Abstract
Glycosaminoglycans (GAGs) have numerous applications in the fields of pharmaceuticals, cosmetics, nutraceuticals, and foods. GAGs are also critically important in the developmental biology of all multicellular animals. GAGs were isolated from chicken egg components including yolk, thick egg white, thin egg white, membrane, calcified shell matrix supernatant, and shell matrix deposit. Disaccharide compositional analysis was performed using ultra high-performance liquid chromatography-mass spectrometry. The results of these analyses showed that all four families of GAGs were detected in all egg components. Keratan sulfate was found in egg whites (thick and thin) and shell matrix (calcified shell matrix supernatant and deposit) with high level. Chondroitin sulfates were much more plentiful in both shell matrix components and membrane. Hyaluronan was plentiful in both shell matrix components and membrane, but were only present in a trace of quantities in the yolk. Heparan sulfate was plentiful in the shell matrix deposit but was present in a trace of quantities in the egg content components (yolk, thick and thin egg whites). Most of the chondroitin and heparan sulfate disaccharides were present in the GAGs found in chicken eggs with the exception of chondroitin and heparan sulfate 2,6-disulfated disaccharides. Both CS and HS in the shell matrix deposit contained the most diverse chondroitin and heparan sulfate disaccharide compositions. Eggs might provide a potential new source of GAGs.
Keywords: chicken egg, glycosaminoglycans, keratan sulfate, chondroitin sulfate, hyaluronan, heparan sulfate
Introduction
Glycosaminoglycans (GAGs) are linear, negatively charged polysaccharides composed of a variable number of repeating disaccharide units. Based on the structure of their repeating disaccharides, GAGs can be classified into four families, hyaluronan (HA), chondroitin sulfates (CS), heparan sulfate (HS), and keratan sulfate (KS). The disaccharide repeating units of HA, CS, HS and KS are comprised hexosamine residue, of N-galactosamine (GalN) or N-glucosamine (GlcN), and for HA, CS and HS residue, uronic acid D-glucuronic acid (GlcA) or L-iduronic acid (IdoA), and for KS, galactose (Gal). HA, the simplest GAG, contains neither sulfo groups nor is it attached to a core protein and has the structure →3) β-GlcNAc (1→4) β-GlcA (1→ (where Ac is acetyl). CS is an O-sulfo group substituted GAG with →3) β-GalNAc (1→4) β-GlcA or α-IdoA (1→ repeating units [1]. HS is an O-sulfo or N-sulfo group substituted GAG with repeating units of →4) α-GlcNAc or α-GlcNS (1→4) β-GlcA or α-IdoA (1→ (where S is sulfo). KS is an O-sulfo group containing GAG with a backbone structure of →4) β-GlcNAc (1→3) β-Gal (1→ [2].
Typically, GAGs are located on various animal cell surfaces and in the extracellular matrix (ECM), they are not only the major structural components of ECM and tissues, but also critical biological regulators. GAGs are involved in cell growth, differentiation, adhesion, and motility through acting with various receptors, such as growth factors, enzymes, cytokines [2, 3]. Because of intriguing biological activities of GAGs, they have numerous applications in the pharmaceutical, nutraceutical, cosmetic, and food industries. For example, HA is used in treating osteoarthritis [4], tendon disorders [5], and in cancer nanomedicine conjugates [6]; in plastic surgery, and as anti-wrinkle hydration cosmetics [7]. In addition to being used as an emulsifying agent in condiments and mayonnaise [8], CS has nutraceutical and pharmaceutical applications in arthritis herpes virus infection, malaria, nervous tissue repair, liver regeneration, and for tumors [9]. Heparin, a highly sulfated HS, has potent functions in allergy, inflammation [10] and anticoagulation [11], and has a long history of being used as anticoagulants in advanced medical and surgical procedures [12]. Finally, KS plays a fundamental role in ocular inflammation, corneal injury, keratitis, and uveitis [13].
Traditionally, GAGs are commercially produced from animal tissues as a by-product of the meat and livestock industry, coming from rooster combs, bovine and swine tracheas/nasal tissues, umbilical cords [14], bovine and porcine intestines and lungs [15], and bovine corneas [3]. These sources are generally limited to low value tissues, and the composition and chain lengths of the GAGs produced depend on their tissue sources and are critical for their proper biological functions [15-18]. Increasing demand for GAGs has resulted in a shortage of appropriate source-tissues and the quality and identity of many of the currently used tissues are difficult to control. Therefore, it is necessary to broaden the tissue sources of commercial GAGs.
Chicken egg contains various important nutrients, such as proteins, monounsaturated fatty acids, polyunsaturated fatty acids, minerals, vitamins, and peptides of unique biological activities, making eggs an important human food source throughout history [19]. An estimated 60 million tons of commercial eggs were produced worldwide in 2013, with China being the largest egg producer, at ~27 million tons.
