Abstract
The incidence of obesity and its related conditions, including non-alcoholic fatty liver disease (NAFLD), has dramatically increased in all age groups worldwide. Given the health consequences of these conditions, and the subsequent economic burden on healthcare systems, their prevention and treatment have become major priorities. Because standard dietary and lifestyle changes and pathogenically-oriented therapies (e.g., antioxidants, oral hypoglycemic agents, and lipid-lowering agents) often fail due to poor compliance and/or lack of efficacy, novel approaches directed toward other pathomechanisms are needed. Here we present several lines of evidence indicating that, by increasing energy extraction in some dysbiosis conditions or small intestinal bacterial overgrowth, specific gut microbiota and/or a “low bacterial richness” may play a role in obesity, metabolic syndrome, and fatty liver. Under conditions involving a damaged intestinal barrier (“leaky gut”), the gut-liver axis may enhance the natural interactions between intestinal bacteria/bacterial products and hepatic receptors (e.g., toll-like receptors), thus promoting the following cascade of events: oxidative stress, insulin-resistance, hepatic inflammation, and fibrosis. We also discuss the possible modulation of gut microbiota by probiotics, as attempted in NAFLD animal model studies and in several pilot pediatric and adult human studies. Globally, this approach appears to be a promising and innovative add-on therapeutic tool for NAFLD in the context of multi-target therapy.
Keywords: Probiotics, Gut-liver axis, Intestinal microbiota, Barrier function, Small intestinal bacterial overgrowth, Bacterial translocation, Non-alcoholic fatty liver disease
Core tip: Modulation of gut microbiota by probiotics is supported by a number of studies conducted with non-alcoholic fatty liver disease animal models and in several pilot pediatric and adult human studies. Globally, this approach appears to be a promising add-on therapeutic tool to be used in the context of a tailored multi-target therapy especially in cases where standard dietary and lifestyle changes have failed.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and the leading cause of chronic liver disease in pediatric and adult individuals living in industrialized countries. NAFLD includes steatosis and non-alcoholic steatohepatitis (NASH), which is characterized by steatosis and periportal and lobular inflammation. Progression to fibrosis and cirrhosis represents the primary complications of NAFLD[1]. The pathogenetic mechanisms that lead to NAFLD seem to be strictly linked to peripheral insulin resistance (IR) and oxidative stress in hepatocytes. In fact, reduced cell responses to insulin lead to increased levels of insulinemia. Consequently, hyperactivating hormone-sensitive lipase increases lipolysis in adipose tissue, which in turn increases free fatty acid (FFA) levels. Regarding the liver, IR causes gluconeogenesis and decreased glycogen synthesis, thereby increasing the FFA production rate and inhibiting beta-oxidation. The consequences of this IR-dependent “first hit” may be compensated by antioxidant hepatic mechanisms in the cell until the FFA surplus leads to a mitochondrial overload of oxygen free radicals, which, in turn, causes lipid peroxidation (“second hit”). Finally, the activation of multiple inflammatory pathways results in necroinflammatory events, fibrosis, and liver cirrhosis.
A growing body of evidence has begun to indicate that gut-liver axis malfunction [small intestinal bacterial overgrowth (SIBO), intestinal dysbiosis, and increased intestinal permeability (“leaky gut”)] is another leading factor in the development and progression of NAFLD[2-5]. This information is particularly important because of: (1) the high resistance of obese patients to the standard treatment of obesity and of its complications (i.e., lifestyle changes and hypocaloric diets); (2) poor effectiveness of other NAFLD pathomechanism-driven pharmacological treatments in reducing steatosis and its complications; and (3) the possibility of modulating the gut-liver axis[6,7].
Here we review the most recent data about the gut-liver axis and its role in NAFLD pathogenesis and progression. We also review experimental studies in animal models and preliminary results from several randomized clinical trials conducted to establish whether probiotic-induced modulation of the intestinal microbiota (IM) improves liver disease outcome.
