUDs than females in each instance.
Conflict of interest. None.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–812. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, D.C: Author; 1987. revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Arnett JJ. Emerging adulthood: What is it, and what is it good for? Child Development Perspectives. 2007;1:68–73. [Google Scholar]
- Beseler CL, Hasin DS. Cannabis dimensionality: Dependence, abuse and consumption. Addictive Behaviors. 2010;35:961–969. doi: 10.1016/j.addbeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Harford TC, Nunes E, Grant B, Hasin D. The latent structure of marijuana and cocaine use disorders: Results from the National Longitudinal Alcohol Epidemiologic Survey (NLAES) Drug and Alcohol Dependence. 2007;91:91–96. doi: 10.1016/j.drugalcdep.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ. Illicit drug use and dependence in a New Zealand birth cohort. Australian and New Zealand Journal of Psychiatry. 2006;40:156–163. doi: 10.1080/j.1440-1614.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- Brook JS, Kessler RC, Cohen P. The onset of marijuana use from preadolescence and early adolescence to young adulthood. Development and Psychopathology. 1999;11:901–914. doi: 10.1017/s0954579499002370. [DOI] [PubMed] [Google Scholar]
- Brook JS, Lee JY, Finch SJ, Koppel J, Brook DW. Psychosocial factors related to cannabis use disorders. Substance Abuse. 2011a;32:242–251. doi: 10.1080/08897077.2011.605696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Zhang C, Brook DW. Antisocial behavior at age 37: Developmental trajectories of marijuana use extending from adolescence to adulthood. American Journal on Addictions. 2011b;20:509–515. doi: 10.1111/j.1521-0391.2011.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria B, Degenhardt L, Briegleb C, Vos T, Hall W, Lynskey M, Callaghan B, Rana U, McLaren J. Systematic review of prospective studies investigating “remission” from amphetamine, cannabis, cocaine or opioid dependence. Addictive Behaviors. 2010;35:741–749. doi: 10.1016/j.addbeh.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview: Test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug and Alcohol Dependence. 2005;79:11–22. doi: 10.1016/j.drugalcdep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Maisto SA. Relapse to alcohol and other drug use in treated adolescents: Review and reconsideration of relapse as a change point in clinical course. Clinical Psychology Review. 2006;26:149–161. doi: 10.1016/j.cpr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Clark DB. The natural history of adolescent alcohol use. Addiction. 2004;99 (Suppl 2):5–22. doi: 10.1111/j.1360-0443.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey MT, Sanci LA, Patton GC. Cannabis dependence in young adults: An Australian population-based study. Addiction. 2002;97:187–94. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Lynskey MT, Li N, Patton GC. Adolescent precursors of cannabis dependence: Findings from the Victorian Adolescent Health cohort Study. British Journal of Psychiatry. 2003;182:330–336. doi: 10.1192/bjp.182.4.330. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, Rojas M, Brook J, Streuning EL. An epidemiological study of disorders in late childhood and adolescence: Age- and gender-specific prevalence. Journal of Child Psychology and Psychiatry. 1993;34:851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. Journal of the American Medical Association. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W. Cannabis use disorder: Epidemiology and management. International Review of Psychiatry. 2009;21:96–103. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Kosty DB, Seeley JR, Olino TM, Lewinsohn PM. Aggregation of lifetime axis I psychiatric disorders through age 30: Incidence, predictors, and associated psychosocial outcomes. Journal of Abnormal Psychology. 2013;122:573–586. doi: 10.1037/a0031429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR, Kosty DB, Lewinsohn PM. Refinements in the hierarchical structure of externalizing psychiatric disorders: Patterns of lifetime liability from mid-adolescence through early adulthood. Journal of Abnormal Psychology. 2009;118:699–710. doi: 10.1037/a0017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Cannabis use and dependence in a New Zealand birth cohort. New Zealand Medical Journal. 2000;113:156–158. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders–Non-Patient Edition. New York: Biometrics Research Department; 1994. [Google Scholar]