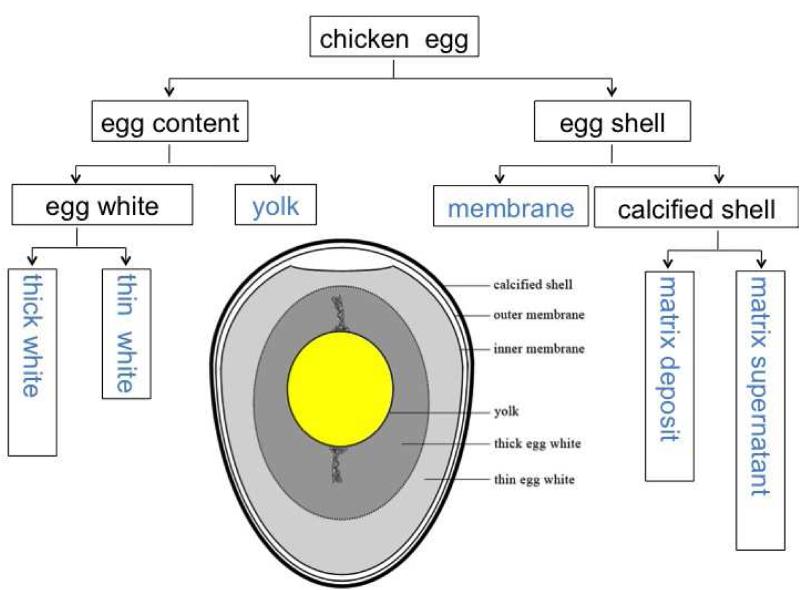
Chicken eggs are comprised of yolk, egg white (thick and thin), and eggshell (Fig. 1). The eggshell is ultrastructurally composed of a pair of shell membranes, calcified shell and a cuticle (Fig. 1) [20]. The yolk is formed in the ovary, while other egg components are formed in a specific region of the oviduct as the ovum passes through. After oviposition, the ovum first enters into the magnum, which secretes albumen (thick egg white), into the isthmus where shell membranes are formed, and finally into shell gland (uterus), which forms the calcified shell and the cuticle, and additionally adds fluid into the albumen to form thin egg white [21].
Fig. 1.
Schematic figure of a chicken egg and a flow chart of sample processing.
Most previous research has focused on the GAGs present in eggshell, especially in calcified shell and shell membrane, while research on GAGs in egg yolk and egg white are very limited. Data suggests that chondroitin-4-sulfate (CS-A), dermatan sulfate (CS-B), and HA are all present in the calcified eggshell [22-25], while KS has been detected in both the calcified shell and in the membrane [26]. However, the presence of these GAGs was based primarily on indirect immunohistochemical data, providing little information on the composition and structure of egg-derived GAGs.
In the present study, the composition and structure of GAGs in the chicken egg components (including yolk, thick egg white, thin egg white, shell membrane, and calcified shell) have been elucidated following their enzymatic conversion to GAG disaccharides using ultra-high-performance liquid chromatography-mass spectrometry (HPLC-MS), providing GAG composition and structure [27]. These results should help broaden our understanding of egg GAG composition and potentially provide a new, readily available source for GAGs.
Materials and methods
Materials
The twenty-two eggs used in these experiments were specific pathogen free (SPF) premium eggs laid by White Leghorn chickens (Charles River, NY, USA). Unsaturated disaccharide standards of CS (0SCS-0: ΔUA-GalNAc; 4SCS-A: ΔUA-GalNAc4S; 6SCS-C: ΔUA-GalNAc6S; 2SCS: ΔUA2S-GalNAc; 2S4SCS-B: ΔUA2S-Gal-NAc4S; 2S6SCS-D: ΔUA2S-GalNAc6S; 4S6SCS-E: ΔUA-GalNAc4S6S; TriSCS: ΔUA2S-GalNAc4S6S), unsaturated disaccharide standards of HS (0SHS: ΔUA-GlcNAc; NSHS: ΔUA-GlcNS; 6SHS: ΔUA-GlcNAc6S; 2SHS: ΔUA2S-GlcNAc; 2SNSHS: ΔUA2S-GlcNS; NS6SHS: ΔUA-GlcNS6S; 2S6SHS: ΔUA2S-GlcNAc6S; TriSHS: ΔUA2S-GlcNS6S), and unsaturated disaccharide standard of HA (0SHA: ΔUA-GlcNAc), where ΔUA is 4-deoxy-α-l-threo-hex-4-enopyranosyluronic acid, were purchased from Seikagaku (Japan). The saturated disaccharide standards, Gal-GlcNAc6S (NSKS) and Gal6S-GlcNAc6S (2SKS) for KS were prepared from bovine corneal KS in our laboratory using keratanase II. Actinase E was obtained from Kaken Biochemicals (Japan). Chondroitin lyase ABC from Proteus vulgaris and chondroitin lyase ACII from Arthrobacter aurescens; Keratanase II from Bacillus sp. Ks 36 were obtained from Seikagaku (Japan). Recombinant Flavobacterial heparin lyases I, II, and III were expressed in our laboratory using Escherichia coli strains provided by Jian Liu (College of Pharmacy, University of North Carolina). AMAC and sodium cyanoborohydride (NaCNBH3) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of HPLC grade. Vivapure Q Mini H strong anion exchange spin columns were from Sartoriou Stedim Biotech (Bohemia, NY, USA).