GUT-LIVER AXIS COMPONENTS
The gut-liver axis refers to the close anatomical and functional relationship between the gastrointestinal tract and the liver. The interaction between the two organs, whether healthy or diseased, includes the transfer of IM-associated molecules to the liver[8]. Alterations of this axis (constituted by the intestinal barrier, IM, and liver) may occur due to changes in intestinal permeability and microbiome composition in several clinically relevant conditions, including NASH and cirrhosis[9].
The intestinal barrier is a complex functional unit composed of luminal and mucosal elements (e.g., epithelial cells layer; mucosal barrier; innate and acquired immune components); the neuroenteric, vascular, and endocrine systems; digestive enzymes; and the IM (Figure 1). This barrier plays a key role in protecting against enteric organisms, potentially harmful toxins, and bacterial bio-products closely associated with health and susceptibility to disease.
Figure 1.
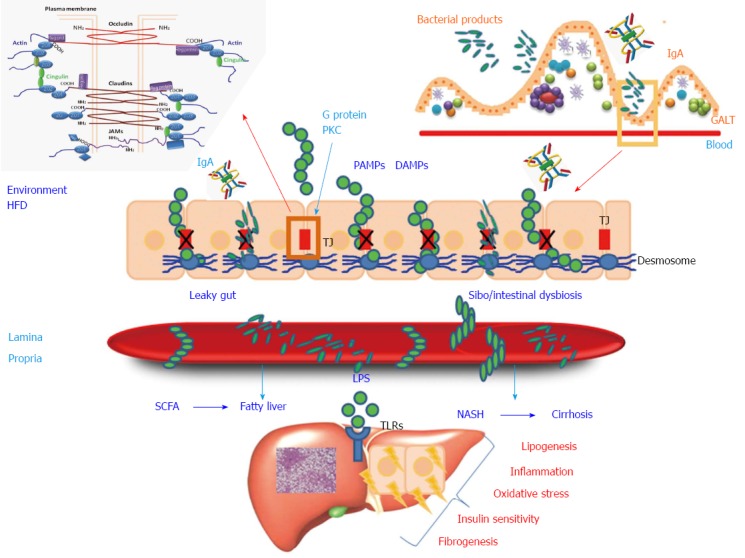
Intestinal barrier and liver. The intestinal microbiota plays an important role in the development of gut-associated lymphoid tissue (GALT), IgA secretion, and the production of antimicrobial peptides. Environmental factors (injury, infection, or high fat diet) may induce small intestinal bacterial overgrowth (SIBO)/intestinal dysbiosis and increased intestinal permeability that promote the translocation of bacteria and bacterial products, pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs). Malfunction of tight junctions (TJ), composed of occludin, claudin, and tricellulin proteins, and under the influence of proteins involved in the cascade of the signal-transduction pathways (G protein and protein kinase C), probably play a critical role in gut “leakiness”. Activation of toll-like receptors (TLRs) induces hepatic inflammation, lipogenesis, fibrogenesis, oxidative stress, and insulin sensitivity. In particular, activation of TLRs on stellate cells determines hepatic fibrosis and activation of TRLs on Kupffer cells promotes hepatic inflammation. In some dysbiosis conditions an high proportion of ethanol producing bacteria (E. Coli) may lead to high levels of endogenous alcohol, whose effects further reflect on intestinal permeability and hepatic damage. HFD: High fat diet; LPS: Lipopolysaccharide; PKC: Protein kinase C; SCFA: Short chain fatty acids.
