Abstract
Objectives
Trimethoprim (TMP)/sulfamethoxazole (SMX) has consistently demonstrated great interindividual variability. Therapeutic drug monitoring may be used to optimize dosing. Optimal peak SMX concentration has been proposed as 100 to 150 μg/mL. The objective of our work was to determine the success rate of a TMP/SMX dosing guideline in achieving a targeted serum peak SMX concentration range.
Methods
Our retrospective cohort study enrolled 305 adult hospitalized patients who received treatment with TMP/SMX and underwent serum peak SMX concentration monitoring from January 2003 to November 2011. Patients receiving low-dose TMP/SMX therapy (TMP <15 mg/kg/d) were compared with those receiving high-dose therapy (TMP >15 mg/kg/d).
Results
Patients were classified into peak and modified peak SMX concentration cohorts based on time between TMP/SMX dose and SMX quantification. The association between dosing group and the outcome of the SMX level within the goal range was measured using logistic regression models. The primary outcome measured was serum peak SMX concentration 100 to 150 μg/mL. Serum peak SMX concentrations were attained within range for the peak and modified peak cohort 29% and 26% of the time, respectively. The median peak SMX concentration was 144 μg/mL (range 25–471 μg/mL). The low daily dose cohort demonstrated a trend toward improvement in the odds of target peak concentration range attainment. The results were similar regardless of the method used to adjust for baseline characteristics. The pure peak and modified peak cohorts had 44% and 46% of patients with above-target SMX peak concentrations, respectively.
Conclusions
Attainment of the intended target concentration range was low with no difference in attainment between the low-dose and high-dose cohorts. Higher proportions of patients had an above-target SMX peak, which may indicate that the dosing algorithm is overly aggressive in obtaining the therapeutic goal.
Key words: Serum peak concentration, Sulfamethoxazole, Therapeutic drug monitoring, Trimethoprim
Introduction
Trimethoprim (TMP)/sulfamethoxazole (SMX) is a broad-spectrum antimicrobial combination effective in treating a variety of microorganisms and has been used in clinical practice for more than 50 years.1 High response rates demonstrated in clinical studies support TMP/SMX as the drug of choice for serious infections such as Stenotrophomonas maltophilia, Pneumocystis jiroveci, and Nocardia spp.2–4,5
Since its discovery, pharmacokinetic studies of TMP/SMX serum concentrations have consistently demonstrated great interindividual variability6,7 and the use of therapeutic drug monitoring was attempted to optimize clinical efficacy while minimizing adverse effects.7–9 Hughes et al10 determined low peak SMX concentration was associated with treatment failure in a study of 55 randomized pediatric patients with Pneumocystis carinii pneumonia (PCP pneumonia) and proposed 100 to 150 μg/mL as the optimal therapeutic range based on concentrations assayed from the 26 patients assigned to the TMP/SMX arm combined with any levels drawn from patients who crossed between treatments. This therapeutic range was further investigated for clinical efficacy in a study of treatment for AIDS-associated PCP pneumonia in HIV-positive adult patients, of whom 21 were assigned to receive TMP/SMX. Mortality in TMP/SMX-treated patients was similar to previous studies with a high rate of TMP/SMX-related adverse events.7 Additionally, peak SMX concentrations >200 μg/mL demonstrated an association with severe adverse effects.9,11,12 Despite extensive clinical experience, difficulties remain in achieving the proposed therapeutic SMX peak serum concentration of 100 to 150 μg/mL.8 The limited data surrounding a relationship between peak SMX serum concentrations and clinically meaningful outcomes call into question the use of routine therapeutic drug monitoring during high-dose TMP/SMX therapy.
The use of TMP/SMX therapy at our institution is frequently coupled with therapeutic drug monitoring in an effort to attain a targeted SMX peak serum concentration. Our institutional dosing algorithm has not been verified for its accuracy of attainment of the prespecified peak serum SMX concentration goal of 100 to 150 μg/mL. The purpose of our study is to compare the performance of a TMP/SMX dosing algorithm in achieving targeted serum peak SMX concentrations in hospitalized patients who received therapeutic doses of TMP/SMX.
Materials and Methods
Eligible patients
This single-center, retrospective cohort study was approved by the Mayo Clinic Institutional Review Board and conducted at Mayo Clinic in Rochester, Minnesota. Consecutive adult patients who received therapeutic doses of TMP/SMX between January 2003 and November 2011 were evaluated. Patients were included if they were aged 18 years or older and underwent serum peak SMX monitoring during TMP/SMX therapy. Intravenous or oral administration was allowed due to the nearly complete bioavailability of the oral formulations.13 Patients were excluded if they received TMP/SMX for infection prophylaxis, underwent TMP/SMX desensitization, or had the serum SMX plasma concentration monitored outside of their hospitalization. Data collection was limited to the earliest chronologic occurrence of therapeutic TMP/SMX administration with concurrent therapeutic drug monitoring for the purposes of maintaining independent data. Only the first peak serum SMX concentration was considered during this evaluation.
