Abstract
Patients with schizophrenia exhibit deficient response monitoring as indexed by blunted activation of the dorsal anterior cingulate cortex (dACC) and functionally related regions during error commission. This pattern may reflect heritable alterations of dACC function. We examined whether the hypofunctional 677C>T variant in MTHFR, a candidate schizophrenia risk gene, contributed to our previous findings of blunted error-related dACC activation and reduced microstructural integrity of dACC white matter. Eighteen medicated outpatients with schizophrenia underwent diffusion tensor imaging and performed an antisaccade paradigm during functional magnetic resonance imaging (fMRI). T allele carriers exhibited significantly less error-related activation than C/C patients in bilateral dACC and substantia nigra, regions that are thought to mediate dopamine-dependent error-based reinforcement learning. T carrier patients also showed significantly lower fractional anisotropy in bilateral dACC. These findings suggest that the MTHFR 677T allele blunts response monitoring in schizophrenia, presumably via effects on dopamine signaling and dACC white matter microstructural integrity.
Keywords: Schizophrenia, Functional MRI, Genetics, Executive function, Anterior cingulate cortex, Diffusion tensor imaging
Introduction
Schizophrenia is consistently characterized by deficient response monitoring, which involves detecting errors, evaluating what went wrong, and adjusting behavior (Carter et al. 2001; Kerns et al. 2005; Laurens et al. 2003). Extensive evidence from functional neuroimaging, electrophysiological, (Carter et al. 2001; Fornito et al. 2009; Kerns et al. 2005). Further, variation in ACC function during response monitoring shows evidence of heritability (Albrecht et al. 2008), including in twin studies (Anokhin et al. 2008), and has been related to specific genetic polymorphisms that affect dopamine neurotransmission in healthy individuals (Klein et al. 2007; Kramer et al. 2007).
Here, we examined whether the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism contributes to ACC dysfunction during response monitoring in schizophrenia. MTHFR is a key regulator of intracellular methylation reactions, and the 677T variant, each copy of which reduces MTFHR activity by 35%, may influence dopamine signaling (Roffman et al. 2008a, b). The 677T allele has been associated with increased schizophrenia risk (Allen et al. 2008; Gilbody et al. 2007). It has also been associated with more severe executive dysfunction in schizophrenia, including perseverative errors on the Wisconsin Card Sort Test; these errors reflect a failure to use feedback to adjust behavior, a fundamental component of response monitoring (Roffman et al. 2007). Finally, the 677T allele has been associated with more impaired recruitment of the dorsolateral prefrontal cortex during working memory performance in schizophrenia as measured by functional magnetic resonance imaging (fMRI) (Roffman et al. 2008a). Given the functional nature of the C>T variant and the low frequency of T allele homozygotes (approximately 10% in Caucasians), as in prior work, we combined C/T and T/T participants as T allele carriers.
We focused on MTHFR effects in the dorsal ACC (dACC), which is theorized to mediate dopamine-dependent learning from errors (Holroyd and Coles 2002). In this theory, the striatum detects a mismatch between the intended (correct) versus actual (error) outcome. This mismatch or ‘prediction error’ results in a phasic decrease in mesencephalic dopamine release that disinhibits dACC neurons (Schultz 2002) and elicits the error related negativity (ERN), an event-related potential that follows error commission. According to this theory, both increased dACC activation and the ERN reflect the use of prediction error signals to modify the associative strength of stimulus-response mappings in the service of optimizing behavioral outcomes. Thus, both the ERN and dACC activation following errors have been theorized to index ‘error-based reinforcement learning’ (Holroyd et al. 2003; Holroyd et al. 2004).
We previously reported blunted error-related fMRI activation in the dACC, striatum, and substantia nigra in schizophrenia, which was inversely correlated with error rate (Polli et al. 2008). This same sample of patients also showed reduced microstructural integrity of the white matter underlying dACC based on diffusion tensor imaging (DTI) measures of fractional anisotropy (FA, Manoach et al. 2007), a potential structural correlate of the observed dACC dysfunction. This finding is consistent with several prior reports of reduced FA in the cingulum bundle in schizophrenia (Ardekani et al. 2003; Hao et al. 2006; Kubicki et al. 2003; Sun et al. 2003; Wang et al. 2004). In the present study, we reanalyzed the schizophrenia data to determine whether MTHFR genotype accounted for variability in these functional and structural dACC abnormalities. We expected that compared to C homozygotes, patients who carried the hypofunctional 677T allele, which may exacerbate their already deficient dopamine signaling (Roffman et al. 2008a) and which was previously associated with decreased use of error feedback to modify performance (Roffman et al. 2007), would exhibit more substantial blunting of error-related activation and greater reductions of dACC white matter microstructural integrity.
Materials and methods
The present study represents a reanalysis of fMRI and DTI data from two prior reports (Manoach et al. 2007; Polli et al. 2008), which provide detailed descriptions of the brain imaging data acquisition and analysis procedures. The studies were approved by the Partners HealthCare Human Research Committee and all participants gave written informed consent.