The intestinal epithelium consists of a single layer of columnar cells, which are mainly absorptive cells (80%), with the remaining 20% being Paneth, goblet, and enteroendocrine cells. Intestinal cells are bound together by tight junctions (TJs) and the zonula adherens (known collectively as the “apical junction complex”), as well as gap junctions and desmosomes. TJs form a multifunctional complex characterized by a series of fusion points on the cell membranes of adjacent cells. Tight junction tetra- and single-span transmembrane proteins mediate cell-to-cell adhesion and seal the paracellular space between epithelial cells. In addition to providing a barrier to noxious molecules, TJs can also operate as pores for the permeation of ions, solutes, and water, as appropriate. Indeed, among the tetra-span proteins (occludin, claudin, and tricellulin), specific claudin isoforms (claudin-2, -7, -12, and -15) determine selective barrier permeability. Single-span transmembrane proteins, on the other hand, are mostly junctional adhesion molecules. Being an immunoglobulin superfamily, junctional adhesion molecules participate in the regulation and maintenance of the TJ barrier. These proteins are associated with peripheral membrane (plaque) proteins such as zonula occludens (ZO) proteins 1, 2, and 3. The latter, located in the intracellular side of the plasma membrane, contribute to anchoring the TJ protein complex to the actin component of the cytoskeleton (Figure 1). Intestinal epithelium homeostasis requires coordination between TJ proteins, the actin cytoskeleton, endocytosis, and the intracellular signaling pathways[10]. The structure of TJs is constantly remodeled consequent to interactions with external stimuli, such as food residues, and pathogenic or intestinal bacteria. Regulation of TJs depends also on several signal transduction networks, including those of the G protein and a number of specific kinases[11-14].
Besides regulating paracellular permeability, the intestinal barrier can activate the innate immune cells (e.g., dendritic cells), thereby preventing systemic infections triggered by intestinal microorganisms. The protrusions of these “sentinel cells” reach the intestinal lumen, where they are able to sense the presence of pathogens; however they also reach the normal intestinal flora and nutrients, and induce immune responses by activating specific B-cells (“acquired immunity”)[15,16]. The health of the intestinal tract is also maintained by Paneth cells, a type of specialized secretory epithelial cell that inhabits the mucosal surface of the small intestine and produces high quantities of defensins, and antimicrobial and antibiotic peptides[17,18].
Another critical element in the homeostasis of the intestinal barrier is the mucosal barrier, which is constituted by a set of glycosylated molecules and enzymes. The intestinal mucus produced by goblet cells consists of two layers: an outer layer that is an ideal habitat for microbial colonization, and an inner, denser layer containing relatively few bacteria that exerts a protective function[18]. The mucosal barrier interacts directly with the overlying components and may therefore influence the microbial balance. The release of mucus containing antimicrobial molecules prevents bacterial contact. Moreover, intestinal mucus provides transduction signals that modulate the pro-inflammatory and apoptotic pathways. However, intestinal microorganisms have developed several smart strategies to bypass these mechanisms, namely the release of mucolytic enzymes, inhibition of mucin synthesis, and damage of TJs. Interestingly, low levels of Akkermansia Muciniphila have been linked to obesity as they live in mucus on intestinal epithelium, and reduce the absorption by promoting the mucus layer thickness.
Intestinal barrier malfunction allows translocations of dangerous gut bacteria and/or their products into the mesenteric portal bloodstream[19]. Intestinal barrier damage, increased intestinal permeability, dysbiosis, and SIBO appear to play a pivotal role in NAFLD pathogenesis and development[3].
The gut microbiota consists of trillions of microorganisms (i.e., approximately 1014 bacteria and archaea) from more than 1000 species, with a total weight of approximately 1-2 kg[20]. Dysbiosis of the gut microbiota refers to an imbalance in the microbial community in terms of qualitative and quantitative changes, metabolic activity, and topographic distribution[19,21,22]. The human gut microbiota is mainly composed of Bacteroidetes and Firmicutes. Proteobacteria, Verrucomicrobia, Actinobacteria, Fusobacteria, and Cyanobacteria are present in minor proportions. Physiologically, they contribute 5%-15% of the dietary energy harvest by fermenting undigested alimentary residues[23]. The microbiota of obese subjects have a higher capacity to harvest energy from the diet, thereby providing substrates that can activate the lipogenic pathways.
The IM is influenced by both exogenous (dietary habits, food containing plant fibers and non-digestible carbohydrates that exert “prebiotic” effects, lifestyle, drugs, and method of birth delivery) and endogenous (bacterial mucosal receptors and interactions, intestinal pH, and immune response) factors[24].
