Abstract
Background
Poor adherence to antiretroviral therapy contributes to pharmacokinetic variability and is the major determinant of virological failure. However, measuring treatment adherence is difficult, especially in children. We investigated the relationship between plasma lopinavir concentrations, pretreatment characteristics and viral load >400 copies/ml.
Methods
A total of 237 HIV-infected children aged 4–42 months on lopinavir/ritonavir oral solution were studied prospectively and followed for up to 52 weeks. Viral load and lopinavir concentration were measured at clinic visits 12, 24, 36 and 52 weeks after starting treatment. Cox multiple failure events models were used to estimate the crude and adjusted effect of lopinavir concentrations on the hazard of viral load >400 copies/ml.
Results
The median (IQR) pretreatment CD4+ T-lymphocyte percentage was 18.80% (12.70–25.35) and 53% of children had a pretreatment viral load >750,000 copies/ml. The median (IQR) weight-for-age and height-for-age z-scores were −2.17 (−3.35–−2.84) and −3.34 (−4.57–−3.41), respectively. Median (IQR) lopinavir concentrations were 8.00 mg/l (4.11–12.42) at median (IQR) 3.50 h (2.67–4.25) after the dose. The hazard of viral load >400 copies/ml was increased with lopinavir concentrations <1 mg/l versus ≥1 mg/l (adjusted hazard ratio 2.3 [95% CI 1.63, 3.26]) and lower height-for-age z-scores.
Conclusions
Low lopinavir concentrations (<1 mg/l) are associated with viraemia in children. This measure could be used as a proxy for adherence and to determine which children are more likely to fail.
Introduction
Approximately 20–50% of children on antiretroviral therapy (ART) do not achieve virological suppression during the first year of treatment [1–3]. Failure to achieve virological suppression may be due to the presence of HIV quasispecies resistant to antiretroviral drugs [4] or inadequate adherence, amongst other factors.
A first-line ART regimen, including ritonavir-boosted lopinavir (LPV/r), is recommended for children exposed to non-nucleoside reverse transcriptase inhibitors used for the prevention of mother-to-child transmission (PMTCT) of HIV [5,6]. LPV/r has a high barrier for the development of resistance. However, the oral suspension of LPV/r has poor palatability [7,8], which may result in poor adherence. Most children with virological failure on a first-line LPV/r regimen do not have protease inhibitor (PI) mutations, suggesting that adherence rather than resistance is the cause of failure [9]. Establishing that adherence rather than resistance as the reason for virological failure will reduce inappropriate ART switches and expenditure on resistance testing. In a small study of South African adults, low lopinavir concentrations were shown to be associated with virological failure [10]. However, wide inter-individual variability is observed in the concentrations of lopinavir even after observed doses and few data exist on the relationship between lopinavir concentrations and virological failure in children.
We measured lopinavir concentrations in plasma samples collected at the same time as viral load (VL) tests in a cohort of children initiated on a first-line LPV/r-based ART regimen and followed them prospectively to determine whether plasma lopinavir concentrations measured in the first 52 weeks after starting therapy are associated with virological response.
Methods
Study participants
Plasma lopinavir concentrations were retrospectively analysed in samples collected at clinic visits during the pre-randomization period from participants of the Neverest2 trial [11,12]. The Neverest2 trial was a randomized open-label clinical trial investigating treatment options for nevirapine-exposed children who initiated PI-based ART when <24 months of age. Treatment responses during the pre-randomization phase have been previously described [13]. The study population included HIV-infected children attending the Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa. Treatment eligibility criteria included WHO stage III or IV disease, CD4+ T-lymphocyte percentage (CD4%) of <25% if <12 months or <20% if >12 months of age, or recurrent (>2× yearly) or prolonged (>4 weeks) admission to hospital for HIV-related complications. Children being treated for opportunistic infections including tuberculosis were excluded from this analysis. All children received 230/57.5 mg/m2 LPV/r (Kaletra® oral solution, Abbott Laboratories, North Chicago, IL, USA), 1 mg/kg stavudine and 4 mg/kg lamivudine as oral solutions every 12 h. At each visit, drug doses were adjusted according to growth. The caregivers of the children were provided with comprehensive counselling about treatment adherence. Treatment doses were typically taken in the morning prior to the clinic visit. The time of dosing was as reported by the caregiver and the time of sample collection was recorded.