Laboratory assessment
SMX serum concentration levels were determined by HPLC by our institution’s Clinic Toxicology and Drug Monitoring Laboratory.14
Definitions
The goal peak SMX concentration range of 100 to 150 μg/mL was defined per our institution’s antimicrobial therapy guide.15 Serum SMX concentrations were considered peak concentrations if they were measured between 1 and 2 hours after intravenous administration or between 2 and 3 hours after administration of an oral dose at the onset of steady-state conditions.15 Serum SMX concentrations were considered modified peak if they were measured within 4 hours after intravenous administration and within 5 hours of oral administration. The peak values in the modified peak group served as surrogate peak levels in the absence of multilevel sampling and pharmacokinetic estimation. The peak and modified peak cohorts were categorized into 2 dosing groups—high dose or low dose—according to our institution’s dosing algorithm for the prespecified subgroup analysis (Table I). The high-dose group received a total daily dose ≥15 mg/kg actual body weight of the TMP component. The low-dose group received a total daily dose <15 mg/kg actual body weight of the TMP component. The dosing intervals ranged from every 6 hours to every 24 hours, depending on the patient’s creatinine clearance (Table I).16,17 Patients were assessed for dosage adjustments based on estimated creatinine clearance using the Cockcroft-Gault formula.18
Table I.
Trimethoprim/sulfamethoxazole dosing algorithm.*
| Creatinine clearance | High dose | Low dose |
|---|---|---|
| ≥30 mL/min | 15–20 mg/kg/d in 3–4 divided doses | <15 mg/kg/d in 2–4 divided doses |
| <30 mL/min† | 7–10 mg/kg/24 h in 2 divided doses | <7 mg/kg/24 h divided q12h–q24h |
Dose based on trimethoprim component.
Patients receiving dialysis were recommended to receive the dose indicated for patients with CrCl < 30 ml/min with the dose scheduled after dialysis on dialysis days.
Data collection
Clinical and demographic data were retrospectively abstracted from medical records and divided into 3 categories: demographic data, infectious disease data, and TMP/SMX data. Demographic data included age, sex, body weight, serum creatinine, estimated creatinine clearance calculated by the Cockcroft-Gault formula, and absence/presence of preexisting chronic kidney disease or immunocompromised status as documented in electronic medical records before the date of admission. Additionally, chronic kidney disease was verified through a search for ICD-9-CM and ICD-10-CM diagnosis codes for chronic kidney disease (585 and N18, respectively). Infectious disease data included site of infection and infectious organism. Lastly, TMP/SMX data included TMP/SMX start date and time; TMP/SMX dose (milligrams per kilogram TMP component); and interval, date, time, and value of peak serum peak SMX concentration. The primary outcome measured was the frequency of serum peak SMX concentration within the defined goal of 100 to 150 μg/mL.
Statistical analysis
Variables were summarized as median (range or interquartile range) or frequency (%), as appropriate. Baseline comparisons between high- and low-dose groups were done using the Wilcoxon rank-sum test for continuous variables or Pearson χ2 test for discrete variables. To adjust for possible covariate imbalance between the high- and low-dose groups, propensity scores of the probability of receiving a high dose were computed for each patient using a multivariable logistic regression model that included variables for chronic kidney disease, dialysis, immunocompromised status, intensive care unit admission, pulmonary diagnosis, identified infectious organism (eg, Stenotrophomonas maltophilia, Pneumocystis jiroveci, Nocardia spp, Staphylococcus spp, or polymicrobial), body mass index (BMI), and creatinine clearance.19 The association between dosing group and the outcome of the SMX level being within the therapeutic range was measured using 4 different logistic regression models: including only the dose group as a predictor (unadjusted model), including the dose group and all the variables that went into the propensity score computation (standard covariate adjustment model), including the dose group and the propensity score as a covariate (propensity score covariate adjustment model), and model stratified by propensity score quintile groups with a predictor of dose group (propensity score stratified model). Models 2 through 4 adjust for the covariates, but in different ways. Model 1 is provided for comparison to assess the level of covariate adjustment. An odds ratio with 95% CI for being in range, comparing the high- and low-dose groups, is provided for each model. These 4 models are fit for 2 different cohorts. The first cohort consisted of patients with SMX levels that were measured during the pure peak period. The second cohort included patients who had a SMX level measured during the pure peak period or the modified peak period.