Characterization of the gut microbiota profile is becoming increasingly more accurate thanks to new molecular microbiology techniques (next-generation sequencing) that supplement the results of culturomics-based investigations. Studies of the IM have also, at a functional level, started to depict the complex metabolic interplay between bacterial activities (metabolome) and the host. These studies revealed that the IM can influence conditions that involve not only the gastrointestinal tract (celiac disease, inflammatory bowel diseases, and irritable bowel syndrome), but also a growing number of extra-intestinal pathologies including obesity, IR, diabetes, cardiovascular disease, allergic diseases, and autism[25]. The concept that the IM is involved in obesity-related NAFLD was recently re-proposed, however various aspects of this association remain to be elucidated[26,27].
INTESTINAL MICROBIOTA IN OBESITY AND NAFLD
Obesity impacts on gastrointestinal health in various ways: by interfering with IM composition, reducing bowel movement, increasing SIBO, causing nutritional deficiency, and damaging barrier function which may result in bacterial translocation[28]. The composition of the IM in obese individuals has been the object of several controversial studies[26,29] (Table 1). The IM profile in NAFLD[32] and NASH[38] is less well documented. With a few exceptions (probably due to ethnic and/or dietary differences), most studies reported an increased proportion of Firmicutes over Bacteroidetes[37,38]. Some drugs (e.g., proton pump inhibitors) may influence the IM profile, promoting an higher proportion of Firmicutes. However, one specific member of the Firmicutes phylum (Oscillibacter spp.) may be significantly under-represented in NAFLD patients[41]. In addition, investigators have recently started to focus on IM gene “richness” (i.e., the number of gut microbial genes), which appears to be reduced in individuals with obesity-related metabolic syndrome features (IR/type 2 diabetes, hyperlipidemia, and hypertension)[42] and with chronic inflammatory stigmata[43].
Table 1.
Intestinal microbiota composition in obese individuals
| Ref. | Subjects | Method | Firmicutes | Bacteroidetes | Actinobacteria | Proteobacteria | Archaea |
| Turnbaugh et al[30] | 12 ob/2 nw | 16 S RNA pyrosequencing 454 | + | - | / | / | / |
| Turnbaugh et al[31] | 31 MZ twin pairs/23 DZ twin pairs/46 mo | 16 S RNA pyrosequencing 454 | == | -- | ++ | == | == |
| Mouzaki et al[32] | 17 nw/11 ss/22 NASH | qPCR | ++ | -- | No statistical difference | No statistical difference | / |
| Armougom et al[33] | 20 ob/20 nw | qPCR | ++ | -- | / | / | + |
| Million et al[34] | 68 ob/47 nw | qPCR; cell counts | ++ | == | - | / | |
| Nadal et al[35] | 39 ob adolescents low calorie 10 wk | FISH | ++ | -- | ++ | ++ | ++ |
| Santacruz et al[36] | 36 ob adolescents low calorie 10 wk | RT-PCR | ++ | -- | ++ | / | / |
| Zhang et al[37] | 3 ob/3 nw | qPCR + 16 S RNA pyrosequencing 454 | +-(Lachnospiraceae) | +(Prevotellaceae) | +(Coriobacteriaceae) | + | +(Methanobrevibacter smithii) |
| Wong et al[38] | 16 ob NASH/22 nw ctrls | 16 S RNA pyrosequencing 454 | -- | ++ | -- | ++ | / |
| Zhu et al[39] | 16 nw/25 I/22 NASH | 16 S RNA pyrosequencing 454 | -- | ++ | -- | ++ | / |
| Schwiertz et al[40] | 33 ob/35 ow/30 nw | qPCR | -- | == | - | / | - |
Firmicutes: Clostridia, Lactobacillales, Coccacea; ob: Obese; nw: Normal weight; ss: Simple steatosis; ow: Overweight; MZ: Monozygotic; DZ: Dizygotic.
Intestinal epithelium, microbes, and dietary pattern share several multi-directional communications[29,39,40]. For example, a high fat diet (HFD) promotes a proinflammatory microbiota, but also interferes with intestinal permeability as a consequence of the increased secretion of bile acids. High fructose consumption favors an IM able to harvest energy, and also increases intestinal permeability[43].