Data collected included age at starting LPV/r therapy, sex, pretreatment VL, pretreatment CD4% and WHO stage. Pretreatment weight-for-age z-score (WAZ) and height-for-age z-score (HAZ) were calculated using WHO software [14]. Blood samples were collected pretreatment and at clinic visits 12, 24, 36 and 52 weeks after starting treatment, and at unscheduled clinic visits. Caregivers were requested to return medication bottles at each visit. The bottles were weighed and the contents reconciled with the expected usage of each medication to determine the extent of adherence. Adherence was defined as returning <20% of the expected volume of any of the three drugs whereas returning >20% was defined as non-adherence. Children exited the pre-randomization phase of the study when they maintained viral suppression (VL≤400 copies/ml) for two consecutive visits, and were followed as part of the post-randomization study (not analysed here). Some children were retained for longer than the planned 52 weeks in an attempt to achieve viral suppression. These children were not eligible for randomization but were included in this analysis.
Laboratory methods
Plasma HIV-1 RNA measurement (Roche Amplicor assay version 1.5, Roche, Branchburg, NJ, USA; quantification range 400–750,000 copies/ml) and CD4+ T-cell counts were determined on pretreatment samples. The ultrasensitive assay (quantification range 50–150,000 copies/ml) was used for VL determination post ART initiation.
Plasma lopinavir concentrations were assayed using a validated mass spectrometry method developed in the Division of Clinical Pharmacology, University of Cape Town, Cape Town, South Africa. An AB Sciex 4000 mass spectrometer was operated at unit resolution in the multiple reaction monitoring mode. The assay was validated over the concentration range of 0.16–20 mg/l. Inter- and intra-day coefficients of variation were <10% for all quality control concentrations. The laboratory participates in the International Interlaboratory Control Program for Therapeutic Drug Monitoring in HIV Infection (KKGT; Hague, the Netherlands) and the AIDS Clinical Trial Group (ACTG) Pharmacology Quality Control Program.
Statistical analyses
Children with a pretreatment WAZ<−3 (that is, >3 sd below the average weight of comparable children in the reference population) were categorized as severely underweight; a WAZ from −3 to −2 was defined as moderate underweight and a WAZ >−2 was regarded as normal. A HAZ <−3, from −3 to −2 and >−2 were defined respectively as severe stunting, moderate stunting and normal. Pretreatment immunity was categorized as low (CD4%<25%) or high (CD4%≥25%). Pretreatment VL was expressed on a log scale and categorized as low or high for log10 VL>5 or ≤5, respectively. We defined WHO stages 1 and 2 as early disease and stages 3 and 4 as moderate disease. Lopinavir concentrations reported as below the limit of quantification (BLQ) were assigned a value of 0.08 mg/l (half the limit of quantification).
Pretreatment characteristics were described with summary statistics (median, IQR and proportions). Individual lopinavir concentrations during follow-up were presented by means of time-series plots and summary statistics. To account for missing data, 10 multiple imputations were conducted using the Amelia II software package [15] in R. We imputed 10 datasets for pretreatment WAZ, pretreatment HAZ, pretreatment CD4%, WHO stage, log10 pretreatment VL, adherence and lopinavir concentrations. The imputation model included all pretreatment (WAZ, HAZ, CD4%, WHO stage and VL) and follow-up (adherence and lopinavir concentration) variables, as well as time (weeks on treatment) and VL (≤400 or >400 copies/ml). All results of our multivariate analysis are based on the imputed datasets and combined using Rubin’s rules [16].
Cox proportional hazard regression modelling for multiple failure events was used to estimate the crude and adjusted hazard ratios (HR) of VL>400 copies/ml for the following pre-determined pretreatment and follow-up variables: age at starting ART, pretreatment WAZ and HAZ respectively, pretreatment log10 VL, pretreatment CD4%, pretreatment WHO stage and lopinavir concentrations. HRs are reported together with the 95% CI. In addition to the crude and adjusted HRs, we also present the HR obtained for a model, with variables selected by Akaike’s information criterion (AIC). We assumed the model to include log10 pretreatment VL and adjusted the AIC with inverse probability weights (AICw) due to missing data [17].
We modelled the effect of lopinavir concentration on the hazard of VL>400 copies/ml in the adjusted models as a dichotomous variable based on cutoffs of 1 mg/l and 4 mg/l. Additionally, we modelled the effect of lopinavir concentrations on VL>400 copies/ml non-linearly via penalized splines, representing this in a figure. Finally we compared two adjusted models by means of AICw: the first model included all pretreatment variables and lopinavir concentrations at each visit, whereas the second model also included all pretreatment variables and percentage adherence at each visit. Data was analysed using the statistical software package R [18].