Preparation of egg components
Each egg was broken into half, the egg content was put into a clean plastic culture plate, and eggshell was washed with distilled water and dried at room temperature.
Yolk, thick egg white and thin egg white were prepared from each egg. Briefly, the egg white embracing yolk was carefully torn, then the whole yolk was carefully recovered from egg white, after being rinsed with distilled water and dried using a paper towel the vitelline membrane was broken to release the yolk. The thick egg white was continuously transferred from one clean plate to another four-times and the thick white remaining on the final plate was freeze-dried. The residue left during each egg white transfer was poured four-times into another clean plate and the thin egg white obtained was freeze-dried. After freeze-drying, the yolk sample was successively defatted by solution A (2 chloroform/1 methanol, V/V), solution B (1 chloroform/1 methanol, V/V), and solution C (1 chloroform/2 methanol, V/V); then the defatted yolk was freeze-dried again.
The samples of membrane, calcified shell matrix (including supernatant and deposit) were then obtained from eggshell. Briefly, eggshell was rinsed into 5% EDTA for 30 min, then washed with distilled water and the membranes were isolated. The eggshells were next rinsed into 5% EDTA for another 7min to completely remove the membrane. The membrane samples were combined and freeze-dried. The shells (calcified eggshell without membrane) were dried at room temperature and powdered. The eggshell powder was then decalcified by stirring with excess of 10% acetic acid at 4°C for 30 h. After dialysis against distilled water at 4°C for 40 h, the mixture was centrifuged at 17,500 × g for 20 min; the deposit was washed with distilled water and again centrifuged, the pellet was freeze-dried and designated as shell matrix deposit (water-insoluble component), and the supernatant was concentrated using a spin column (molecular weight cutoff (MWCO) 10 kDa, YM-10 membrane) and freeze-dried and designated as shell matrix supernatant (water-soluble component).
GAG recovery from egg components
The freeze-dried organic matter of each egg component was individually subjected to proteolysis at 55 °C for 40h using actinase E (1 mg actinase E/mg dry membrane, 0.5 mg actinase E/mg dry weight of the other egg components). After centrifugation at 6500 × g for 20 min, each sample was transferred into YM-10 spin columns to further remove small peptides then freeze-dried. The dried samples were dissolved into 8M urea containing 2% CHAPS (pH 7.8) and bound to Vivapure Q Mini H spin columns, after washing 4-times with 200 mM NaCl, crude GAGs were eluted using 16% NaCl. Finally, the crude GAGs samples were desalted using YM-10 spin column and freeze-dried.
Disaccharide preparation of GAGs in egg components
The crude GAGs in the various egg components were individually and completely depolymerized using polysaccharides lyases and hydrolase. Chondroitin lyase ABC (20 mU) and chondroitin lyase ACII (10 mU) in 0.1% BSA were added to ~200 μg crude GAGs in buffer of 100 mM ammonium containing 10mM CaCl2 (pH 7.5; final volume 100 μL). Keratanase II (60 mU) in 0.1% BSA was added to ~200 μg crude GAGs in 50mM Tris-HCl buffer (pH7.5; final volume 100 μL). Heparin lyase I, II, III (40 mU/each enzyme) in 25 mM Tris, 500 mM NaCl, 300 mM imidazole buffer (pH 7.4) were added to ~500 μg crude GAGs (both shell matrix deposit and supernatant crude GAGs were ~200 μg) (final volume 100 μL). Reacting mixtures were incubated at 35°C for 8 h. After boiling to inactivate the enzymes at 100°C for 2 min, the mixtures were centrifuged with 14,500 × g for 15 min, and then the supernatants were freeze-dried.