The IM in obese and NAFLD patients is particularly likely to serve as an additional source of energy by fermenting unused energy substrates (e.g., indigestible fibers and polysaccharides), thereby resulting in the production of organic acids [the short chain fatty acids (SCFAs) butyrate, acetate, and propionate] (Figure 1). SCFAs also play an important adipogenic role by activating two G protein-coupled receptors (Gpr40 and Gpr43) that are expressed in the small intestine, colon, adipose tissue, and immune cells[44]. While increased levels of SCFAs enhance intestinal barrier integrity[12], they are also responsible for reduced gut motility and transit time, which may promote small intestinal bacterial overgrowth. A high prevalence of SIBO has been observed in obese subjects[28], as well as in adult[27], and pediatric[45] NAFLD in parallel with the severity of hepatic steatosis, which may be associated with elevated blood lipopolysaccharides (LPS)[45] (Figure 1). See Figure 2 for a summary of the major mechanisms of the interplay between the IM and NAFLD.
Figure 2.
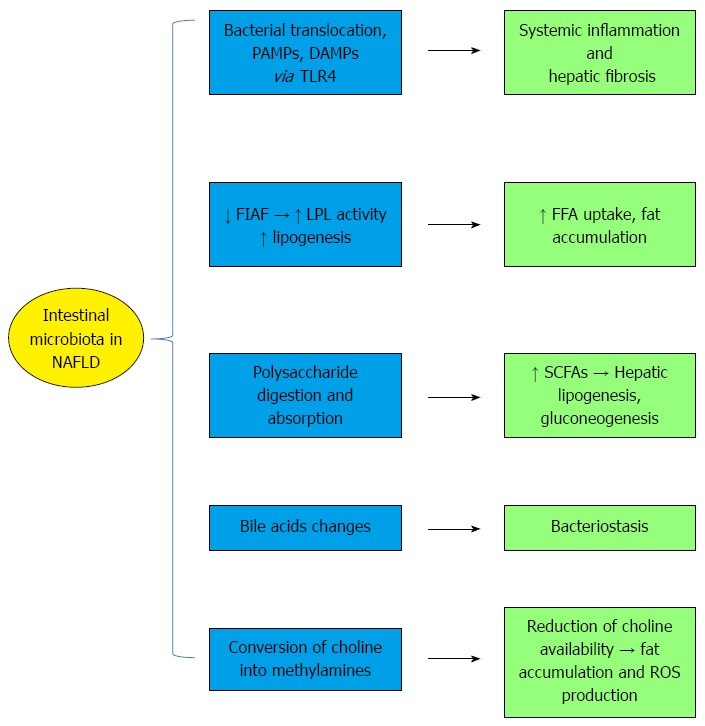
Mechanisms of the interplay between the intestinal microbiota and non-alcoholic fatty liver disease. Intestinal dysbiosis promotes the translocation of bacterial products [e.g., damage associated molecular patterns (DAMP) and pathogen-associated molecular patterns (PAMPs)] from the intestinal lumen into the lamina propria and to the bloodstream. This event is associated with the activation of toll-like receptor 4 (TLR-4), which causes hepatic fibrogenesis and systemic inflammation. Furthermore, the intestinal microbiota reduces fasting-induced adipose factor (FIAF) expression, lipogenesis, and free fatty acids (FFA) uptake. The gut microbiota have an increased capacity to harvest energy from non-digestible indigestible complex polysaccharides into monosaccharides and short chain fatty acids (SCFAs), which are substrates for hepatic lipogenesis and gluconeogenesis. Endogenous ethanol production by some bacteria is another mechanism damaging the liver. The properties of bile acids, which exert bacteriostatic activity, are also altered. The conversion of choline into methylamines leads to insulin resistance, fat accumulation, and ROS production (modified from refs 4 and 91). LPL: Lipoprotein lipase.