Results
Study population
A total of 322 children exposed to nevirapine for PMTCT who met clinical and immunological criteria were enrolled into the study. All participants were initiated on an LPV/r-based regimen. Overall, 38 (12%) children died and 40 (12%) were lost to follow-up before samples were collected for lopinavir concentration measurement. In addition, 4 (2%) children on tuberculosis treatment and 3 (1%) on full-dose ritonavir (previously used instead of LPV/r for treatment of children <6 months of age) were excluded from this analysis. The sample size for analysis was thus reduced to 237 children. Table 1 shows the pretreatment characteristics of the study population.
Table 1.
Characteristics of the 237 HIV-infected children initiating LPV/r-based antiretroviral therapy and included in the analysis
| Variable | Value | Missing data, % |
|---|---|---|
| Age on starting ART, months | 10 (9–25.50) | 0 |
| Pretreatment viral load | 13 | |
| <100,000 copies/ml | 20 (8) | – |
| 100,000–750,000 copies/ml | 61 (26) | – |
| >750,000 copies/ml | 125 (53) | – |
| Pretreatment body weight, kg | 6.81 (5.32–7.90) | – |
| LPV/r dose, mg/m2 | 230/57.5 | – |
| Pretreatment WAZ | −2.17 (−3.35–−1.21) | 11 |
| Pretreatment HAZ | −3.34 (−4.57–−3.41) | 12 |
| Pretreatment CD4% | 18.80 (12.70–25.35) | 5 |
| Sex | 0 | |
| Male | 109 (46) | – |
| Female | 128 (54) | – |
| WHO stage | 18 | |
| Early | 42 (22) | – |
| Moderate | 147 (78) | – |
Data are median (IQR) or n (%). ART, antiretroviral therapy; CD4%, CD4+ T-cell percentage; HAZ, height-for-age z-score; LPV/r, ritonavir-boosted lopinavir; WAZ, weight-for-age z-score.
Plasma lopinavir concentrations
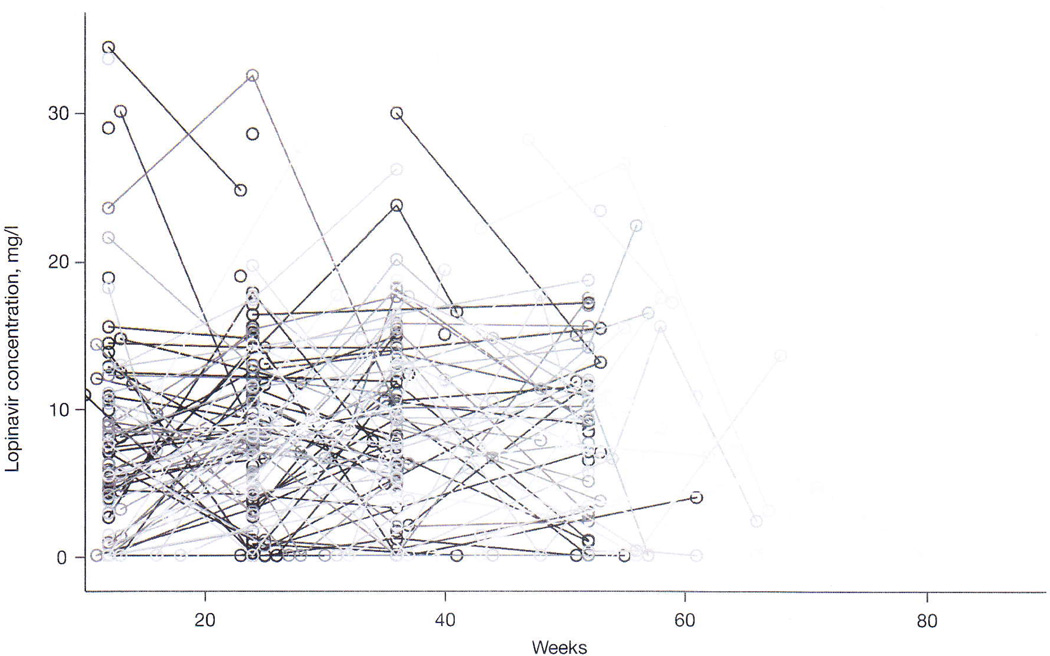
A total of 487 plasma samples from 237 children with a median number of 2 samples per child were analysed to determine plasma concentrations of lopinavir. The median (IQR) sampling time was 3.50 h (2.67–4.25) after the dosing time reported by the caregiver, and 12% of the samples were BLQ. Figure 1 presents plasma lopinavir concentrations of all children from 10 to 80 weeks of the study. We determined the population median lopinavir concentrations at each scheduled visit and found it to be similar for all visits. Sampling times after the dose were similar for samples <1 mg/l versus >1 mg/l (median [IQR] 3.37 h [2.60–4.42] versus 3.50 h [2.67–4.25]) and for samples <4 mg/l versus >4 mg/l (median [IQR] 3.33 h [2.58–4.17] versus 3.50 h [2.75–4.25]). The percentage of samples <1 mg/l and <4 mg/l at each clinic visit are shown in Figure 1 and Table 2.