Participant characterization
Patients with schizophrenia (n=18) were recruited from an urban mental health center, and schizophrenia diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (First et al. 2002). Participants with a history of substance abuse or dependence within the previous 6 months, or any other medical or neurologic condition affecting brain function, were excluded. All patients were taking atypical antipsychotics, except one participant taking fluphenazine. In patients, MTHFR 677C>T genotype was determined with the MassArray platform (Sequenom, San Diego) using previously described primers (Roffman et al. 2008a). Given the low frequency of T/T genotype (n=1), C/C participants (n=8) were contrasted against T allele carriers (C/T + T/T, n=10). Genotype groups did not differ in age, gender, handedness as measured by the modified Edinburgh Handedness Inventory (Oldfield 1971; White and Ashton 1976), estimated verbal IQ (American National Adult Reading Test, Blair and Spreen 1989), symptom ratings on the Positive and Negative Syndrome Scale (PANSS, Kay et al. 1987), or antipsychotic medication dose as measured by chlorpromazine (CPZ) equivalents (Woods 2003) (Table 1). However, consistent with previous reports (Gilbody et al. 2007), Caucasians were overrepresented among T carriers.
Table 1.
Demographic and clinical description of patient participants
| Subject characteristics | C/C Subjects (n=8) | T Carriers (n=10) | Statistics | p |
|---|---|---|---|---|
| Demographics | ||||
| Age at fMRI | 38.9±4.3 | 42.9±4.1 | t=−0.67 | 0.52 |
| Sex | 5 Male/3 Female | 8 Male/2 Female | X2=0.68 | 0.41 |
| Race | 4 Caucasian/4 African-American | 9 Caucasian/1 African-American | X2=3.55 | 0.06 |
| Length of Illness (yrs) | 14.9±3.3 | 19.0±3.7 | t = 0.82 | 0.42 |
| Estimated Verbal IQ | 101 ±4 | 106±4 | t = −.072 | 0.48 |
| Edinburgh Handedness Inventory | 92±3 | 88±5 | t=0.62 | 0.55 |
| Clinical | ||||
| PANSS Positive | 13.3±2.1 | 15.4±1.9 | t=−.076 | 0.46 |
| PANSS Negative | 18.3±2.0 | 15.4±2.0 | t=1.00 | 0.33 |
| PANSS General | 28.0±2.0 | 28.9±1.3 | t=−.039 | 0.70 |
| PANSS Total | 59.5±4.2 | 59.7±4.3 | t=−.033 | 0.97 |
| CPZ Equivalents | 365±87 | 507±149 | t=−.077 | 0.45 |
| Performance and Motion | ||||
| Antisaccade Latency (ms) | ||||
| - correct trials | 295±15 | 345±28 | t=−1.58 | 0.14 |
| - error trials | 251 ±28 | 283±24 | t=−0.87 | 0.40 |
| Antisaccade % Error | 20.7±5.1 | 18.6±3.9 | t=0.33 | 0.75 |
| Error Self-correction % | 79.5 | 77.6 | t=0.19 | 0.85 |
| Total Motion (mm) | 1.71±0.26 | 1.62±0.19 | t=0.28 | 0.79 |
Values are reported as mean±standard error
fMRI, functional magnetic resonance imaging; PANSS, Positive and Negative Syndrome Scale; CPZ, chlorpromazine
Comparisons were also made between patient genotype groups and healthy volunteers (fMRI, n=15; DTI, n=19), who were not genotyped. Patients and healthy participants were demographically matched as previously described (Polli et al. 2008; Manoach et al. 2007). The healthy control group contained a mixture of Caucasian (n=17) and Asian (n=2) participants.
Antisaccade paradigm
We used an antisaccade paradigm to examine error-related activation. Antisaccades require one to inhibit the prepotent response of looking toward a suddenly appearing visual target (i.e., a prosaccade) and to instead look in the opposite direction. Patients with schizophrenia reliably show increased antisaccade errors (i.e., failures to suppress the prepotent prosaccade, for reviews see Gooding and Basso 2008; Levy et al. 1998). Participants practiced in a mock scanner prior to scanning. The paradigm consisted of a pseudorandom sequence of prosaccade and antisaccade trials balanced for left- and right-sided movements, and fixation intervals lasting 2, 4, or 6 seconds (Supplemental Figure 1). Six runs of the task produced 211 prosaccade trials, 211 antisaccade trials, and 80 fixation intervals. Participants were encouraged to respond as quickly and accurately as possible. In addition to a base rate of pay, they received 5 cents for each correct response. The ISCAN fMRI Remote Eye Tracking Laboratory (ISCAN, Burlington, MA) recorded eye position during scanning. The directional accuracy of each saccade with respect to the required response was scored using a MATLAB (Mathworks, Natick, MA) program.
fMRI acquisition and analysis
Images were acquired using a 3.0T Tim Trio whole body high speed imaging device (Siemens, Erlangen, Germany). Two high-resolution 3D structural images were acquired using an MPRAGE sequence (TR 2530 ms, TE 3 ms, flip angle 7°). Functional images were collected for 20 contiguous horizontal slices parallel to the intracommisural plane using a gradient echo T2*-weighted sequence with prospective acquisition correction (PACE) for head motion (TR 2000 ms, TE 30 ms, flip angle 90°, voxel size 3.13×3.13×5 mm3).
Analyses were conducted using FreeSurfer (Fischl et al. 1999a) and FreeSurfer Functional Analysis Stream (FS-FAST) (Burock and Dale 2000) software. To register data across participants, anatomical and functional scans were spatially normalized using a surface-based spherical coordinate system that employs a non-rigid alignment algorithm based on sulcal and gyral patterns (Dale et al. 1999; Fischl et al. 1999a, b) and smoothed with a 2D 4.6 mm FWHM Gaussian kernel. Cortical activation was localized using an automated surface-based parcellation system (Fischl et al. 2004). To investigate subcortical activation, structural and functional volumes were registered to the Montreal Neurological Institute (MNI305) atlas (Collins et al. 1994), and coordinates were transformed to standard Talairach space using an algorithm developed by Matthew Brett (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). All analyses included race as between-group factor to control for population stratification. Functional scans were motion corrected, intensity normalized, smoothed using a 3D 8 mm FWHM Gaussian kernal, and aligned to each participant’s averaged structural scans. Finite impulse response (FIR) estimates (Burock and Dale 2000) of the event-related hemodynamic responses (HDR) were calculated separately for correct and error antisaccade trials for each participant at 12 time points (4 s prior to the start of the trial to 18 s after the start).