Derivatization of unsaturated disaccharides with AMAC
The freeze-dried biological sample containing GAG-derived disaccharides or a mixture of unsaturated disaccharide standards (5 μg/per each disaccharide) was combined with 10 μL 0.1 M AMAC solution in acetic acid (AcOH)/dimethyl sulfoxide (DMSO) (3:17, v/v) and mixed by transient vortexing and standing at room temperature for 30 min. Next, 10 μL of 1 M NaBH3CN was added in the reaction mixture and incubated at 45°C for 4.5 h. Finally, the AMAC-tagged disaccharide mixtures were centrifuged with 14,500 × g for 15 min, and the supernatants were used for HPLC-MS analysis.
HPLC-MS
HPLC-MS analyses were performed on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Inc., Wilmington, DE) equipped with a 6300 ion-trap and a binary pump. The column used was a Poroshell 120 C18 column (3.0 × 150 mm, 2.7 m, Agilent, USA) at 45°C. Eluent A was ammonium acetate solution (80 mM) and eluent B was methanol. Solution A and 15% solution B was flowed (150 μL/min) through the column for 5 min followed by linear gradients 15-30% solution B from 5 to 30 min.
The column effluent entered the ESI-MS source for continuous detection by MS. The electro spray interface was set in negative ionization mode with a skimmer potential of -40.0 V, a capillary exit of -40.0 V, and a source temperature of 350°C, to obtain the maximum abundance of the ions in a full-scan spectrum (300-1200 Da). Nitrogen (8 L/min, 40 psi) was used as a drying and nebulizing gas.
Quantification analysis of AMAC-labeled HS, CS, HA disaccharides was performed using calibration curves established by separation of increasing amounts of disaccharide standards (0.1, 0.5, 1, 5, 10, 20, 50, 100 ng/each). Linearity was assessed based on the amount of disaccharide and peak intensity in extracted ion chromatogram (EIC).
Quantification analysis of AMAC-labeled KS disaccharides in the samples was based on the KS-disaccharides in the bovine corneal KS acquired by Keratanase II digestion. It was reported that the molar proportion of 2SKS/NSKS was 38: 53 [29]. According these different concentrations of bovine corneal KS disaccharides analyzed by LC-MS, the KS disaccharides in the samples were analyzed and quantified.
Results and analysis
Characterizations of egg components
Based on careful manual dissection, each egg yielded an average of 8.04 g dried yolk, 2.04 g dried thick egg white, 1.62 g dried thin egg white, 137 mg dried membrane, and 5.95 g calcified shell (Table 1).
Table 1.
Characterizations of the egg components
| parameters | yolk | Thick egg white | Thin egg white | membrane | calcified shell |
|---|---|---|---|---|---|
| Dry weight in each egg (mg) | 8040±26 | 2040±99 | 1620±65 | 137±4 | 5950±160 |
| Water content (%) | 48.7±0.2 | 88.01±0.17** | 87.96±0.18** | ND | ND |
| lipid content (%) | 65.6±0.6* | ND | ND | ND | ND |
| Matrix supernatant content (%) | ND | ND | ND | ND | 0.12±0.04 |
| Matrix deposit content (%) | ND | ND | ND | ND | 1.55±0.01 |
Note: ND, not detected,
the proportion based on freeze-dried yolk,
there was a significant difference between the values (P=0.009).
The fresh yolk contained 48.7% water, and in dried yolk, there was 65.6% of lipid (Table 1).
After centrifugation of 17,500 × g, the eggshell matrix was isolated into two parts, supernatant and deposit. The contents of shell matrix supernatant and deposit in calcified shell were respectively 0.12% and 1.55% (Table 1). The calcified shell contained a total of 1.67% organic matrix, lower than the 2.29% previously reported on combusting at 580°C in a muffle furnace [30]. The present results suggest that each calcified eggshell affords ~99.6 mg organic matrix, slightly higher than the previous estimates of 50-75 mg [31]. Some of this difference may be attributable to eggshell size and different methods used to obtain the organic matrix.
KS and its composition characterizations in various egg components
After digestion of bovine corneal KS with keratanase II, two saturated disaccharide standards, 2SKS (Gal6S(1→4)GlcNAc6S) and NSKS (Gal(1→4)GlcNAc6S), could be obtained [28]. The HPLC-MS results showed that in all egg components examined, the disaccharide 2SKS was undetectable (Table 2, Fig. 2). This suggests that KS in various chicken egg components is only comprised of the NSKS disaccharide. Since the egg KS just contained disaccharide NSKS, the KS content in each egg component could be directly reflected by NSKS content (Table 2).
Table 2.