Figure 1.
Lopinavir concentrations at scheduled visits for all children in the study
The lines connect the individual concentrations for each child.
Table 2.
Lopinavir concentrations, time of sampling, body weight and lopinavir dose, by study week
| Weeks |
|||||
|---|---|---|---|---|---|
| 12 (n=74) | 24 (n=132) | 36 (n=128) | 52 (n=100) | ≥52 (n=53) | |
| LPV, mg/l | 7.03 (3.36–10.95; 0.08–34.40) | 8.21 (4.18–12.20; 0.08–32.50) | 8.57 (4.97–13.00; 0.08–30.00) | 8.22 (3.12–12.00; 0.08–33.60) | 6.76 (2.59–13.20; 0.08–26.60) |
| Samples <1 mg/l, n (%) | 13 (18) | 16 (12) | 18 (14) | 16 (16) | 16 (30) |
| Samples <4 mg/l, n (%) | 17 (23) | 16 (17) | 23 (18) | 19 (19) | 16 (30) |
| Time after dose, h | 3.58 (3.00–4.17; 1.25–9.00) | 3.50 (2.50–4.17; 0.50–6.41) | 3.41 (2.58–4.25; 1.25–6.91) | 3.50 (2.75–4.42; 0.42–6.08) | 3.33 (2.37–4.23; 0.58–6.67) |
| Total body weight, kg | 8.38 (7.49–9.50; 4.20–12.50) | 9.25 (8.04–10.25; 4.03–15.44) | 9.80 (8.62–10.80; 5.85–16.40) | 10.10 (8.90–11.00; 5.52–16.40) | 10.60 (9.75–11.93; 6.30–15.55) |
| LPV dose, mg/m2 | 225.24 (220.17–232.75; 135.48–287.89) | 221.83 (213.20–229.26; 174.41–442.48) | 224.04 (216.72–231.26; 195.95–355.40) | 224.72 (216.67–231.73; 192.02–321.08) | 224.89 (221.84–230.51; 204.46–302.30) |
Data are median (IQR; range), unless otherwise indicated. LPV, lopinavir.
Predictors of viral load >400 copies/ml
We performed Cox proportional hazards regression analysis to evaluate the risks for VL>400 copies/ml. The results showed reduced risk of virological failure (VL>400 copies/ml) for increased lopinavir concentrations (HR 0.96 [95% CI 0.93, 0.98] for each 1 mg/l; P=0.002) and increased risk of VL>400 copies/ml for pretreatment HAZ (HR 2.24 [95% CI 1.17, 4.28; P=0.015] for moderate stunting and HR 2.92 [95% CI 1.67, 5.03; P=0.0001] for severe stunting, relative to those with normal HAZ). After adjustment for other covariates, both lopinavir concentrations and pretreatment HAZ remained significant (Table 3). Utilizing model selection with AICw yielded a model with similar estimated HRs for low lopinavir concentrations (HR 0.96 [95% CI 0.93, 0.99]; P=0.005) as well as moderate (HR 2.19 [95% CI 1.19, 4.05]; P=0.011) and severe (HR 0.45 [95% CI 0.24, 0.84]; P=0.009) stunting, confirming the stability of the adjusted model. A high log10 pretreatment VL was associated with HRs>1 although these did not reach significance in either the crude (HR 1.62 [95% CI 0.77, 3.34]; P=0.205) or adjusted (HR 1.56 [95% CI 0.91, 3.54]; P=0.269) models. Due to the high percentage (38%) of missing data, adherence was not included in the primary analysis. However, in a sensitivity analysis we compared two adjusted models using AICw, where the first model included lopinavir concentrations and the second model included recorded adherence. The results revealed that the AICw favours the model including lopinavir concentrations (AICw 1,220.7) compared with the model including adherence (AICw 1,228.4).