Analyses of Registered group data
We compared C/C and T allele carriers on error-related activation based on the contrast of error versus correct antisaccade trials at 6 s following trial onset, the time point of maximal difference in both patients and controls (Polli et al. 2005, 2008). To correct for multiple comparisons, 10,000 Monte Carlo simulations of synthesized white Gaussian noise were run on the cortical surface and in the volume, using the smoothing, resampling, and averaging parameters of the functional analyses. This determines the likelihood that a cluster of a certain size at a certain threshold (p<.01) would be found by chance (cluster-wise probability, CWP). These methods set the corrected overall probability level to 0.05.
Region-of-interest (ROI) analyses
ROIs were defined for the left and right dACC in all 18 participants using both anatomical and functional constraints. The (entire) ACC was defined using an automated parcellation for the cortical surface (Fischl et al. 2004). The ACC was divided into dorsal and rostral regions by drawing a line perpendicular to the intercommisural plane at the anterior boundary of the genu of the corpus callosum (Devinsky et al. 1995). For each participant, the ROIs were functionally constrained to vertices within the dACC that showed error-related activation at p<.05 based on the averaged group of all 18 participants. The dACC is a functionally and anatomically heterogeneous region, and this constraint limited our inquiry to the dACC region involved in error processing. Because we selected vertices based on analysis of all participants, the region was not biased towards our hypothesis of more blunted error-related dACC activation in T-carriers than C/C patients. We averaged activation across voxels in the left and right dACC for each participant and used these values in repeated measures ANOVAs, with genotype and race as between-subject factors and condition (error or correct) and hemisphere as within-subject factors.
DTI acquisition and analysis
A subset of patients (n=6 C/C, n=9 T carrier) also underwent high-resolution DTI scans, using methods that are detailed in the original study (Manoach et al. 2007). Briefly, single-shot EPI DTI was acquired using a twice refocused spin echo sequence (Reese et al. 2003) with the following sequence parameters: TR/TE=8400/82 ms; b=700 s/mm2; NEX=1; ten T2 images acquired with b=0; 72 diffusion directions; 128×128 matrix; 2×2 mm in-plane resolution; 64 axial oblique (AC-PC) slices; 2 mm (0 mm gap) slice thickness; scan duration 12′44″. dACC ROIs were defined for each participant using the same anatomical boundaries as the fMRI analysis.
DTI data were analyzed as follows: (1) raw diffusion data were corrected for head motion and residual eddy current distortion via co-registration to a T2 image aquired with b=0, using FLIRT from the FSL software library (http://www.fmrib.ox.ac.uk/fsl); (2) diffusion tensor and FA values were reconstructed using the standard least squares fit to the log diffusion signal (Basser et al. 1994); (3) FA volumes were registered to high-resolution T1 volumes for each participant using the T2 volume as an intermediary; (4) following delineation of the white/gray matter boundary using Freesurfer (Dale et al. 1999), FA was sampled in the undeformed white matter 2 mm below the white/gray boundary for each vertex on the surface and then projected onto the white/gray interface. FA values were smoothed using n=50 iterations of replacement by nearest neighbors. This corresponds to smoothing by approximately 10 mm FWHM on the surface. This strategy minimizes partial volume contributions from cortical gray matter and preserves regional specificity; (5) average FA values across the left and right dACC ROIs were determined for each participant; (6) mean dACC FA for C/C and T carrier patients were compared using a mixed model ANOVA, with genotype and race as between-subject factors and hemisphere as a within-subject factor.
Relations of error-related activation with FA
To determine whether the microstructural integrity of dACC white matter influenced error-related activation (error minus correct), and whether these relations differed as a function of either diagnosis or MTHFR genotype, we conducted two linear regressions of error-related activation on FA. The first included the entire sample and an interaction term of diagnosis by FA. The second regression included only patients and an interaction term of genotype by FA.
Control analyses
Motion
To characterize average motion for each participant, the total translational motion in mm for three directions (x,y,z), as determined by the AFNI motion correction algorithm, was averaged across the six runs of the task and compared between groups.
Specificity
To examine whether genotype differences in dACC activation were specific to response monitoring or represented a more general blunting of dACC function, we compared genotype groups on dACC activation in the contrast of correct antisaccades vs. fixation at 4 s, which is the time point showing maximal activation in ocular motor regions in both groups.
Comparisons to demographically-matched controls
We compared dACC activation and FA in C/C and T carrier patients to that of the demographically-matched healthy control participants of our prior studies (n=15 for fMRI, n=19 for DTI, Manoach et al. 2007; Polli et al. 2008) to determine whether T carriers contributed disproportionately to the observed differences between schizophrenia patients and controls.