Results
One thousand four hundred sixty-three individual serum SMX concentrations were recorded from 686 patients between January 2003 and November 2011. Of these, a total of 381 patients met our inclusion and exclusion criteria (Figure 1). From the 381 patients, 76 patients were excluded because the serum SMX concentration was taken outside the modified peak window, leaving 305 patients for analysis in the modified peak group and 119 patients for analysis in the peak group (Figure 1).
Figure 1.
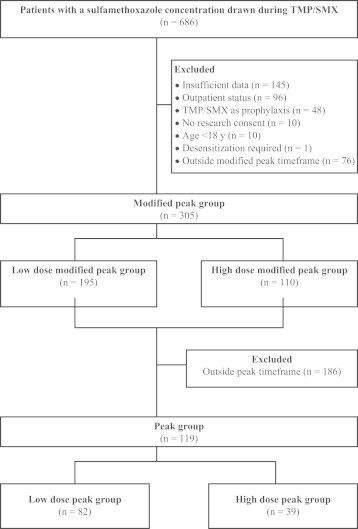
Consort diagram. TMP/SMX = trimethoprim/sulfamethoxazole.
Peak group
The peak group consisted of 119 patients with 82 patients in the low-dose group and 37 patients in the high-dose group. Baseline characteristics of the peak cohort are shown in Table II and Table III. The median age was 65.2 years (interquartile range = 53.8–74.9 years) and the majority (90%) were immunocompromised. Most patients experienced a pulmonary infection (70%) with Stenotrophomonas maltophilia (32%) and Pneumocystis carinii (36%) as the predominant causative organisms. Baseline characteristics were similar between patients when comparing the low- and high-dose groups except for BMI and type of infection. The low-dose group members were found to have a higher median BMI compared with members of the high-dose group (25.3 vs 23.0, respectively; P = 0.01).
Table II.
Baseline demographic characteristics.
| Characteristic | Peak (n = 119) |
Modified peak (n = 305) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Low-dose (n = 82) | High-dose (n = 37) | P | Total | Low-dose (n = 195) | High-dose (n= 110) | P | |
| Male gender* | 82 (68.9) | 55 (67.1) | 27 (73.0) | 0.52 | 208 (68.2) | 134 (68.7) | 74 (67.3) | 0.79 |
| Age† | 65.2 (53.8–74.0) | 64.5 (53.8–74.1) | 68.2 (54.8–72.8) | 0.91 | 63.6 (49.8–72.6) | 64.6 (53.5–74.1) | 60.7 (45.5–69.4) | 0.0067 |
| Race* | ||||||||
| White | 102 (85.7) | 71 (86.6) | 31 (83.8) | 0.6860 | 260 (85.2) | 167 (85.6) | 93 (84.5) | 0.7956 |
| Hispanic | 2 (1.7) | 2 (2.4) | 0 (0) | 4 (1.3) | 4 (2.1) | 0 (0) | ||
| African American | 1 (0.8) | 1 (1.2) | 0 (0) | 2 (0.7) | 2 (1.0) | 0 (0) | ||
| Asian/Pacific Islander | 0 (0) | 0 (0) | 0 (0) | 2 (0.7) | 0 (0) | 2 (1.8) | ||
| Native American | 0 (0) | 0 (0) | 0 (0) | 1 (0.3) | 1 (0.5) | 0 (0) | ||
| Other/not reported | 14 (11.8) | 8 (9.8) | 6 (16.2) | 36 (11.8) | 21 (10.8) | 15 (13.6) | ||
| Weight† (kg) | 74.0 (63.3–85.8) | 75.1 (65.8–87.7) | 71.5 (60.4–79.8) | 0.058 | 75.4 (63.4–88.1) | 74.8 (64.6–90.0) | 73.3 (61.1–84.5) | 0.087 |
| Body mass index† | 24.8 (21.5–27.9) | 25.3 (22.0–29.7) | 23.0 (20.3–25.1) | 0.011 | 25.0 (21.9–29.2) | 25.4 (22.7–30.1) | 24.6 (21.5–27.9) | 0.036 |
| Creatinine clearance‡ | 68.4 (44.9–97.6) | 65.3 (37.5–95.9) | 78.5 (52.0–106.8) | 0.065 | 69.8 (46.3–103.3) | 64.1 (39.6–94.1) | 78.0 (53.2–110.5) | 0.001 |
| Comorbidities | ||||||||
| Chronic kidney disease* | 25 (21.0) | 17 (20.7) | 8 (21.6) | 0.91 | 62 (20.3) | 42 (21.5) | 20 (18.3) | 0.48 |
| Dialysis* | 17 (14.3) | 15 (18.3) | 2 (5.4) | 0.063 | 42 (13.8) | 33 (16.9) | 9 (8.2) | 0.033 |
| Hemodialysis | 11 (9.2) | 11 (13.4) | 0 (0) | 27 (8.9) | 22 (11.3) | 5 (4.5) | ||
| Continuous venovenous hemofiltration | 6 (5.0) | 4 (4.9) | 2 (5.4) | 15 (4.9) | 11 (5.6) | 4 (3.6) | ||
| Diabetes* | 30 (25.2) | 24 (29.3) | 6 (16.2) | 0.13 | 70 (23.0) | 56 (28.7) | 14 (12.7) | 0.0014 |
| Immunocompromised* | 107 (89.9) | 71 (86.6) | 36 (97.3) | 0.073 | 267 (87.5) | 161 (82.6) | 106 (96.4) | 0.0005 |
Values are given as n (%).