Table 3.
Cox proportional hazards regression analysis for risk of VL>400 copies/ml for crude and adjusted models after multiple imputation of all covariates
| Crude model |
Adjusted mode |
AICw |
||||
|---|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Lopinavir, mg/l | 0.96 (0.93, 0.98) | 0.005 | 0.96 (0.94, 0.99) | 0.019 | 0.96 (0.93, 0.99) | 0.005 |
| Age | ||||||
| >10 months | Reference | – | Reference | – | – | – |
| <10 months | 1.07 (0.75, 1.52) | 0.70 | 1.14 (0.80, 1.62) | 0.46 | – | – |
| Pretreatment WAZ | ||||||
| Normal | Reference | – | Reference | – | – | – |
| Moderate | 1.06 (0.75, 1.48) | 0.72 | 0.91 (0.61, 1.33) | 0.63 | – | – |
| Severe | 1.87 (0.61, 1.25) | 0.45 | 1.15 (0.77, 1.72) | 0.49 | – | – |
| Pretreatment HAZ | ||||||
| Normal | Reference | – | Reference | – | Reference | – |
| Moderate | 2.24 (1.17, 4.28) | 0.015 | 2.20 (1.18, 4.09) | 0.012 | 2.19 (1.19, 4.05) | 0.011 |
| Severe | 2.92 (1.69, 5.03) | 0.0001 | 2.83 (1.66, 4.82) | 0.0001 | 2.83 (1.67, 4.78) | 0.0001 |
| Pretreatment log10 VL | ||||||
| <5 | Reference | – | Reference | – | Reference | – |
| >5 | 1.62 (0.77, 3.44) | 0.21 | 1.56 (0.91, 3.54) | 0.27 | 1.58 (0.72, 3.44) | 0.252 |
| Pretreatment CD4% | ||||||
| >25% | Reference | – | Reference | – | – | – |
| <25% | 1.02 (0.68, 1.53) | 0.91 | 1.09 (0.73, 1.65) | 0.65 | – | – |
| WHO stage | ||||||
| Early | Reference | – | Reference | – | – | – |
| Moderate | 1.22 (0.70, 2.13) | 0.47 | 1.21 (0.69, 2.01) | 0.49 | – | – |
AICw, Akaike's information criterion with inverse probability weights; CD4%, CD4+ T-cell percentage; HAZ, height-for-age z-score; VL, viral load; WAZ, weight-for-age z-score.
We also fitted separate models where lopinavir concentrations were dichotomized based on the cutoffs of 1 mg/l and 4 mg/l, respectively. The results showed that children with lopinavir concentrations of <1 mg/l (Table 4) or 4 mg/l (crude HR 2.3 [95% CI 1.63, 3.26] and adjusted HR 1.74 [95% CI 1.36, 2.23]) had an increased hazard of VL>400 copies/ml in both crude and adjusted models. Similarly we showed that moderate and severe stunting were significantly associated with increased hazards of VL>400 copies/ml in both crude and adjusted models. We compared the two models by means of AICw and showed that the model with 1 mg/l cutoff (AICw 1,326.34) described the data better than the model with 4 mg/l cutoff (AICw 1,331.03).
Table 4.