Results
Genotype groups did not differ in antisaccade performance (i.e., the latency of correct or erroneous antisaccades, antisaccade error rate, or rate of error self-correction) or in motion during fMRI scanning (Table 1).
fMRI
Group comparisons of error-related activation on the cortical surface and in the volume are presented in Fig. 1 and Table 2. Significant C/C>T effects were observed in bilateral dACC and other regions including the substantia nigra and right dorsolateral prefrontal cortex, which are thought to coordinate with dACC to mediate error-based reinforcement learning (Holroyd and Coles 2002). No significant T>C/C effects were observed.
Fig. 1.
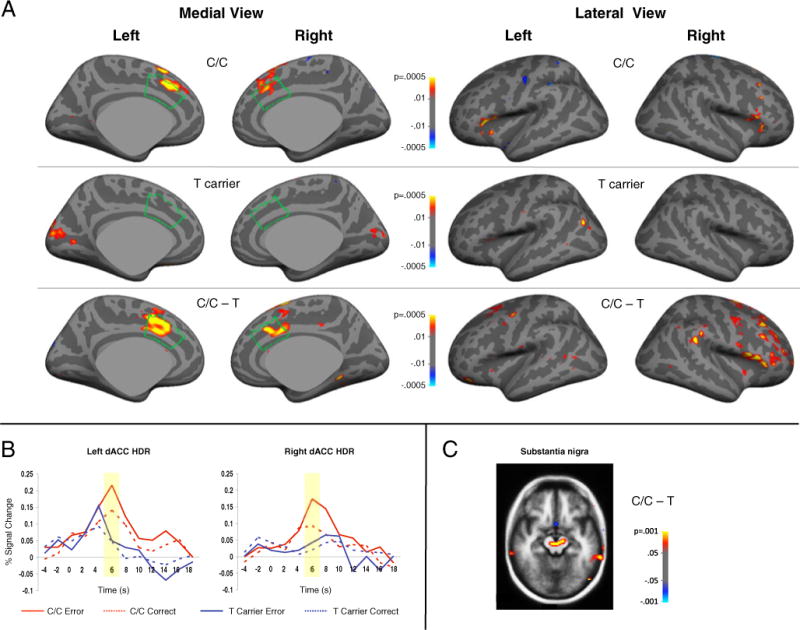
a Statistical maps of MTHFR 677C>T genotype effects on error-related activation at 6 s displayed on the inflated medial and lateral cortical surfaces. The dACC is outlined in green. b Hemodynamic responses (HDRs) of the vertices with the maximum group difference in left and right dACC separated by genotype group and condition. c Statistical map showing greater error-related activation for C/C vs. T carriers in the substantia nigra at 6 s
Table 2.
Regions showing greater activation for MTHFR 677C/C genotype compared to T carriers in the contrast of error minus correct antisaccade trials at 6 seconds. There were no regions that were more activated for T-carriers
| SURFACE ANALYSIS (cortical regions) | |||||
|---|---|---|---|---|---|
| Hemisphere | Region | Maximal activation (p-values) | Talairach coord. (x, y, z) | Size (mm2) | Cluster-wise probability |
| Left | Dorsal ACC (BA 32) | 1.0× 10−4 | −11, 15, 38 | 855 | 1.0× 10−4 |
| Premotor cortex (BA 6) | 1.3 × 10−3 | −19, 9, 49 | 462 | 1.3 × 10−2 | |
| Right | Dorsal ACC (BA 32) | 2.0× 10−5 | 12, 23, 32 | 393 | 3.3 × 10−2 |
| Frontal eye field (BA 8) | 1.6 × 10−5 | 31, 29, 37 | 858 | 2.0 × 10−4 | |
| Premotor cortex (BA 6) | 4.1 × 10−5 | 22, 5, 59 | 779 | 4.0 × 10−4 | |
| DLPFC (BA 9) | 6.1 × 10−4 | 45, 1, 42 | 372 | 4.2 × 10−2 | |
| VLPFC (BA 45) | 2.1 × 10−7 | 50, 21, 8 | 900 | 1.0× 10−4 | |
| VLPFC (BA 47) | 1.3 × 10−4 | 47, 35, −2 | 375 | 4.1 × 10−2 | |
| VOLUME ANALYSIS (subcortical regions) | |||||
| Hemisphere | Region | Maximal activation (p-values) | Talairach coord. (x, y, z) | Size (mm³) | Cluster-wise probability |
| Right | * Thalamus / substantia nigra | 3.5× 10−5 | 20, −21, −5 | 1712 | 5.2× 10−2 |
This cluster contains a local maximum in the substantia nigra (Talariach coordinates −2, −21, −10), p=4.2×10−4
ACC anterior cingulate cortex; BA Brodmann Area; DLPFC dorsolateral prefrontal cortex; VLPFC ventrolateral prefrontal cortex
ROI analysis results are presented in Table 3. The main effect of MTHFR genotype approached significance and there was a significant genotype × condition interaction. This interaction reflected that only C homozygotes showed significantly greater dACC activation for error compared to correct trials, and that the difference between C homozygotes and T carriers was greater for errors than correct trials. No main effects of race, condition, or hemisphere were observed. A significant genotype × condition × race interaction was seen; however, this interaction should be viewed with caution since there was only one African American participant in the T-carrier group.
Table 3.