Disaccharide composition of the egg components
| Disaccharide standards |
yolk | thick egg white |
thin egg white | membrane | shell matrix supernatant |
shell matrix deposit |
|---|---|---|---|---|---|---|
| 2SKS | ND | ND | ND | ND | ND | ND |
| NSKS | 14.5 | 252 | 76.1 | 48.0 | 713 | 153 |
|
| ||||||
| Total (KS) | 14.5 | 252 | 76.1 | 48.0 | 713 | 153 |
|
| ||||||
| 0SHA | 0.18 | 5.19 | 4.98 | 364 | 798 | 325 |
|
| ||||||
| Total (HA) | 0.18 | 5.19 | 4.98 | 364 | 798 | 325 |
|
| ||||||
| TriSCS | ND | ND | ND | ND | ND | 5.80 |
| 2S4SCS-B | ND | ND | ND | ND | ND | 3.93 |
| 2S6SCS-D | ND | ND | ND | ND | ND | ND |
| 4S6SCS-E | ND | ND | 0.20 | 0.22 | ND | 97.6 |
| 2SCS | ND | ND | 0.02 | ND | ND | ND |
| 4SCS-A | 9.35 | 2.23 | 0.80 | 43.8 | 592 | 3600 |
| 6SCS-C | 0.15 | 0.55 | 0.45 | 1.65 | 33.7 | 364 |
| 0SCS-O | 8.73 | 8.23 | 6.88 | 45.3 | 853 | 1500 |
|
| ||||||
| Total (CS) | 18.2 | 11.0 | 8.4 | 91.0 | 1480 | 5570 |
|
| ||||||
| TriSHS | ND | ND | ND | ND | ND | 67.5 |
| NS6SHS | ND | ND | ND | ND | ND | 67.5 |
| NS2SHS | ND | ND | ND | 0.23 | 1.32 | 50.0 |
| NSHS | ND | ND | ND | 1.96 | 10.7 | 204 |
| 2S6SHS | ND | ND | ND | ND | ND | ND |
| 6SHS | ND | ND | ND | 0.13 | 1.63 | 38.1 |
| 2SHS | ND | ND | ND | ND | ND | 4.36 |
| 0SHS | 0.14 | 0.09 | 0.09 | 8.14 | 31.2 | 380 |
|
| ||||||
| Total (HS) | 0.14 | 0.09 | 0.09 | 10.5 | 44.9 | 811 |
Note: Disaccharides and GAG contents expressed as nanogram per milligram freeze-dried matter; ND, not detected.
Fig. 2.
Extracted ion chromatographs (EICs) of AMAC-labeled KS and its disaccharides in various egg components analyzed by LC-MS.
A. KS disaccharide standards; B. yolk; C. thick egg white; D. thin egg white; E. membrane; F. shell matrix supernatant; G. shell matrix deposit.
There were major differences for NSKS content among various egg components. The content of KS in the yolk was the lowest, just 14.5 ng per mg defatted yolk (Table 2). The concentrations of KS in both egg whites were much higher, especially in thick egg white (252 ng/mg dried matter), which was over 3-times higher than in thin egg white (Table 2).
Among three eggshell components, the concentration of KS in the membrane was lowest (48.0 ng/mg dried matter) (Table 2), while the shell matrix supernatant contained much higher KS levels, 713 ng per mg dried matter, nearly 5-times the level of KS in the shell matrix deposit (Table 2). These results demonstrate that calcified shell contained much more KS than membrane (since each egg contained nearly 137 mg of membrane and ~99.6 mg of shell matrix), and that a considerable amount of KS in the shell matrix was water-soluble.
Among the egg components, the concentration of KS in the shell matrix supernatant was the highest; however, based on dry organic matter (Table 1), the thick egg white represents the largest source of KS in the egg.
CS/DS and its disaccharide characterizations in various egg components
All egg components examined contain CS (Table 2, Fig. 3), but there were major differences in the CS content in the various components (Table 2). The amount of CS content in the yolk, thick egg white and thin egg white was similar, i.e. 18.2, 11.0, and 8.4 ng/mg dried matter; while the components of eggshell contained much higher CS, the content in the membrane was 91.0 ng/mg dried matter, shell matrix supernatant was 1480 ng/mg dried matter, and the content in the shell matrix deposit was the highest, even up to 5570 ng/mg dried matter (Table 2).
Fig. 3.
EICs of AMAC-labeled CS and HA and its disaccharides in various egg components analyzed by LC-MS.
A. CS and HA disaccharide standards; B. yolk; C. thick egg white; D. thin egg white; E. membrane; F. shell matrix supernatant; G. shell matrix deposit.
Of the eight possible CS disaccharides, three disaccharides of 4SCS, 6SCS, and 0SCS-0 were common to all egg components (Table 2, Fig. 3). The 2S6SCS-D was absent in all egg components, and 2SCS was present only in the thin egg white and then only in trace amounts (0.02 ng/mg) (Table 2, Fig. 3). Of the remaining three disaccharides, 4S6SCS-E was present in the thin egg white, membrane, and shell matrix deposit (Table 2; Fig. 3D, 3E, 3G); and both TriSCS and 2S4SCS-B were only present in shell matrix deposit (Table 2, Fig. 3G).