Values are given as median (interquartile range).
Values are given as median (mL/min).
Table III.
Microbiology characteristics.*
| Characteristic | Peak (n = 119) |
Modified peak (n = 305) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Low-dose (n = 82) | High-dose (n = 37) | P | Total | Low-dose (n = 195) | High-dose (n= 110) | P | |
| Site of infection | N/A | N/A | ||||||
| Central nervous system | 1 (0.8) | 1 (1.2) | 0 (0.0) | 2 (0.7) | 2 (1.0) | 0 (0.0) | ||
| Intra-abdominal | 6 (5.0) | 5 (6.1) | 1 (2.7) | 11 (3.6) | 10 (5.1) | 1 (0.9) | ||
| Pulmonary | 83 (69.7) | 55 (67.1) | 28 (75.7) | 194 (63.6) | 120 (61.5) | 74 (67.3) | ||
| Bone | 5 (4.2) | 4 (4.9) | 1 (2.7) | 9 (3.0) | 8 (4.1) | 1 (0.9) | ||
| Skin, soft tissue | 1 (0.8) | 1 (1.2) | 0 (0.0) | 8 (2.6) | 8 (4.1) | 0 (0.0) | ||
| Urine | 1 (0.8) | 1 (1.2) | 0 (0.0) | 1 (0.3) | 1 (0.5) | 0 (0.0) | ||
| Bloodstream | 6 (5.0) | 4 (4.9) | 2 (5.4) | 23 (7.5) | 15 (7.7) | 8 (7.3) | ||
| Empiric therapy | 15 (12.6) | 10 (12.2) | 5 (13.5) | 54 (17.7) | 29 (14.9) | 25 (22.7) | ||
| Multifocal infection | 1 (0.8) | 1 (1.2) | 0 (0.0) | 3 (1.0) | 2 (1.0) | 1 (0.9) | ||
| Organism | 0.0002‡ | <0.0001‡ | ||||||
| Stenotrophomonas maltophilia | 40 (33.6) | 32 (39.0) | 8 (21.6) | 104 (34.1) | 88 (45.1) | 16 (14.5) | ||
| Pneumocystis (carinii) jiroveci | 43 (36.1) | 20 (24.4) | 23 (62.2) | 108 (35.4) | 47 (24.1) | 61 (55.5) | ||
| Empiric therapy | 15 (12.6) | 10 (12.2) | 5 (13.5) | 53 (17.4) | 28 (14.4) | 25 (22.7) | ||
| Other† | 21 (17.6) | 20 (24.4) | 1 (2.7) | 40 (13.1) | 32 (16.4) | 8 (7.3) | ||
N/A, not applicable.
Values are given as n (%).
Other organisms include Staphylococcus spp: methicillin-sensitive Staphylococcus aureus (peak n = 1, modified peak n = 1), methicillin-resistant Staphylococcus aureus (peak n = 1, modified peak n = 1), Staphylococcus epidermidis (peak n = 1, modified peak n = 2) and Nocardia spp (peak n = 12, modified peak n = 21).
Based on the combination of all organism groups within each cohort.
Overall, 28.6% of patients were found to be within the therapeutic range on the first peak SMX concentration. For the remaining patients, 27.7% were below the target and 43.7% were above the target. The median SMX concentration was 144 μg/mL (range = 25–471 μg/mL). Median time from the start of TMP/SMX therapy to the first level was 2 days (range = 1–32 days). The low-dose group had 32% of patients within the therapeutic range, whereas the high-dose group had 22% of patients within the target range (Table IV). In the peak cohort, 31.5% of those receiving oral dosing (23 out of 73 patients) were in range compared with 23.9% of those receiving intravenous dosing (11 out of 46 patients) (P = 0.37 by Pearson χ2 test). The propensity score analysis revealed no statistical differences between the 2 dosing groups in regard to therapeutic concentration range attainment. Comparison of the dosing groups showed consistent results across all the multiple propensity score models (Table V).