Repeated measures ANOVA of response monitoring activation of the dACC ROI at 6 seconds
| Between-subjects effects | Statistics | p | Partial η2 |
|---|---|---|---|
| Genotype | F(1,14)=3.75 | p=.07 (C/C>T) | 0.21 |
| Race | F(1,14)=0.12 | p=.73 | 0.01 |
| Within-subjects effects | Statistics | p | Partial η2 |
| Condition | F(1,14)=0.68 | p=.43 | 0.05 |
| Hemisphere | F(1,14)=0.75 | p=.40 | 0.05 |
| Interactions | Statistics | p | Partial η2 |
| Genotype × Condition | F(1,14)=9.23 | p=.009 | 0.40 |
| Genotype × Race | F(1,14)=0.57 | p=.46 | 0.04 |
| Genotype × Hemisphere | F(1,14)=2.26 | p=.16 | 0.14 |
| Genotype × Condition × Race | F(1,14)= 10.46 | p=.006 | 0.43 |
| Genotype × Condition × Hemisphere | F(1,14)=0.26 | p=.62 | 0.02 |
| Genotype × Condition × Hemisphere × Race | F(1,14)=0.48 | p=.50 | 0.03 |
| Post hoc tests for Genotype × Condition | Statistics | p | Partial η2 |
| C/C error vs C/C correct | F(1,28)=4.96 | p=.03 (error>correct) | 0.15 |
| T error vs T correct | F(1,36)=0.33 | p=.57 | 0.01 |
| C/C error vs T error | F(1,32)=12.28 | p=.001 (C/C>T) | 0.28 |
| C/C correct vs T correct | F(1,32)=3.45 | p=.07 (C/C>T) | 0.10 |
For post hoc tests, no significant main or interactive effects of race were observed (p>.05)
The genotype by condition interaction is depicted in Fig. 2a. Although the genotype groups significantly differed in dACC activation only on error trials, it is possible that the reduced activation for T carriers on correct trials might have reached significance with a larger sample, which would be consistent with a more blunted dACC response for T carriers during response monitoring in general, not just for errors.
Fig. 2.
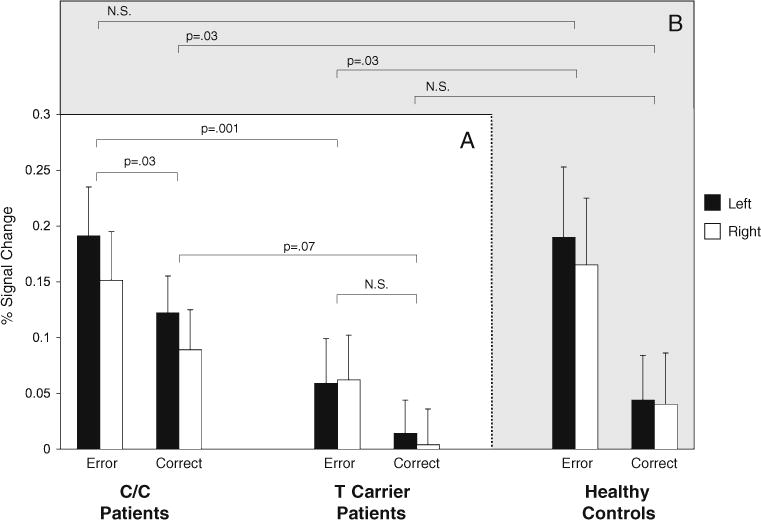
a Bar graphs of mean dACC activation in C/C and T carrier patients during error and correct antisaccade trials with standard error bars. b Bar graphs for demographically matched healthy control participants. Levene’s tests supported the assumption of equal variance between groups for each condition/hemisphere combination (p’s>.2). N.S. not significant
To determine whether dACC function in T carriers was blunted for functions other than response monitoring, we compared genotype groups on dACC activation in the contrast of correct antisaccades vs. fixation at 4 s. This contrast identifies activation related to having to perform an antisaccade (i.e., inhibition of the prepotent response and executing a volitional motor program). Both genotype groups showed robust activation in the ocular motor network that did not differ significantly between groups, including in the dACC (Supplemental Figure 2). This suggests that the observed reduction in dACC activation during response monitoring for T carriers does not represent a more global pattern of dACC hypoactivation.
ROI-based comparisons of the two patient groups with the healthy controls of the prior study (Fig. 2b) revealed similar dACC activation during error commission for C homozygotes [F(1,21)=0.03, p=.87], but greater activation in C/C patients during correct antisaccades [F(1,21)=5.11, p=.03]. T carrier patients showed significantly reduced activation for errors [F(1,23)=5.08, p=.03] and no difference on correct trials [(F(1,23)=1.09, p=.31].
DTI
T carriers showed significantly reduced FA in the dACC compared to C homozygotes [Fig. 3, F(1,12)=6.59, p=.03]. There was a main effect of hemisphere reflecting greater FA in the left than right dACC [F(1,12)=11.20, p=.006], but no genotype by hemisphere interaction [F(1,12)=0.10, p=.76]. There was no significant effect of race [F(1,12)= 0.40, p=.84]. Since there were no African Americans in the T carrier group we could not test for a race × genotype interaction.
Fig. 3.