The relevant role played by the IM in various pathological conditions has recently been proven in fecal transplantation experiments. Fecal transplantation appears to be capable of modulating the gut microbiota not only in patients with gastrointestinal diseases (inflammatory bowel diseases and Clostridium difficile infection), but also in pathological conditions of distant organs, including obesity and its associated metabolic phenotypes[46]. As an example, Ridaura et al[47] confirmed that fat mass and obesity-associated metabolic phenotypes were transmissible from human twins (one obese and one lean) to germ-free mice with uncultured fecal communities and with their corresponding fecal bacterial culture collections. Furthermore, mice that initially received the obese twin’s microbiota were also able to transmit the pathological metabolic condition to co-housed mice that had received the lean twin’s microbiota provided the latter had not received a healthy diet. Other extra-intestinal diseases such as multiple sclerosis, IR, and idiopathic thrombocytopenic purpura could also become targets for this experimental treatment[46].
GUT-LIVER AXIS MALFUNCTION AND NAFLD
As previously mentioned, obesity and diet-related intestinal barrier damage may favor gut-liver axis malfunction, thereby allowing further liver steatosis and a deranged passage of bacterial components into circulation (the so-called “leaky” gut). Hepatotoxic bacterial products [pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs)] reaching the liver via the portal circulation have been shown to activate specific toll-like receptors (TLRs) present in many different liver cells, including Kupffer cells, stellate cells, and hepatocytes. Kesar and Odins[48] extensively reviewed the location of TLRs in the liver and their specific PAMP/DAMP ligands.
Lipopolysaccharide, a cell wall component of gram-negative bacteria, is the prototypical ligand for TLR4 and one of the most widely studied PAMPs. It initiates the pro-inflammatory cascade that indirectly activates the MyD88-dependent pathway (nuclear factor kappa B, the protein-1-dependent pathway of the mitogen-activated protein kinase activator), and LPS-induced TNF alpha factor (Figure 1). TLR2 also senses other bacterial products, such as lipoteichoic acid from gram-positive bacteria, to regulate the maintenance of barrier function and intestinal permeability. In mice, TLR2 deficiency is associated with increased absorption of bacterial LPS and metabolic syndrome[49,50].
Gut dysbiosis and a phenotype characterized by obesity, IR, hyperlipidemia, and hypertension occur in TLR5-knockout mice[51]. Interestingly, transplantation of TLR5-/- fecal microbiota to germ-free wild-type mice was associated with obesity and metabolic syndrome. TLR9 ligand, which recognizes the unmethylated CpG motifs of bacterial DNA, was documented in the blood of a murine NAFLD model[52]. Moreover TLR-9-deficient mice showed less IR and a less pronounced fibrogenic response[52]. A HFD may also favor liver sensitivity to LPS by increasing the expression of TLR2, TRL4, and CD14. This pathway is involved in NAFLD pathogenesis, particularly in TLR4 induction of hepatic fibrogenesis. TRL4 can induce hepatic fibrosis by activating stellate cells and by enhancing radical oxidative species together with TNF-α production and systemic inflammation[49,50] (Figure 1).
Finally, also inflammasomes (i.e., large intracellular multiprotein complexes that play a central role in innate immunity) detect and respond to a large range of PAMPs, including bacterial flagellin and DAMPs. Inflammasomes include a member of the NOD-like receptor family that recruits the inflammasome-adaptor protein ASC, which in turn interacts with caspase-1. This cascade of events may lead to inflammasome activation and subsequent maturation of the proinflammatory cytokines interleukin (IL)-1β and IL-18. This activation, which is crucial for host defense against pathogens, also appears to play a role in the pathogenesis of the inflammatory component of obesity and NAFLD[53].