Table IV.
Therapeutic attainment.*
| Value of peak sulfamethoxazole concentration | Peak cohort |
Modified peak cohort |
||||
|---|---|---|---|---|---|---|
| Overall (n = 119) | Low dose (n = 82) | High dose (n = 37) | Overall (n = 305) | Low dose (n = 195) | High dose (n = 110) | |
| Below target (<100 μg/mL) | 33 (27.7) | 32 (39.0) | 1 (2.7) | 91 (28.8) | 85 (43.6) | 6 (5.5) |
| Target (100-150 μg/mL) | 34 (28.6) | 26 (31.7) | 8 (21.6) | 75 (25.6) | 52 (26.7) | 23 (20.9) |
| Above target (>150 μg/mL) | 52 (43.7) | 24 (29.3) | 28 (75.7) | 139 (45.6) | 58 (29.7) | 81 (73.6) |
Values are given as n (%).
Table V.
Logistic regression models comparing the high- to low-dose groups for therapeutic attainment.
| Cohort | Odds ratio (95% CI) | P |
|---|---|---|
| Peak cohort | ||
| Statistical model | ||
| Unadjusted | 0.594 (0.239–1.477) | 0.26 |
| Standard covariate adjustment | 0.864 (0.282–2.650) | 0.80 |
| Propensity score covariate adjustment | 0.852 (0.285–2.543) | 0.77 |
| Propensity score stratified | 0.793 (0.268–2.350) | 0.68 |
| Modified peak cohort | ||
| Unadjusted | 0.729 (0.420–1.264) | 0.26 |
| Standard covariate adjustment | 0.681 (0.358–1.293) | 0.24 |
| Propensity score covariate adjustment | 0.650 (0.348–1.212) | 0.18 |
| Propensity score stratified | 0.67 (0.363–1.263) | 0.22 |
Modified peak group
The modified peak group consisted of 305 patients with 195 categorized as receiving a low dose and 110 as receiving a high dose (Figure 1). The median age within the cohort was 63.6 years. Comparison of the baseline characteristics between the 2 dosing arms showed statistically significant higher frequencies of patients with diabetes (P = 0.0014) and patients undergoing dialysis (P = 0.033) in the low-dose group (Table II). Additionally, the low dose group had a higher BMI compared with the high-dose group (P = 0.0355). The high-dose group was characterized by more immunocompromised patients (P = 0.0005) and more patients with higher creatinine clearance (P = 0.0010).
The attainment in the modified peak group had 26% of patients within the therapeutic range on the first peak SMX concentration. The remaining patients were found to be below the target 29% of the time and above the target 46% of the time. A higher proportion of patients within the low-dose group was in the therapeutic range (28%) compared with the high-dose group (22%). The modified peak cohort had a median peak SMX concentration of 144 μg/mL (range = 25–471 μg/mL). Median time from the start of TMP/SMX therapy to the first level in the modified peak cohort was 2 days (range = 0–32 days). In the modified peak cohort, 27.6% of those receiving oral dosing (34 out of 123 patients) were in range compared with 24.2% of those (44 out of 182 patients) receiving intravenous dosing (P = 0.50 by Pearson χ2 test). The propensity score matching analyses demonstrated no difference in therapeutic attainment between the 2 dosing arms (Table V). The results were unchanged regardless of the method.
Discussion
TMP/SMX is a commonly prescribed antimicrobial agent for the treatment of many different infections. Our institution commonly pairs TMP/SMX with therapeutic drug monitoring for serious infections such as Stenotrophomonas maltophilia, Pneumocystis jiroveci, and Nocardia spp. The results of our study demonstrate that our institution’s dosing algorithm was unsuitable to attain the suggested goal therapeutic range for the majority of patients (71.4%) in the peak cohort. Additionally, peak SMX concentrations exhibited high variability despite weight-based dosing adjusted for renal function whereby a majority of the results in the peak cohort (44%) were above the target.