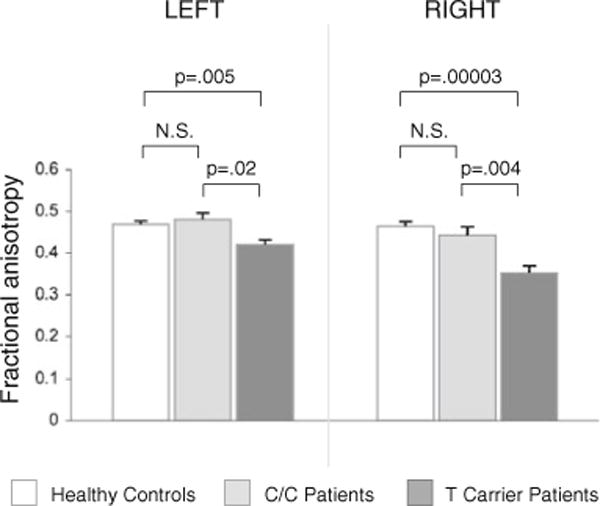
Bar graphs of mean dACC fractional anisotropy (FA) with standard error bars in healthy controls, and C/C and T carrier patients. Levene’s tests supported the assumption of equal variance between groups for each hemisphere (p’s>.1). N.S. not significant
Comparisons of the two patient groups with the healthy controls of the prior study revealed greater FA in controls than in T carrier patients bilaterally [left: t(26)=3.06, p=.005, right: t(26)=5.01, p=.00003], but no differences in FA between controls and C/C patients [left: t(23)=0.76, p=.45, right: t(23)=1.12, p=.27)].
Relations of error-related activation with FA
FA was not significantly related to error-related activation in either the left (β=−0.72, p=0.33) or right (β=−0.22, p=0.79) hemisphere, and this relation did not differ as a function of diagnosis (diagnosis × FA interaction, left: β=2.27, p=0.26; right: β=0.28, p=0.82). Among patients, genotype did not interact with FA to influence error-related activation (left: β=0.83, p=0.89; right: β=−1.06, p=0.82).
Discussion
Recent neuroimaging studies of healthy individuals demonstrate that common genetic polymorphisms affecting dopa-mine and serotonin neurotransmission contribute to variation in neural indices of response monitoring, including dACC activation and dACC functional connectivity with the striatum (Fallgatter et al. 2004; Klein et al. 2007; Kramer et al. 2007). To our knowledge, this is the first demonstration that a common polymorphism associated with schizophrenia risk contributes to a neural index of abnormal response monitoring in patients and to reduced microstructural integrity of the white matter underlying the implicated region. Patients with the 677T allele exhibited reduced FA of dACC white matter and significantly less error-related activation than C/C patients in the dACC, substantia nigra, and dorsolateral prefrontal cortex. These regions show error-related activation in healthy individuals (Polli et al. 2008; Taylor et al. 2007) and are hypothesized to mediate dopamine-dependent error-based reinforcement learning (Holroyd and Coles 2002), although others have theorized that error-related dACC activation and error related negativity could instead reflect response monitoring regardless of error commission (Carter et al. 1998), or index other action-regulation functions within the Papez circuit (Luu et al. 2003).
Because response monitoring is the product of coordinated activity in ACC networks, we also examined the microstructural integrity of the white matter underlying the dACC using DTI measures of FA. Similar to our findings with fMRI, T allele carriers showed reduced microstructural integrity of dACC white matter compared to C/C patients, and only the T carriers showed reduced FA relative to healthy controls. Thus, the FA differences between patients and controls in our prior study (Manoach et al. 2007) were largely driven by T carriers. It is also possible, though speculative, that differences in sample composition with regard to T allele frequency might have contributed to discrepancies in the literature between positive (Ardekani et al. 2003; Hao et al. 2006; Kubicki et al. 2003; Sun et al. 2003; Wang et al. 2004) and negative (Agartz et al. 2001; Burns et al. 2003; Foong et al. 2002) reports of reduced FA in the cingulum bundle in schizophrenia. Along with our prior finding that MTHFR 677T was specifically related to a behavioral index of response monitoring (i.e., perseverative errors, Roffman et al. 2007), our current findings concerning dACC function and structure demonstrate a detrimental influence of MTHFR 677T on behavioral and functional indices of response monitoring in schizophrenia and the structural integrity of the implicated region.
Blunted error-related activation in schizophrenia is thought to be disadvantageous and may reflect deficient error-based reinforcement learning. In these same patient and control samples, we previously reported that blunted error-related dACC activation predicted a higher error rate (Polli et al. 2008). This finding is consistent with prior reports in healthy individuals that both error-related ACC activation and the ERN are inversely related to error rate (Brown and Braver 2005; Fitzgerald et al. 2009; Hajcak et al. 2003; Holroyd and Coles 2002). It is also consistent with the theory that both increased dACC activation and the ERN reflect the use of dopamine-dependent error signals to modify the associative strength of stimulus-response mappings in the service of optimizing behavioral outcomes (Holroyd et al. 2003, 2004). Thus, both dACC activity following errors and the ERN can be conceptualized as dopamine-dependent training signals that are used to learn from errors (Brown and Braver 2005; Holroyd and Coles 2002). A more blunted dACC response in T carriers suggests reduced learning from errors that may contribute to more severe perseverative behavior, a common feature of schizophrenia.
Compared to control participants, C/C patients exhibited a significant increase in dACC activation following correct responses. This may represent a hemodynamic correlate of the correct-related negativity (CRN), an event-related potential that is sometimes observed following correct responses, and that is enhanced in schizophrenia (Mathalon et al. 2002). It may also reflect greater response uncertainty (Scheffers and Coles 2000) or greater or more persistent effects of response conflict from the requirement to perform an antisaccade (Carter and van Veen 2007).