Cox proportional hazards regression analysis for risk of VL>400 copies/ml for the crude model and the adjusted model after multiple imputation of all covariates using lopinavir with a cutoff of 1 mg/l
| Crude model |
Adjusted model |
AICw |
||||
|---|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Lopinavir | ||||||
| >1 mg/l | Reference | – | Reference | – | Reference | – |
| <1 mg/l | 2.3 (1.63, 3.26) | 0.0001 | 2.11 (1.62, 2.75) | 0.001 | 2.14 (1.52, 3.02) | 0.0001 |
| Age | ||||||
| >10 months | Reference | – | Reference | – | – | – |
| <10 months | 1.07 (0.75, 1.52) | 0.70 | 1.04 (0.73, 3.95) | 0.81 | – | – |
| Pretreatment WAZ | ||||||
| Normal | Reference | – | Reference | – | – | – |
| Moderate | 1.06 (0.75, 1.48) | 0.72 | 0.91 (0.61, 1.33) | 0.63 | – | – |
| Severe | 1.87 (0.61, 1.25) | 0.45 | 1.15 (0.77, 1.72) | 0.49 | – | – |
| Pretreatment HAZ | ||||||
| Normal | Reference | – | Reference | – | Reference | – |
| Moderate | 2.24 (1.17, 4.28) | 0.015 | 2.20 (1.18, 4.09) | 0.012 | 2.32 (1.24, 4.37) | 0.009 |
| Severe | 2.92 (1.69, 5.03) | 0.0001 | 2.83 (1.66, 4.82) | 0.0001 | 2.90 (1.67, 5.05) | 0.0001 |
| Pretreatment logl0 VL | ||||||
| <5 | Reference | – | Reference | – | Reference | – |
| >5 | 1.67 (0.79, 3.50) | 0.17 | 1.69 (0.81, 3.53) | 0.16 | 1.58 (0.72, 3.44) | 0.252 |
| Pretreatment CD4% | ||||||
| ≥25% | Reference | – | Reference | – | – | – |
| <25% | 1.09 (0.69, 1.72) | 0.71 | 1.15 (0.74, 1.77) | 0.53 | – | – |
| WHO stage | ||||||
| 1 and 2 | Reference | – | Reference | – | – | – |
| 3 and 4 | 1.26 (0.75, 2.11) | 0.38 | 1.25 (0.73, 2.13) | 0.49 | – | – |
AICw, Akaike's information criterion with inverse probability weights; CD4%, CD4+ T-cell percentage; HAZ, height-for-age z-score; VL, viral load; WAZ, weight-for-age z-score.
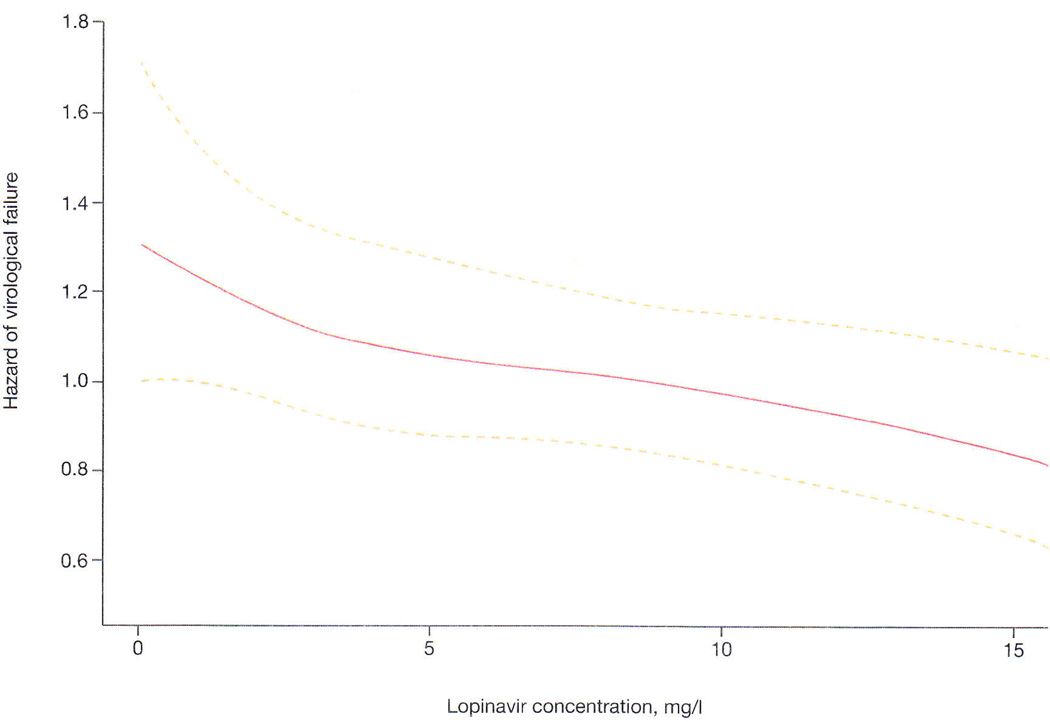
Non-linear effect of lopinavir concentrations on the risk of viraemia
We modelled the non-linear effect of lopinavir concentrations on the hazard of VL>400 copies/ml and failed to show any distinct threshold. Nonetheless, we showed that increasing lopinavir concentrations were associated with reduced hazard of VL>400 copies/ml across the full range of LPV concentrations studied (Figure 2).
