Abstract
The open reading frame contained within the long terminal repeat (LTR) of mouse mammary tumor virus encodes Naf, a negative regulator of transcription, as well as a superantigen activity, Sag, which causes the deletion of specific classes of T cells. In the present study, the effect of Naf expression on different promoters and the coding requirements for Naf and Sag have been investigated. Sag activity was found to require only sequences in the LTR, whereas sequences located within the gag gene were additionally required for functional Naf activity. Surprisingly, both the classic promoter and a recently described promoter located in the LTR can give rise to both functional Naf and Sag. Further analysis of Naf revealed that the downregulatory effect was mediated by sequences located in the LTR and that heterologous promoters were also affected by Naf.
Full text
PDF
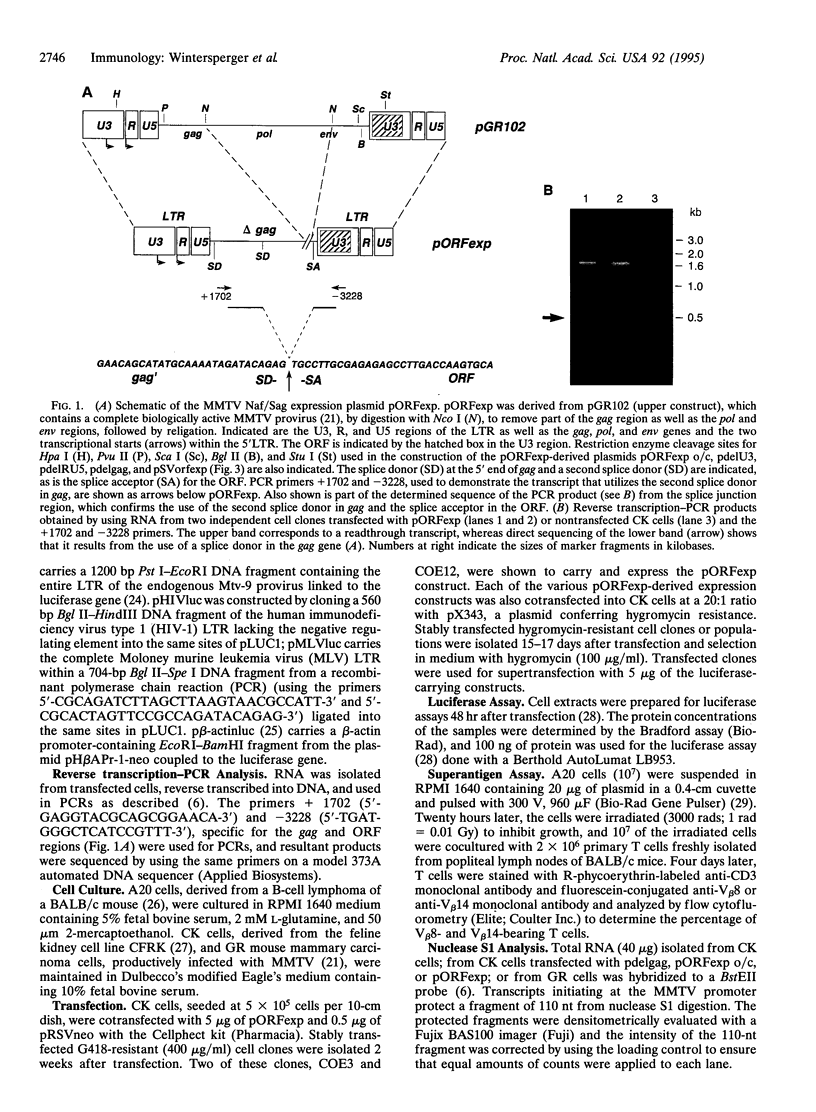
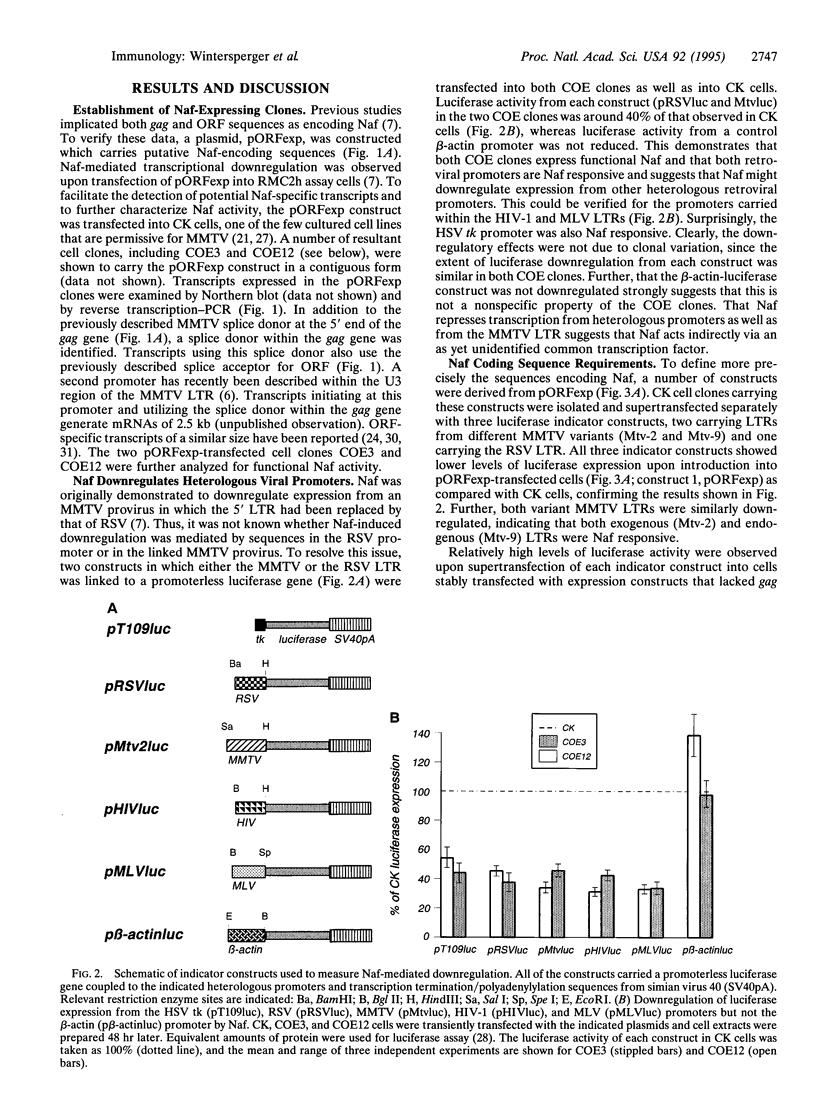
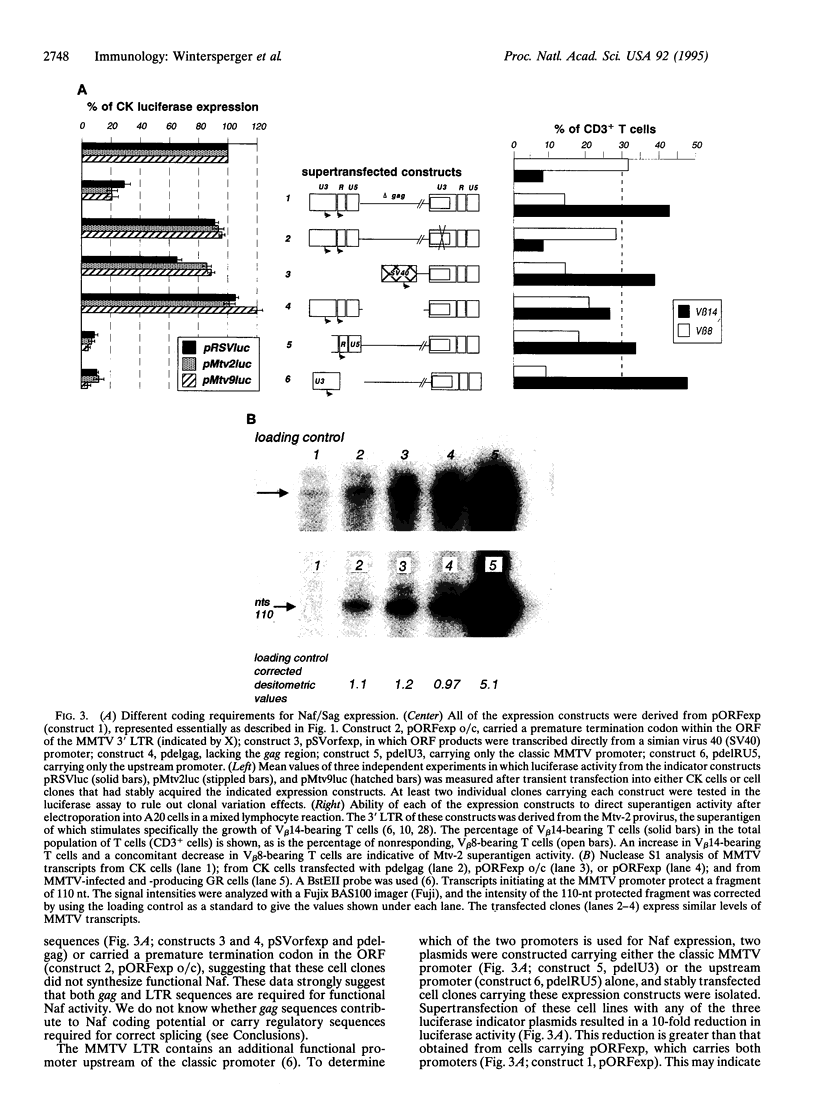

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., MacDonald H. R. Subversion of host immune responses by viral superantigens. Trends Microbiol. 1993 Apr;1(1):32–34. doi: 10.1016/0966-842x(93)90022-j. [DOI] [PubMed] [Google Scholar]
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Brandt-Carlson C., Butel J. S. Detection and characterization of a glycoprotein encoded by the mouse mammary tumor virus long terminal repeat gene. J Virol. 1991 Nov;65(11):6051–6060. doi: 10.1128/jvi.65.11.6051-6060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt-Carlson C., Butel J. S., Wheeler D. Phylogenetic and structural analyses of MMTV LTR ORF sequences of exogenous and endogenous origins. Virology. 1993 Mar;193(1):171–185. doi: 10.1006/viro.1993.1113. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzburg W. H., Heinemann F., Wintersperger S., Miethke T., Wagner H., Erfle V., Salmons B. Endogenous superantigen expression controlled by a novel promoter in the MMTV long terminal repeat. Nature. 1993 Jul 8;364(6433):154–158. doi: 10.1038/364154a0. [DOI] [PubMed] [Google Scholar]