Delays of obtaining appropriately timed serum peak SMX concentrations may be unavoidable in clinical practice. There are numerous clinical situations, such as procedures or imaging studies, that may impede the proper timing of laboratory serum draws. For this reason, we further described a modified peak cohort to account for varying clinical situations and the time frame for peak serum SMX concentration was extended to within 4 hours after intravenous administration and within 5 hours after oral administration. The extension of the peak time window was performed to better represent real clinical practice. Regardless of the allowed time frame of serum peak SMX concentration quantification, the 2 dosing regimens we evaluated did not have any significant influence on the rate of target range attainment. The modified peak cohort was consistent in results returning above the target range (46%).
Interestingly, the low-dose group did show a trend of higher tendency to achieve the target range but this was not found to be statistically significant in all of the propensity score models. The results were consistent across the unadjusted and adjusted propensity score models, strengthening the result of no significant difference between the high- and low-dose groups for attainment of therapeutic peak SMX concentrations. Despite no apparent difference in attainment rates, the pure peak and modified peak cohorts had 44% and 46% of patients with above-target SMX peak concentrations, respectively. This may indicate that the dosing algorithm is overly aggressive in regard to obtaining a goal-directed range.
Our study supports previous clinical and pharmacokinetic studies that have shown high interindividual variability of SMX in regard to plasma concentrations.6,12 The peak SMX concentrations in our study are similar to serum levels of SMX reported in other clinical studies.6,16,17 Remarkable interpatient variability was shown by Blaser et al6 in a study of high-dose TMP/SMX in PCP pneumonia where TMP/SMX was dosed at 15 to 22 mg/kg/d of the TMP component and resulted in a median peak SMX concentration of 198 μg/mL with multiple patients found to have concentrations >300 μg/mL. Many studies have attempted to establish a relationship between TMP/SMX dose and resultant serum concentration without success; however, these studies were limited by small sample size.12,20–22 High variability has been seen despite controlled administration and weight-specific dosing without a correlation to TMP, SMX, or N-acetyl-SMX.6 Another study showed no correlation between serum creatinine and plasma concentrations. As a renally eliminated antimicrobial agent, even subclinical changes in glomerular filtration rate (GFR) may significantly alter the plasma concentrations of TMP/SMX. Unfortunately, clinically applicable bedside surrogates of renal function incompletely reflect true GFR.23–25 Serum creatinine in specific is affected by age, sex, race, and habitus. Furthermore, TMP itself alters serum creatinine concentrations (therefore altering creatinine clearance) independent of changing true GFR. Drug-induced inhibition of the renal tubular secretion of creatinine results in an artificial increase in serum creatinine concentrations and an underestimation of true GFR by creatinine clearance. Lastly, recent evidence suggests that contemporary methods of renal replacement substantially removes TMP/SMX, leading to below-target SMX concentrations and a potential need for revised dosing in this population.26,27 In the peak cohort, 16.7% were within range while receiving hemodialysis, whereas 27.3% achieved the target range while receiving continuous venovenous hemofiltration. In the modified peak cohort, 20% were in range while receiving hemodialysis, whereas 22.2% were in range while receiving continuous venovenous hemofiltration. Unfortunately, the numbers of patients receiving dialysis were too few to allow any inferences about being in range within dialysis subgroups. All of these factors likely contribute to the challenges of accurate renal function interpretation in these patients and the significant interindividual variability noted in our study.
Chin et al28 found similar attainment rates in their investigation of the influence of monitoring serum TMP/SMX concentrations in patients with PCP pneumonia. TMP/SMX was dosed at 20 mg/kg/d of the TMP component with an intended goal peak SMX concentration range of 150 to 200 μg/mL. Dose adjustments were performed in the intervention arm to improve the therapeutic range attainment; however, researchers demonstrated that only 28% of SMX concentrations in the dose-adjustment group were within the therapeutic range compared with 32% without adjustment. Despite the higher established target therapeutic range, the results were similar to our study’s attainment of 26% within the therapeutic range.
There are a number of limitations to our study. First is the potential for selection bias given the single-center, retrospective nature of our study design. Our patient population consisted of a majority of white, male patients. The enrollment of patients consecutively during the study period and implementation of the propensity score matching method were our attempt to minimize the effects of confounding variables when comparing the low- and high-dose groups. The peak SMX concentrations from both oral and intravenous administration were combined for analysis, which may lead to difficulties in interpretation. Peak SMX concentrations were combined from 2 different routes of administration because of oral administration results in almost complete absorption. Analysis demonstrated similar distribution of levels between different routes of administration. Further, there was no statistical difference in target concentration attainment when comparing the intravenous versus oral route (24% and 27%, respectively). Third, therapeutic drug monitoring of serum peak SMX levels are obtained at the physicians’ and pharmacists’ discretion as opposed to following a standardized algorithm; however, the median time of 2 days of administration before concentration quantification ensured that SMX levels were drawn at steady state concentrations. The potential for drug accumulation after the time at which steady state occurs may augment the already high percentage of patients in the above-target classification and make our analysis an overestimation of patients within the target range. Daily processing and reporting of serum peak SMX concentrations by our institution’s laboratory allows dose adjustments to occur in a timely fashion if necessary; however, routine laboratory monitoring is not available in all institutions so real-time laboratory assessment may be a challenge.