Figure 2.
Non-linear effect of lopinavir concentrations on the hazard of virological failure
Discussion
We used Cox regression models to describe the association of lopinavir concentrations during the first year of treatment and pretreatment characteristics with the hazard of viraemia (VL>400 copies/ml) in a cohort of young, nevirapine-exposed South African children initiated on a PI-based regimen. Our data suggests that with increasing lopinavir concentrations the hazard of VL>400 copies/ml is reduced. We also found a significant association with moderate (HAZ −2 to −3) and severe (HAZ<−3) pretreatment stunting with a greater chance of VL>400 copies/ml, while no association was found for the other pretreatment characteristics, including WAZ.
Using the Cox regression models we found that children with lopinavir concentrations below the cut-offs of 1 mg/l or 4 mg/l have an increased hazard of virological failure, but the effect was stronger at the lower threshold. Moreover, we determined the non-linear effect of lopinavir concentrations on the hazard of VL>400 copies/ml (Figure 2) and showed that decreased concentrations correlated with increased risk of virological failure across the full range of lopinavir concentrations studied. This suggests that in addition to adherence-related changes in drug exposure, individual variability in lopinavir concentrations may be important for therapeutic outcomes. However, high lopinavir concentrations would likely increase the risk of toxicity. Low lopinavir concentrations, especially <1 mg/l, are likely to reflect poor adherence and could provide an objective measure of non-adherence. ART adherence is difficult to assess in paediatric patients as there is considerable social pressure for caregivers to report complete adherence and measuring returned medication is difficult, when compared to pill counts which can be done for adults. An objective adherence measure would be useful in children failing an LPV/r-based regimen as PI mutations are rarely found, provided that there was no prior exposure to other PIs [9]. Antiretroviral resistance testing, which is expensive, could be limited to children with lopinavir concentrations that are > 1 mg/l, as has been suggested in a pilot study in adults [10].
HIV infection adversely affects growth. Prior to ART, studies demonstrated that perinatally acquired HIV is associated with poor growth and high mortality. In our data, we found a significant association with pretreatment HAZ, but not with WAZ. This suggests that children who are stunted have a higher hazard of virological failure. Our data is consistent with other reports in the literature with regard to the effect of stunting on virological failure [19].
Our study has several limitations that are worth highlighting. Firstly, in our study, there was missing data, which we dealt with by multiple imputation. This approach has been shown to be superior to complete case analysis in which only subjects who do not have missing values are analysed [16]. If data are missing at random and thus the probability for a value to be missing depends only on observed quantities, then no bias is introduced. We found the missing at random assumption to be reasonable in our study given that the missing data related mainly to data not being measured, or due to insufficient sample volume or a lost sample. Secondly, we did not observe the time of morning dose prior to sampling for lopinavir concentration. Hence our analysis did not include adjustment of lopinavir concentrations for the time after the dose. Nevertheless we have shown that the lopinavir concentrations in samples taken 0.42–9.00 h after the last dose predict VL>400 copies/ml, which would allow laboratories to conduct lopinavir assays to decide whether to proceed to the much more expensive genotypic resistance tests. To exclude potential bias due to th inclusion of early VL data, which (if >400 copies/ml) may indicate failure to suppress at that time point, rather than virological failure, and children followed-up to >52 weeks, we conducted two sensitivity analyses (data not shown); the first excluding visits before 24 weeks, and the second excluding visits after the planned follow-up period. Our findings were not substantially altered in either analysis.
The use of therapeutic drug monitoring is complicated by insufficient knowledge of the target plasma concentrations particularly in children on ART in whom the optimal drug concentrations have not been clearly defined [20]. In this study, we used reference values for plasma lopinavir concentrations derived largely from adult studies. The recommended minimum lopinavir trough concentrations are 1 mg/l in treatment-naive patients and 4 mg/l in treatment-experienced patients [21]. We found that lopinavir concentrations 0.42–9.00 h after the last dose (analogous to the time after dose for samples collected at a typical clinic visit when the child has taken his/her ART in the morning) predicted the risk of viraemia. The lopinavir concentrations taken during the clinic visits were in keeping with those described in other studies amongst children of a similar age [22,23].