Previous work from our group and others has indicated that in addition to increasing overall risk for schizophrenia (Allen et al. 2008), the 677T allele is associated with earlier age of onset (Vares et al. 2009), more pronounced negative symptoms (Roffman et al. 2008c), reduced verbal fluency, and more severe executive dysfunction (Roffman et al. 2007), as well as with blunted working memory load-dependent dorsolateral prefrontal cortex recruitment (Roffman et al. 2008a) in schizophrenia patients. Effects of the 677T allele on executive dysfunction (Roffman et al. 2008b) and related brain activation (Roffman et al. 2008a) are augmented by the high-activity 158Val variant of COMT, which catabolizes cortical dopamine more efficiently than the 158Met variant. These findings suggest that the 677T allele may exacerbate underlying cortical dopa-mine deficiency in schizophrenia. While the size of our cohort precluded the study of interaction of COMT 158Val>Met with MTHFR on dACC function during response monitoring, it would be of interest to examine these effects, and those of other genes that putatively influence dopamine signaling in schizophrenia.
We observed MTHFR genotype effects on error-related activation in three reciprocally connected areas that comprise a circuit for dopamine-dependent error-based reinforcement learning (Holroyd and Coles 2002): dACC, substantia nigra, and dorsolateral prefrontal cortex. We also saw greater activation for C homozygotes in the ventrolateral prefrontal cortex, which has been associated with posterror adjustments of performance (Li et al. 2008). Based on these findings we are not able to determine whether these effects reflect the actions of MTHFR in one of these regions or elsewhere in the brain, or whether they reflect the effects of MTHFR on dopamine, glutamate, GABA, and/or other neurotransmitter systems that have been implicated in schizophrenia. In prior work we observed that MTHFR interacts with COMT 158Val>Met to affect fMRI activation in the dorsolateral prefrontal cortex (Roffman et al. 2008a) suggesting that it affects prefrontal dopamine. Another variant affecting prefrontal dopamine, DRD4 SNP -521, has been shown to affect the ERN (Kramer et al. 2007). In addition, DRD2-TAQIA, which modulates dopamine D2 receptor density and has been associated with dopamine synthesis in the striatum (Laakso et al. 2005), also affects dACC activation during response monitoring (Klein et al. 2007). More basic research will be necessary to understand the mechanisms of MTHFR effects.
We report a T allele-related reduction in FA in the white matter underlying the dACC in schizophrenia. Given the small sample, we view this finding as preliminary. FAwas not significantly related to error-related activation, and this relation did not differ as a function of diagnosis or genotype, but we again note that the study may have been underpowered to detect meaningful relations between dACC function and structure. The mechanism through with MTHFR may influence white matter integrity in schizophrenia remains uncertain; however, the lack of an interactive effect of MTHFR genotype and FA on error-related dACC activation suggests that the two deficits may be relatively independent sequelae of having the T allele. Interestingly, MTHFR deficiency has been associated with leukoencephalopathy (Bishop et al. 2008; Tallur et al. 2005; Walk et al. 1994), and the 677T allele has been linked to white matter lesions in large cohorts of elderly subjects (Hong et al. 2009; Kohara et al. 2003). These reports speculate that the observed white matter changes are due to homocysteine elevations, a direct metabolic consequence of low MTHFR activity. Although folate and homocysteine levels were not available for the present cohort, it would be of interest to examine interactive effects of these markers with MTHFR genotype in future schizophrenia neuroimaging studies.
It is well-accepted that genetic variation influences brain function and contributes to cognitive deficits in schizophrenia. However, such genetically-mediated alterations in brain function are not always manifest at the level of behavior. In the present study, despite the significant differences in dACC activation between genotype groups, there were no differences in task performance (antisaccade error rate, latency, or rate of error self-correction), a pattern frequently observed in the COMT/working memory literature [e.g., (Ho et al. 2005)]. Intact behavior may reflect the use of an alternate strategy and/or the recruitment of compensatory neural circuitry. It may also reflect that brain function is more proximal to putative genetic mechanisms than behavior, and larger sample sizes might be required to observe a behavioral effect.
Although previous work demonstrated stronger MTHFR effects on DLPFC activation in schizophrenia patients than controls (Roffman et al. 2008a), it is possible that effects of MTHFR on error-related dACC activation and dACC white matter microstructural integrity would also be seen in healthy participants, who were not genotyped for this study. This, the small sample size, and the different racial composition of the genotype groups comprise the primary limitations of this investigation, which should be viewed as preliminary until replicated in a larger and more homogeneous cohort. In the present study, however, there were no significant main effects of race, or race by genotype interactions and the results did not change substantially when race was removed from the model (main effects of MTHFR on fMRI: p changed from .07 to .05; main effects of MTHFR on DTI: p changed from .03 to .007). This suggests that neither race itself, nor adjustment for race, contributed substantially to our findings.
In summary, the present findings that MTHFR 677T genotype is associated with more abnormal dACC structure and function during response monitoring add to a highly convergent body of evidence implicating this allele in executive dysfunction in schizophrenia. We hypothesize that the observed genotype effects are mediated by dopaminergic signaling in the dACC circuitry implicated in error-based reinforcement learning (Holroyd and Coles 2002). The more blunted error-related activation in T allele carriers may exacerbate reinforcement learning deficits in schizophrenia (Waltz et al. 2007) and thereby contribute to rigid, perseverative behavior, a hallmark feature of the disorder.
Acknowledgments
This work was supported by the National Institute for Mental Health (R01 MH67720) and NARSAD Independent Investigator Award (to DSM); the Charles A. King Trust, Bank of America, Co-Trustee and the Bushrod H. Campbell and Adah F. Hall Charity Fund, Alden Trust, and Howard Hughes Medical Institute Physician Scientist Early Career Award (to JLR); the Mental Illness Neuroscience Discovery (MIND) Institute (DOE DE-FG02-99ER62764); and the National Center for Research Resources (P41RR14075). We thank Doug Greve for helpful discussions regarding the manuscript. In memory of Jesse Friedman.