To our knowledge, this study represents the largest reported sample to date of TMP/SMX monitoring. This study adds to the literature on therapeutic drug monitoring of SMX currently centered on Pneumocystis jiroveci pneumonia treatment and provides a robust set of patients using therapeutic drug monitoring of peak SMX concentrations for various infections requiring TMP/SMX therapy. In addition, including a modified peak cohort allows our results to be applicable to routine clinical practice. Clinical outcomes were not assessed in our study, but our results establish a foundation to support further assessment of the influence of SMX concentrations on the efficacy and safety of the high doses of TMP/SMX typically used as infection treatment. Given the heterogeneity of the indications for using TMP/SMX treatment and the low attainment rates of goal-directed therapeutic SMX concentrations, further prospective, randomized studies are needed to elucidate the optimal dose of TMP/SMX, further define the ideal goal therapeutic ranges for Nocardia spp and Stenotrophomonas maltophilia infections, and investigate the association of peak SMX concentration and clinical outcomes.
Conclusions
Peak SMX concentrations demonstrate wide variability, resulting in low overall attainment of intended target concentration ranges. There was no difference in attainment between the low- and high-dose cohorts. Therapeutic range attainment rates were poor regardless of administration of high- or low-dose TMP/SMX. Higher proportions of patients had an above-target SMX peak, which may be an indication that the dosing algorithm is overly aggressive in regard to obtaining the therapeutic goal. The wide therapeutic index of TMP/SMX coupled with the broad range of resultant serum concentrations makes targeted therapy difficult and of questionable necessity. Further investigation into the association between peak SMX concentration monitoring and clinically meaningful outcomes, including treatment response and adverse effects, should be conducted in larger, prospective clinical trials with a more diverse patient population.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
There is no funding to disclose nor any acknowledgments.
Author Contributions: BD Dao participated in concept and study design, data collection and data analysis/interpretation, and manuscript creation involving critical writing and revising of the intellectual content. JN Barreto participated in concept and study design, data collection and data analysis/interpretation, and manuscript creation involving critical writing and revising of the intellectual content. RC Wolf participated in concept and study design, data analysis/interpretation, and manuscript creation involving critical writing and revising of the intellectual content. RA Dierkhising participated in study design, data analysis/interpretation, and manuscript creation involving critical writing and revising of the intellectual content. MF Plevak participated in study design, data analysis/interpretation, and manuscript creation involving critical writing and revising of the intellectual content. Pritish K. Tosh participated in concept and study design, data analysis/interpretation, and manuscript creation involving critical writing and revising of the intellectual content.
References
- 1.Vohringer H.F., Arasteh K. Pharmacokinetic optimisation in the treatment of Pneumocystis carinii pneumonia. Clin Pharmacokinet. 1993;24:388–412. doi: 10.2165/00003088-199324050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Limper A.H., Knox K.S., Sarosi G.A. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 3.Mofenson L.M., Brady M.T., Danner S.P. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 4.Smego R.A., Jr, Moeller M.B., Gallis H.A. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med. 1983;143:711–718. [PubMed] [Google Scholar]
- 5.Abbott I.J., Slavin M.A., Turnidge J.D., Thursky K.A., Worth L.J. Stenotrophomonas maltophilia: emerging disease patterns and challenges for treatment. Expert Rev Anti Infect Ther. 2011;9:471–488. doi: 10.1586/eri.11.24. [DOI] [PubMed] [Google Scholar]
- 6.Blaser J., Joos B., Opravil M., Luthy R. Variability of serum concentrations of trimethoprim and sulfamethoxazole during high dose therapy. Infection. 1993;21:206–209. doi: 10.1007/BF01728888. [DOI] [PubMed] [Google Scholar]
- 7.Wharton J.M., Coleman D.L., Wofsy C.B. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis pneumonia in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:629–630. doi: 10.7326/0003-4819-105-4-629_2. [DOI] [PubMed] [Google Scholar]
- 8.Joos B., Blaser J., Opravil M., Chave J.P., Luthy R. Monitoring of co-trimoxazole concentrations in serum during treatment of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1995;39:2661–2666. doi: 10.1128/aac.39.12.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klinker H., Langmann P., Zilly M., Richter E. Drug monitoring during the treatment of AIDS-associated Pneumocystis carinii pneumonia with trimethoprim-sulfamethoxazole. J Clin Pharm Ther. 1998;23:149–154. doi: 10.1046/j.1365-2710.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes W.T., Feldman S., Chaudhary S.C., Ossi M.J., Cox F., Sanyal S.K. Comparison of pentamidine isethionate and trimethoprim-sulfamethoxazole in the treatment of Pneumocystis carinii pneumonia. J Pediatr. 1978;92:285–291. doi: 10.1016/s0022-3476(78)80028-6. [DOI] [PubMed] [Google Scholar]
- 11.Wong IW. Correlation of Side Effects of Trimethoprim/sulfamethoxazole with blood levels in AIDS patients treated for Pneumocystis carinii pneumonia. In: 28th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC; 1988:328.