- Günzburg W. H., Salmons B. Factors controlling the expression of mouse mammary tumour virus. Biochem J. 1992 May 1;283(Pt 3):625–632. doi: 10.1042/bj2830625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., Shakhov A. N., Izui S., Waanders G. A., Scarpellino L., MacDonald H. R., Acha-Orbea H. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med. 1993 Feb 1;177(2):359–366. doi: 10.1084/jem.177.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby P. J., Yang L., Lala D. S., Cheng C. Y., Salmons B. A modified procedure for replica plating of mammalian cells allowing selection of clones based on gene expression. Biotechniques. 1992 Feb;12(2):244–251. [PubMed] [Google Scholar]
- Huber B. T. Mls genes and self-superantigens. Trends Genet. 1992 Nov;8(11):399–402. doi: 10.1016/0168-9525(92)90302-k. [DOI] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Knight A. M., Harrison G. B., Pease R. J., Robinson P. J., Dyson P. J. Biochemical analysis of the mouse mammary tumor virus long terminal repeat product. Evidence for the molecular structure of an endogenous superantigen. Eur J Immunol. 1992 Mar;22(3):879–882. doi: 10.1002/eji.1830220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Bourgarel P., Meo T., Rieckhof G. E. The mouse mammary tumour virus long terminal repeat encodes a type II transmembrane glycoprotein. EMBO J. 1992 May;11(5):1901–1905. doi: 10.1002/j.1460-2075.1992.tb05242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C., Diggelmann H. The mouse mammary tumor virus long terminal repeat encodes a 47 kDa glycoprotein with a short half-life in mammalian cells. Mol Immunol. 1993 Sep;30(13):1151–1157. doi: 10.1016/0161-5890(93)90133-v. [DOI] [PubMed] [Google Scholar]
- Langer S. J., Ostrowski M. C. Negative regulation of transcription in vitro by a glucocorticoid response element is mediated by a trans-acting factor. Mol Cell Biol. 1988 Sep;8(9):3872–3881. doi: 10.1128/mcb.8.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F. E., Corley R. B. Regulated expression of mouse mammary tumor proviral genes in cells of the B lineage. J Exp Med. 1991 Dec 1;174(6):1439–1450. doi: 10.1084/jem.174.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F. E., Randall T. D., Woodland D. L., Corley R. B. MHC class II limits the functional expression of endogenous superantigens in B cells. J Immunol. 1993 Jan 1;150(1):78–86. [PubMed] [Google Scholar]