Strengths of the study include a relatively large sample size and the cohort design, which provides a higher level of evidence for the relationship between explanatory and outcome variables compared to studies with a cross-sectional or case-control design. Another strength of the study was repeated plasma drug concentration measurement at each follow-up visit, which made it possible to assess each child’s lopinavir concentration profile and its correlation with treatment success.
In conclusion lopinavir concentrations were associated with the hazard of VL>400 copies/ml. Low lopinavir concentrations could be used as a proxy for treatment non-adherence to guide determination of eligibility for resistance testing. Furthermore, our findings provide preliminary data to support developing optimal target concentrations of lopinavir required for viral suppression in children, which could be used as part of therapeutic drug monitoring to optimize the efficacy of ART regimens in children. Moderate and severe stunting were also associated with virological response to LPV/r-based ART suggesting that the reasons for poor responses in stunted children should be investigated further and that this group may be targeted for appropriate interventions.
Acknowledgements
The study was supported in part by grants from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD) HD 47177 and Secure the Future Foundation RES 219 (LK). The authors wish to acknowledge the current support of RRM by the South National Research Foundation and Carnegie Corporation.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Penazzato M, Prendergast A, Tierney J, Cotton M, Gibb D. Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age. Cochrane Database Syst Rev. 2012;7:CD004772. doi: 10.1002/14651858.CD004772.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012;26:929–938. doi: 10.1097/QAD.0b013e328351f5b2. [DOI] [PubMed] [Google Scholar]
- 3.Bain-Brickley D, Butler LM, Kennedy GE, Rutherford GW. Interventions to improve adherence to antiretroviral therapy in children with HIV infection. Cochrane Database Syst Rev. 2011;12:CD009513. doi: 10.1002/14651858.CD009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resino S, Bellón JM, Ramos JT, et al. Salvage lopinavir-ritonavir therapy in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2004;23:923–930. doi: 10.1097/01.inf.0000142170.52155.7f. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach – 2010 revision. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 7.Sharland M, Blanche S, Castelli G, Ramos J, Gibb DM. PENTA guidelines for the use of antiretroviral therapy, 2004. HIV Med. 2004;5(Suppl 2):61–86. doi: 10.1111/j.1468-1293.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 8.Rudin C, Wolbers M, Nadal D, Rickenbach M, Bucher HC. Long-term safety and effectiveness of lopinavir/ritonavir in antiretroviral-experienced HIV-1-infected children. Arch Dis Child. 2010;95:478–481. doi: 10.1136/adc.2009.169375. [DOI] [PubMed] [Google Scholar]
- 9.Taylor BS, Hunt G, Abrams EJ, et al. Rapid Development of antiretroviral drug resistance mutations in HIV-infected children less than two years of age initiating protease inhibitor-based therapy in South Africa. AIDS Res Hum Retroviruses. 2011;27:945–956. doi: 10.1089/aid.2010.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zyl GU, van Mens TE, Mcllleron H, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56:333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn L, Coovadia A, Strehlau R, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12:521–530. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201:1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Child growth standards: WHO Anthro (version 3.2.2, January 2011) and macros. Geneva: World Health Organization; [Accessed 11 August 2010]. Updated January 2011. Available from http://www.who.int/childgrowth/software/en/ [Google Scholar]
- 15.Honaker J, King G. What to do about missing values in time-series cross-section data. Am J Pol Sci. 2010;54:561–581. [Google Scholar]
- 16.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 17.Hens N, Aerts M, Molenberghs G. Model selection for incomplete and design-based samples. Stat Med. 2006;25:2502–2520. doi: 10.1002/sim.2559. [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team. R: a language and environment for statistical computing. Version 2.15.3. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 19.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–251. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 20.Treluyer JM, Burgard M, Cazali N, et al. Relationship between antiretroviral drug plasma concentrations and viral load in children. J Acquir Immune Defic Syndr. 2003;32:112–115. doi: 10.1097/00126334-200301010-00016. [DOI] [PubMed] [Google Scholar]
- 21.La Porte CJL, Back DJ, Blaschke T, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:4–14. [Google Scholar]
- 22.Chadwick EG, Pinto J, Yogev R, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J. 2009;28:215–219. doi: 10.1097/INF.0b013e31818cc053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, Nuttall JJC, Egbers C, et al. Effect of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2008;47:566–569. doi: 10.1097/QAI.0b013e3181642257. [DOI] [PubMed] [Google Scholar]