The yolk, thick egg white, and shell matrix supernatant were comprised of 4SCS, 6SCS, and 0SCS-0with 0SCS-0and 4SCS as major components (Table 2; Fig. 3B, 3C, 3F). The membrane was comprised of 4S6SCS-E, 4SCS, 6SCS, and 0SCS-0 with 0SCS-0 and 4SCS as major components (Table 2, Fig. 3E). The thin egg white was comprised of 4S6SCS-E, 2SCS, 4SCS, 6SCS, and 0SCS-0 with 0SCS-0 as major components (Table 2, Fig. 3D).The shell matrix deposit was comprised of TriSCS, 2S4SCS-B, 4S6SCS-E, 4SCS, 6SCS, and 0SCS-0 as major components with TriSCS and 2S4SCS-B as minor components (Table 2, Fig. 3G).
HA and its disaccharide characterizations in various egg components
HA is the simplest GAG, and is comprised of repeating unit of 0SHA [(1→3)-β-D-GlcNAc-(1→4)-β-D-GlcA]. HA was detectable in all egg components (Table 2, Fig. 3), HA was found in trace amounts, only 0.18 ng per mg dried matter in yolk (Table 2). The HA in both thick and thin egg whites were similar, 5.19 ng per mg and 4.98 ng per mg, respectively (Table 2). The HA contents in the membrane and the shell matrix deposit were similar, 364 and 325 ng per mg dried matter, respectively (Table 2). Shell matrix supernatant with the highest HA content, 798 ng per mg of dried matter (Table 2).
HS and its disaccharide characterizations in various egg components
HS was detectable in all egg components (Table 2, Fig. 4).Only trace amounts of HS were found in yolk, thick egg white, and thin egg white, 0.14, 0.09, and 0.09 ng per mg dried matter, respectively (Table 2). More HS was found in the membrane and the shell matrix supernatant, 10.5 and 44.9 ng per mg of dried matter, respectively (Table 2). Shell matrix deposit, showed the greatest HS content, 811 ng per mg of dried matter (Table 2).
Fig. 4.
EICs of AMAC-labeled HS and its disaccharides in various egg components analyzed by LC-MS.
A. HS disaccharide standards; B. yolk; C. thick egg white; D. thin egg white; E. membrane; F. shell matrix supernatant; G. shell matrix deposit. No. 1 peaks in Fig. 3A, 3B, 3C represented 0S disaccharides.
HS disaccharide analysis showed that 0SHS is present in various egg components, while 2S6SHS was undetectable in all egg components (Table 2, Fig. 4). Of the remaining 6 HS disaccharides, NS2SHS, NSHS, and 6SHS were detected in the three eggshell components, i.e. membrane, shell matrix supernatant, and shell matrix deposit (Table 2; Fig. 4E, 4F, 4G); while TriSHS, NS6SHS, and 2SHS were only detected in the shell matrix deposit (Table 2, Fig. 4G).
The HS compositions varied greatly in different egg components. The HS in the yolk, thick egg white, and thin egg white, was heparosan, affording only the 0SHS disaccharide (Table 2; Fig. 4B, 4C, 4D). The HS in the membrane and the shell matrix supernatant were both comprised of four disaccharides, NS2SHS, NSHS, 6SHS, and 0SHS with 0SHS being the most prominent (Table 2; Fig. 4E, 4F). Finally, the shell matrix deposit showed a much more structurally diverse HS, being comprised of 7 of the 8 HS disaccharides, TriSHS, NS6SHS, NS2SHS, NSHS, 6SHS, 2SHS, and 0SHS and a trace amount of 2SHS (Table 2, Fig. 4G).
Discussion
Previous researchers reported that GAGs were present in chicken eggshell [25, 26]. Immunohistochemical and enzymatic data showed that in the calcified eggshell contained HA [25], CS [25, 26], and KS [26]. The present study demonstrates that the calcified shell, regardless of shell matrix deposit or supernatant, additionally contains HS (Table 2). These results also broaden the GAG compositional data in the eggshell matrix supernatant, since it had been previously reported that only HA was present in the shell matrix supernatant [23]. The differences between the previous and current study may be the results of different methods used as well as improved analytical sensitivity. The shell membrane reportedly only contained KS [26]; however, the current study demonstrates the presence of all four types of GAGs in the shell membrane (Table 2).