PROBIOTICS AND NAFLD TREATMENT: EVIDENCE FROM ANIMAL MODELS AND HUMAN STUDIES
Probiotics, which are defined by the FAO/WHO as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”, have attracted interest given the possibility of positively altering the IM composition and its interactions with the immune system and gut epithelium. The growth and/or activity of bacteria in the digestive system may moreover be stimulated by prebiotics (non-digestible food ingredients) in ways claimed to be beneficial to health. Probiotics can include elements of the normal human flora. They are introduced into the body to increase their dominance in the bowel, thereby reversing the damage or harm caused by detrimental bacteria. Commercialized probiotics include lactic acid bacteria (Clostridium/Bacillus gram-positive bacteria and Actinomycetes gram-positive Bifidobacteria) and spore-forming bacteria (Clostridium-Bacillus gram-positive bacteria). Lactic acid bacteria have been used in both clinical and experimental studies. Obviously, they must be resistant to pH changes, mechanical stress, extreme temperatures, enzymatic activities, and osmotic force to survive until they reach the intestinal colonization site. Less is known about spore-forming bacteria, which, theoretically, are ideal for dietary supplementation given their resistance to harsh conditions[54]. The effectiveness of probiotic delivery to the gastrointestinal tract varies greatly depending on formulation. The microencapsulated formulation, in which probiotic bacteria are enclosed in a coating material, appears to protect them until they reach the gut targets[55].
Many physiological studies have shown that intestinal barrier function may be improved/modulated by probiotics under several conditions. As an example, Streptococcus thermophilus and Lactobacillus acidophilus play a role in the activation of TJ proteins, thereby preventing the development of a “leaky” intestine[56]. In addition, as shown in Figure 1, Lactobacillus rhamnosus GG can prevent inflammation and apoptosis in the lining of intestinal epithelial cells[57]. The biochemical pathways mediating the effect exerted by probiotics on TJ function include the protein kinase C and MAP kinase pathways, which involve both the redistribution and altered expression of the TJ proteins occludin and ZO[12,58,59].
Probiotic administration may repair damaged intestinal barrier and hence restore its function. In particular, Lactobacillus casei DN-114001[60] and VSL#3 (a mixture of pre- and probiotics)[61] seem to restore intestinal barrier function by enhancing the expression of ZO-2 and protein kinase C in TJs. Intraduodenal administration of Lactobacillus plantarum MB452 enhances ZO expression near TJs in healthy individuals[62]. Escherichia coli strain Nissle 1917 restored mucosal permeability in the murine dextran sulfate sodium-induced colitis model by increasing ZO-1 expression[43]. Finally, in vitro studies have shown that probiotics can increase the expression of TJ-related occludin and cingulin[27] and promote mucus secretion. Probiotics also exert antimicrobial activity by producing antibacterial substances called “bacteriocins”.
In the following section we summarize the results of studies published between 2000 and 2014 that evaluated the effect of probiotic treatments in animal models and in NAFLD patients. Data were retrieved from the major data banks (PubMed, Google Scholar, Medscape, and Embase).
Animal studies
Most studies refer to the use of a single probiotic or probiotic mixtures in NAFLD animal models that was mostly obtained by genetic manipulation, or in which the animals received a high-fat diet, a methionine-choline deficient diet (MCD), or a choline-deficient L-amino acid diet.
VSL#3: VSL#3 is a mixture of probiotic bacteria including Lactobacilli that has been used in a number of experimental and human studies of NAFLD treatment. VSL#3 has 450 billion bacteria per sachet, with a mixture of eight different bacterial species (Lactobacilli, Bifidobacteria, and Streptococcus; Table 2). We retrieved five studies that used this probiotic mixture. In 2003, a cornerstone paper showed that VSL#3 treatment significantly decreased hepatic inflammation, serum alanine aminotransferase (ALT) levels, and hepatic oleic acid levels in a genetically obese ob/ob mice NAFLD model. The effects were mediated by modulation of IR, as shown by reduced activity of the c-Jun N-terminal kinase. This is a TNF-regulated kinase that promotes IR and decreases the DNA binding activity of NF-κB, which is the target of I-kappa-B-kinase beta (another TNF-regulated enzyme that probably represents the link between inflammation and obesity-induced IR). Consistent with these treatment-related pathogenetic mechanisms, fatty acid beta-oxidation, and uncoupling protein-2 expression decreased after VSL#3 treatment[63].
Table 2.