- Mohan N., Mottershead D., Subramanyam M., Beutner U., Huber B. T. Production and characterization of an Mls-1-specific monoclonal antibody. J Exp Med. 1993 Feb 1;177(2):351–358. doi: 10.1084/jem.177.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Pullen A. M., Choi Y., Kushnir E., Kappler J., Marrack P. The open reading frames in the 3' long terminal repeats of several mouse mammary tumor virus integrants encode V beta 3-specific superantigens. J Exp Med. 1992 Jan 1;175(1):41–47. doi: 10.1084/jem.175.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Erfle V., Brem G., Günzburg W. H. naf, a trans-regulating negative-acting factor encoded within the mouse mammary tumor virus open reading frame region. J Virol. 1990 Dec;64(12):6355–6359. doi: 10.1128/jvi.64.12.6355-6359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Groner B., Calberg-Bacq C. M., Ponta H. Production of mouse mammary tumor virus upon transfection of a recombinant proviral DNA into cultured cells. Virology. 1985 Jul 15;144(1):101–114. doi: 10.1016/0042-6822(85)90309-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow G. M., Marrack P., Kappler J. W. Processing and major histocompatibility complex binding of the MTV7 superantigen. Immunity. 1994 Apr;1(1):23–33. doi: 10.1016/1074-7613(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Winslow G. M., Scherer M. T., Kappler J. W., Marrack P. Detection and biochemical characterization of the mouse mammary tumor virus 7 superantigen (Mls-1a). Cell. 1992 Nov 27;71(5):719–730. doi: 10.1016/0092-8674(92)90549-r. [DOI] [PubMed] [Google Scholar]
- Wintersperger S., Indraccolo S., Miethke T., Günzburg W. H., Salmons B. A transient assay for gene expression studies in B lymphocytes and its use for superantigen assays. Biotechniques. 1994 May;16(5):882–886. [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]