It was reported that the predominant GAG in the shell palisade region (the major part of calcified shell, about 70% thickness of calcified shell) was dermatan sulfate (CS-B) with a high content of 4-sulfated rather than 6-sulfated disaccharides [26]. Our data, while not distinguishing between CS and dermatan sulfate, were consistent with these results. The CS content (DS is a specialized CS (CS-B) containing IdoA) in the whole shell matrix (considering combined shell matrix supernatant and deposit) was much higher than other three types of GAGs (Table 3). Compared with the disaccharide compositions of CS in the shell matrix supernatant and also in the shell matrix deposit, the concentrations of 4SCS is much higher than 6SCS (592 vs. 33.7 ng/mg; 3600 vs. 364 ng/mg) (Table 2). The concentration of uronic acid in the decalcified eggshell is reportedly 6.34 μg/mg organic matter [25]. Since uronic acid is a moiety in repeating disaccharides of all GAGs except for KS, this suggests that the uronic acid containing GAGs might be two-times higher than the concentration in calcified shell matrix. However, our results showed the content of uronic acid containing GAGs in calcified shell matrix was 6.38 μg/mg organic matter (Table 3). Different detection methods or egg sources might partially account for these differences, alternatively, uronic acid might be a component of other organic non-GAG material.
Table 3.
GAG contents in the whole calcified shell matrix
| GAG | KS | CS | HA | HS |
|---|---|---|---|---|
| Content (ng/mg) | 195 | 5270 | 360 | 755 |
Note: GAG contents expressed as nanogram per milligram freeze-dried matter.
The fine structures of GAGs depend on sources, for example, sulfated disaccharides of CS from chicken cartilage were 4SCS-A and 4S6SCS-E [16]. The results of the current study show that 4S6SCS-E is only present in shell matrix deposit, and in the thin egg white and in trace amounts in the membrane, while 4SCS-A is present in all egg components (Table 2). The CS in the shell matrix deposit contains diversely sulfated disaccharides, including TriSCS, 2S4SCS-B, and 6SCS-C in addition to the above two disaccharides (Table 2).
Egg is a very important food with a large commercial production and consumption, and the present study demonstrates that each egg component, particularly eggshell components, contain all four types of GAGs. However, it is known that the disaccharide constituents and chain lengths of GAGs are critical to their property and biological functions [15, 17, 18]; for example, 4S6SCS-E is a potent antiviral agent [32], HA with high molecular weight demonstrates antiangiogenic activity effect [33] while low molecular weight HA fragments can stimulate angiogenesis [34], heparins are anticoagulants with low molecular weight heparins having higher anti-FXa activity and lower incidence of side effects [12]. Therefore, the properties and biological activities of GAGs in the egg components need further elucidation.
Conclusion
All four types of GAGs are present in yolk, thick egg white, thin egg white, membrane, eggshell matrix supernatant, and shell matrix deposit. KS was rich in the both egg whites and both shell matrix components (Table 2). CS is plentiful in both shell matrix components (Table 2). HA is plentiful in both shell matrix components and membrane, but is only present in trace amounts in the yolk (Table 2). HS is plentiful in shell matrix deposit, but only trace amounts present in three egg content components, yolk, thick whites and thin whites (Table 3).
The 2S6SCS-D disaccharide is absent in all egg components, and 2SCS is only present in trace amounts in the thin egg white (Table 2, Fig 3). The 2S6SHS disaccharide is absent in all egg components, and 2SHS is only present in trace amounts in the shell matrix deposit (Table 2, Fig 4).
Finally, both CS and HS in the shell matrix deposit contained much more diverse disaccharide compositions (Table 2, Table 3).
Acknowledgements
The authors thank the US National Institutes of Health (HL62244, HL094463, HL096972, GM102137), the National Natural Science Foundation of China (NSFC 31372303, 30700567) and Zhejiang Provincial Natural Science Foundation of China (LY12C17002) for supporting this research.