- Flory K, Lynam D, Milich R, Leukefeld C, Clayton R. Early adolescent through young adult alcohol and marijuana use trajectories: Early predictors, young adult outcomes, and predictive utility. Development and Psychopathology. 2004;16:193–213. doi: 10.1017/s0954579404044475. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith-Lendering MF, Wigman JT, Prince van Leeuwen A, Huijbregts SC, Huizink AC, Ormel J, Verhulst FC, van Os J, Swaab H, Vollebergh WAM. Cannabis use and vulnerability for psychosis in early adolescence—A TRAILS study. Addiction. 2012;108:733–740. doi: 10.1111/add.12050. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Gelhorn H, Crowley TJ, Sakai JT, Stallings M, Young SE, Rhee SH, Corley R, Hewitt JK, Hopfer CJ. An item response theory analysis of DSM-IV Cannabis abuse and dependence criteria in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:165–173. doi: 10.1097/chi.0b013e31815cd9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. The family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2012: Volume I, Secondary school students. Ann Arbor: Institute for Social Research, University of Michigan; 2013. [Google Scholar]
- Kandel DB, Chen K. Types of marijuana users by longitudinal course. Journal of Studies in Alcohol. 2000;61:367–378. doi: 10.15288/jsa.2000.61.367. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott PA. The Longitudinal Interval Follow-up Evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM–IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, Chung T. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. Journal of Abnormal Psychology. 2004;113:72–80. doi: 10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Grant JD, Nelson EC, Bucholz KK, Madden PAF, Statham DJ, Martin NG, Heath AC. Duration of cannabis use — A novel phenotype? Addictive Behaviors. 2006;31:984–994. doi: 10.1016/j.addbeh.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Kokaua J, Milne BJ, Polanczyk G, Poulton R. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine. 2010;40:899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb MD, Galaif ER, Locke TF. Substance use diagnosis within a community sample of adults: Distinction, comorbidity, and progression over time. Professional Psychology: Research and Practice. 2001;32:239–247. [Google Scholar]
- Orvaschel H, Puig-Antich J, Chambers WJ, Tabrizi MA, Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-E. Journal of the American Academy of Child Psychiatry. 1982;21:392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Goodwin RD, Fiedler A, Behrendt S, Beesdo K, Lieb R, Wittchen H-U. The natural course of cannabis use, abuse, and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- Poulton R, Moffitt TE, Harrington H, Milne BJ, Caspi A. Persistence and perceived consequences of cannabis use and dependence among young adults: implications for policy. New Zealand Medical Journal. 2001;114(1145):544–547. [PubMed] [Google Scholar]
- Rohde P, Andrews J. Substance use disorders. In: Essau CA, editor. Child and adolescent psychopathology: Theoretical and clinical implications. New York: Routledge; 2006. pp. 184–220. [Google Scholar]
- Roxburgh A, Hall WD, Degenhardt L, McLaren J, Black E, Copeland J, Mattick RP. The epidemiology of cannabis use and cannabis-related harm in Australia 1993–2007. Addiction. 2010;105:1071–1079. doi: 10.1111/j.1360-0443.2010.02903.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Modeling the days of our lives: Using survival analysis when designing and analyzing longitudinal studies of duration and the timing of events. Psychological Bulletin. 1991;110:268–290. [Google Scholar]
- Sobell LC, Ellingstad TP, Sobell MB. Natural recovery from alcohol and drug problems: Methodological review of the research with suggestions for future directions. Addiction. 2000;95:749–764. doi: 10.1046/j.1360-0443.2000.95574911.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Author; 2012. (NSDUH Series H-44; HHS Publication No. (SMA) 12–4713) [Google Scholar]
- ter Bogt T, Schmid H, Nic Gabhainn S, Fotiou A, Vollebergh W. Economic and cultural correlates of cannabis use among mid-adolescents in 31 countries. Addiction. 2006;101:241–251. doi: 10.1111/j.1360-0443.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: Predictors and outcomes. Development and Psychopathology. 2004;16:1007–1027. doi: 10.1017/s0954579404040118. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Behrendt S, Höfler M, Perkonigg A, Rehm J, Lieb R, Beesdo K. A typology of cannabis-related problems among individuals with repeated illegal drug use in the first three decades of life: evidence for heterogeneity and different treatment needs. Drug and Alcohol Dependence. 2009;102:151–157. doi: 10.1016/j.drugalcdep.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Fröhlich C, Behrendt S, Günther A, Lieb R, Perkonigg A, Rehm J, Zimmermann P. Cannabis use and cannabis use disorders and their relationship to mental disorders: A 10-year prospective-longitudinal study in adolescents. Drug and Alcohol Dependence. 2007;88:60–70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics: Neuropsychiatric Genetics. 2000;96:684–695. [PubMed] [Google Scholar]
- Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121:S252–S272. doi: 10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]