- 12.Bowden F.J., Harman P.J., Lucas C.R. Serum trimethoprim and sulphamethoxazole levels in AIDS. Lancet. 1986;1:853. doi: 10.1016/s0140-6736(86)90958-x. [DOI] [PubMed] [Google Scholar]
- 13.Kremers P., Duvivier J., Heusghem C. Pharmacokinetic studies of co-trimoxazole in man after single and repeated doses. J Clin Pharmacol. 1974;14:112–117. doi: 10.1002/j.1552-4604.1974.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 14.Sulfamethoxazole, Serum. Mayo Medical Laboratories. Available at: http://www.mayomedicallaboratories.com/test-catalog/Overview/8238. Accessed August 13, 2013.
- 15.Wilson J.W., Estes L.L. Mayo Clinic Scientific Press; Rochester, MN: 2008. Mayo Clinic. Mayo Clinic antimicrobial therapy: quick guide. [Google Scholar]
- 16.Bactrim™ and Bactrim ™ DS (sulfamethoxazole and trimethoprim) [package insert]. Philadelphia PAS, Inc; 2013.
- 17.Nahata M.C. Dosage regimens of trimethoprim/sulfamethoxazole (TPM/SMX) in patients with renal dysfunction. Ann Pharmacother. 1995;29:1300. [PubMed] [Google Scholar]
- 18.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino R.B., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Lee B.L., Medina I., Benowitz N.L., Jacob P., 3rd, Wofsy C.B., Mills J.T. Dapsone, trimethoprim, and sulfamethoxazole plasma levels during treatment of Pneumocystis pneumonia in patients with the acquired immunodeficiency syndrome (AIDS). Evidence of drug interactions. Ann Intern Med. 1989;110:606–611. doi: 10.7326/0003-4819-110-8-606. [DOI] [PubMed] [Google Scholar]
- 21.McLean I., Lucas C.R., Mashford M.L., Harman P.J. Modified trimethoprim-sulphamethoxazole doses in Pneumocystis carinii pneumonia. Lancet. 1987;2:857–858. doi: 10.1016/s0140-6736(87)91046-4. [DOI] [PubMed] [Google Scholar]
- 22.Medina I., Mills J., Leoung G. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776–782. doi: 10.1056/NEJM199009203231202. [DOI] [PubMed] [Google Scholar]
- 23.Moran S.M., Myers B.D. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 24.Perrone R.D., Madias N.E., Levey A.S. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 25.Robert S., Zarowitz B.J. Is there a reliable index of glomerular filtration rate in critically ill patients? DICP: The annals of pharmacotherapy. 1991;25(2):169–178. doi: 10.1177/106002809102500212. PMID: 2058189. [DOI] [PubMed] [Google Scholar]
- 26.Clajus C., Kuhn-Velten W.N., Schmidt J.J. Cotrimoxazole plasma levels, dialyzer clearance and total removal by extended dialysis in a patient with acute kidney injury: risk of under-dosing using current dosing recommendations. BMC Pharmacol Toxicol. 2013;14:19. doi: 10.1186/2050-6511-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curkovic I., Luthi B., Franzen D., Ceschi A., Rudiger A., Corti N. Trimethoprim/sulfamethoxazole pharmacokinetics in two patients undergoing continuous venovenous hemodiafiltration. Ann Pharmacother. 2010;44:1669–1672. doi: 10.1345/aph.1P160. [DOI] [PubMed] [Google Scholar]
- 28.Chin T.W., Vandenbroucke A., Fong I.W. Pharmacokinetics of trimethoprim-sulfamethoxazole in critically ill and non-critically ill AIDS patients. Antimicrob Agents Chemother. 1995;39:28–33. doi: 10.1128/aac.39.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]