References
- 1.Capila I, Linhardt RJ. Heparin-protein interactions. Angew. Chem. Int. Ed. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 3.Weyers A, Yang B, Solakyildirim K, Yee V, Li L, Zhang F, Linhardt RJ. Isolation of bovine corneal keratan sulfate and its growth factor and morphogen binding. FEBS J. 2013;280:2285–2293. doi: 10.1111/febs.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trigkilidas D, Anand A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann. Royal Col. Surg. Engl. 2013;95:545–551. doi: 10.1308/003588413X13629960049432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abate M, Schiavone C, Salini V. The use of hyaluronic acid after tendon surgery and in tendinopathies. BioMed Res. Int. 2014 doi: 10.1155/2014/783632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arpicco S, Milla P, Stella B, Dosio F. Hyaluronic acid conjugates as vectors for the active targeting of drugs, genes and nanocomposites in cancer treatment. Molecules. 2014;19:3193–3230. doi: 10.3390/molecules19033193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong BF, Blank LM, Mclaughlin R, Nielsen LK. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005;66:341–351. doi: 10.1007/s00253-004-1774-4. [DOI] [PubMed] [Google Scholar]
- 8.Hamano T, Mitsuhashi Y, Acki N, Yamamoto S. High-performance liquid chromatographic assay of chondroitin sulphate in food products. Analyst. 1989;114:891–893. [Google Scholar]
- 9.Yamada S, Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Disc. Technol. 2008;5:289–301. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 10.Oschatz C, Maas C, Lecher B, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34(2):258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Linhardt RJ. Claude S Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J. Med. Chem. 2003;46(13):2551–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa M. What is the best treatment for a cancer patient with thrombosis? Clin. Med. Insights Oncol. 2014;8:49–55. doi: 10.4137/CMO.S13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amjadi S, Mai K, McCluskey P, Wakefield D. The role of lumican in ocular disease. ISRN Ophthalmol. 2013:1–7. doi: 10.1155/2013/632302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vázquez JA, Rodríguez-Amado I, Montemayor M, Fraguas J, González MP, Murado MA. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: characteristics, applications and eco-friendly processes: A review. Mar. Drugs. 2013;11:747–774. doi: 10.3390/md11030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord MS, Whitelock JM. Bioengineered heparin: Is there a future for this form of the successful therapeutic? Bioengineered. 2014;5(4):1–5. doi: 10.4161/bioe.29388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnjanagoonchorn W, Wongekalak L, Engkagul A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Proc. 2007;46:465–471. [Google Scholar]
- 17.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Ann. Rev. Cell Dev. Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 19.Abdou AM, Kim M, Sato K. Functional proteins and peptides of hen’s egg origin. In: Hernández-Ledesma B, Hsieh CC, editors. Bioactive food peptides in health and disease, InTech (JanezaTrdine 9, 51000 Rijeka, Croatia) 2013. pp. 115–144. [Google Scholar]
- 20.Arias JL, Fink DJ, Xiao SQ, Heuer AH, Caplan AI. Biomineralization and eggshells: cell-mediated acellular compartments of mineralized extracellular matrix. Int. Rev. Cytol. 1993;145:217–250. doi: 10.1016/s0074-7696(08)60428-3. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SE. Egg and Eggshell Quality. Wolfe Publ. Ltd; London: 1991. [Google Scholar]
- 22.Baker JR, Balch DA. A study of the organic material of hen’s eggshell. Biochem. J. 1962;82:352–361. doi: 10.1042/bj0820352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heaney RK, Robinson DS. The isolation and characterization of hyaluronic acid in eggshell. Biochim. Biophys. Acta. 1976;451:133–142. doi: 10.1016/0304-4165(76)90265-8. [DOI] [PubMed] [Google Scholar]
- 24.Leach RM. Biochemistry of the organic matrix of the eggshell. Poult. Sci. 1982;61:2040–2047. [Google Scholar]
- 25.Nakano T, Ikawa NI, Ozimek L. Chemical composition of chicken eggshell and shell membranes. Poult. Sci. 2003;82:510–514. doi: 10.1093/ps/82.3.510. [DOI] [PubMed] [Google Scholar]
- 26.Carrino DA, Dennis JE, Wu TM, Arias JL, Fernandez MS, Rodriguez JP, Fink DJ, Heuer AH, Caplan AI. The avian eggshell extracellular matrix as a model for biomineralization. Connect. Tissue Res. 1996;35(1):325–329. doi: 10.3109/03008209609029207. [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ. Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A. 2012;1225:91–98. doi: 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ucakturk E, Cai C, Li L, Li G, Zhang F, Linhardt RJ. Capillary electrophoresis for total glycosaminoglycan analysis. Anal. Bioanal. Chem. 2014;406(19):4617–26. doi: 10.1007/s00216-014-7859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plaas AHK, West LA, Midura RJ. Keratan sulfate disaccharide composition determined by FACE analysis of keratanase II and endo-β-galactosidase digestion products. Glycobiology. 2001;11(10):779–790. doi: 10.1093/glycob/11.10.779. [DOI] [PubMed] [Google Scholar]
- 30.Wang PL, Wang SH, Liu ZG. Structural characterizations of pimpled eggshells. Chinese J. Animal Husbandry. 2014;50(1):79–83. [Google Scholar]
- 31.Carrino DA, Rodriguez JP, Caplan AI. Dermatan sulfate proteoglycans from the mineralized matrix of the avian eggshell. Connect. Tissue Res. 1997;36(3):175–193. doi: 10.3109/03008209709160219. [DOI] [PubMed] [Google Scholar]
- 32.Yamada S, Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulphate. Curr. Drug. Discov. Technol. 2008;5:289–301. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220(4602):1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- 34.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]