Footnotes
Jesse S. Friedman: Deceased
Electronic supplementary material The online version of this article (doi:10.1007/s11682-010-9111-2) contains supplementary material, which is available to authorized users.
Conflict of Interest
None of the authors report any conflicts of interest.
Contributor Information
Joshua L. Roffman, Email: jroffman@partners.org, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA Massachusetts General Hospital, 149 13th St, Room 2606, Charlestown, MA 02129, USA.
David G. Brohawn, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA
Jesse S. Friedman, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA
Kara A. Dyckman, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA
Katharine N. Thakkar, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA
Yigal Agam, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Mark G. Vangel, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston MA 02114 USA Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA 02129, USA.
Donald C. Goff, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA
Dara S. Manoach, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA 02129, USA.
References
- Agartz I, Andersson JL, Skare S. Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. NeuroReport. 2001;12(10):2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M, et al. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: evidence for an endophenotype. Biological Psychiatry. 2008;64(7):615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nature Genetics. 2008;40(7):827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psycho-physiology. 2008;45(4):524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. NeuroReport. 2003;14(16):2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L, Kanoff R, Charnas L, Krenzel C, Berry SA, Schimmenti LA. Severe methylenetetrahydrofolate reductase (MTHFR) deficiency: a case report of nonclassical homocystinuria. Journal of Child Neurology. 2008;23(7):823–828. doi: 10.1177/0883073808315410. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. The British Journal of Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Human Brain Mapping. 2000;11(4):249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. The American Journal of Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, et al. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology. 2004;29(8):1506–1511. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. New York: Biometrics Research, The New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Perkins SC, Angstadt M, Johnson T, Stern ER, Welsh RC, et al. The development of performancemonitoring function in the posterior medial frontal cortex. Neuroimage. 2009;49(4):3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA. Investigating regional white matter in schizophrenia using diffusion tensor imaging. NeuroReport. 2002;13(3):333–336. doi: 10.1097/00001756-200203040-00017. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophrenia Bulletin. 2009;35(5):973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. American Journal of Epidemiology. 2007;165(1):1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain and Cognition. 2008;68(3):371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and posterror compensatory behavior. Psychophysiology. 2003;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, et al. White matter integrity of the whole brain is disrupted in firstepisode schizophrenia. NeuroReport. 2006;17(1):23–26. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O’Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158-Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Molecular Psychiatry. 2005;10(3):229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. Errors in reward prediction are reflected in the event-related brain potential. NeuroReport. 2003;14(18):2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, et al. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7(5):497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Hong ED, Taylor WD, McQuoid DR, Potter GG, Payne ME, Ashley-Koch A, et al. Influence of the MTHFR C677T polymorphism on magnetic resonance imaging hyperintensity volume and cognition in geriatric depression. The American Journal of Geriatric Psychiatry. 2009;17(10):847–855. doi: 10.1097/JGP.0b013e3181aad5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. The American Journal of Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318(5856):1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Kohara K, Fujisawa M, Ando F, Tabara Y, Niino N, Miki T, et al. MTHFR gene polymorphism as a risk factor for silent brain infarcts and white matter lesions in the Japanese general population: The NILS-LSA Study. Stroke. 2003;34(5):1130–1135. doi: 10.1161/01.STR.0000069163.02611.B0. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Cunillera T, Camara E, Marco-Pallares J, Cucurell D, Nager W, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. The Journal of Neuroscience. 2007;27(51):14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, et al. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharma-cogenetics and Genomics. 2005;15(6):387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126(Pt 3):610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Levy DL, Mendell NR, LaVancher CA, Brownstein J, Krastoshevsky O, Teraspulsky L, et al. Disinhibition in antisaccade performance in schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and development of schizophrenia. Washington DC: American Psychological Association; 1998. pp. 185–210. [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20(6):1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Ketwaroo GA, Polli FE, Thakkar KN, Barton JJ, Goff DC, et al. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007;37(2):599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology. 2002;111(1):22–41. [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(43):15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic Resonance in Medicine. 2003;49(1):177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophrenia Research. 2007;92(1–3):181–188. doi: 10.1016/j.schres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, et al. MTHFR 677C–>T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val–>Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, et al. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics. 2008;147B(6):990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Purcell S, Caffalette CA, Freudenreich O, Henderson DC, et al. Contribution of methylenetetrahyrdofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biological Psychiatry. 2008;63(1):42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(1):141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, et al. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. NeuroReport. 2003;14(14):1833–1836. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- Tallur KK, Johnson DA, Kirk JM, Sandercock PA, Minns RA. Folate-induced reversal of leukoencephal-opathy and intellectual decline in methylene-tetrahydrofolate reductase deficiency: variable response in siblings. Developmental Medicine and Child Neurology. 2005;47(1):53–56. doi: 10.1017/s0012162205000095. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. The Neuroscientist. 2007;13(2):160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Vares M, Saetre P, Deng H, Cai G, Liu X, Hansen T, et al. Association between methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and age of onset in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.31030. [DOI] [PubMed] [Google Scholar]
- Walk D, Kang SS, Horwitz A. Intermittent encephalopathy, reversible nerve conduction slowing, and MRI evidence of cerebral white matter disease in methylenetetrahydrofolate reductase deficiency. Neurology. 1994;44(2):344–347. doi: 10.1212/wnl.44.2.344. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. The American Journal of Psychiatry. 2004;161(3):573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]