Studies with probiotics in animal models of non-alcoholic fatty liver disease
| Ref. | Animal model | Probiotic | Weeks | Positive effects | Negative effects |
| Li et al[63] | HFD ob/ob mice | VSL#3 | 4 | Reduced liver inflammation and serum ALT | |
| Esposito et al[64] | HFD Sprague-Dawley rats | VSL#3 | 4 | Significantly reduced TNF alpha levels, MMP-2 and MMP-9 activities, iNOS, and Cox-2 expression. Increased PPAR-alpha expression | NS |
| Ma et al[65] | HFD-WT male C57BL6 | VSL#3 | 4 | Improved NKT cells depletion, insulin resistance, and hepatic steatosis | NS |
| Velayudham et al[66] | MCD mouse | VSL#3 | 10 | Prevented PPAR-induced fibrosis. Increased expression of Bambi, a negative regulator of the TGFβ signaling pathway | Did not prevent NASH. |
| Did not protect against MCD liver injury | |||||
| Mencarelli et al[67] | Apo E-/- mice fed dextran sulfate sodium | VSL#3 | 12 | Reversed IR and prevented steatohepatitis by transactivation of PPARγ | NS |
| Bhathena et al[68] | MCD Bio F1B Golden Syrian hamster | Lactobacillus fermentum ATCC | 12 | Reduced liver fat deposition, decreased total cholesterol, triglycerides, uric acid, and insulin resistance | NS |
| Wagnerberger et al[69] | High fructose intake C57BL/6 L mouse | Lactobacillus Casei-Shirota | 8 | Attenuated the TLR4 signaling pathway and increased PPAR activity | |
| Karahan et al[70] | MCD Wistar rats | Pro1; Pro2 | 2 and 8 | Both probiotics reduced ≥ 50% the incidence of steatohepatitis by modulating apoptosis + anti-inflammatory activity | NS |
| Yalçin et al[71] | Broilers fed low-protein diet | Primalac 454 | 4 | Significantly diminished histological grade, steatosis and cell ballooning scores | Increased serum TG |
| Xu et al[72] | HFD Sprague-Dawley rats | Lactobacillus acidophilus Bifidobacterium longum | 12 | Bifidobacterium longum was superior to Lactobacillus acidophilus in attenuating liver fat accumulation. No variation of intestinal permeability in the treated groups | |
| Nardone et al[74] | Ischemia/reperfusion (I/R) in rats fed a standard or MCD diet | Lactobacillus Paracasei | 8 | Reduced LPS levels. Attenuated I/R-related damage | NS |
| Fazeli et al[75] | Rats on high cholesterol diet | Lactobacillus plantarum A7 | 2 | Significantly reduced levels of cholesterol, TG and LDL | NS |
| Endo et al[77] | Male Fischer CDAA rats | Butyrate producing Clostridium butyricum MIYAIRI 588 | 8, 16, 50 | Delayed CDAA-induced NAFLD progression and liver tumorigenesis. Reduced lipid deposition and improved IR, serum endotoxin levels, and hepatic inflammatory indexes. Improved ZO-1 expression | NS |
| Chiua et al[78] | HepG2 cells exposed to LPS | Lactobacilli bacteria lisate | Suppressed cytokine signaling 1 and PPAR alpha via NOD-NF-κB and cross-regulation of TLR4 | NS | |
| Raso et al[76] | Rats on a HFD | Synbiotic with Lactobacillus paracasei B21060 | 6 | Improved IR parametersReduced cytokine synthesis and restored the HFD-dysregulated TLR 2, 4 and 9 mRNAs.Preserved gut barrier integrity | NS |
Primalac 454: Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Bifidobacterium thermophilus; VSL#3: Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. bulgaricus; Pro1: Lactobacillus fermentum, Lactobacillus plantarum, and Enterococcus faecium; Pro2: Enterococcus faecium and Lactobacillus Pl; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TNF: Tumor necrosis factor; IL: Interleukin; IR: Insulin resistance; PPAR: Peroxisome proliferator-activated receptors; MMP: Matrix metalloproteinase; NOS: Nitric oxide synthase; NOD: Nucleotide-binding oligomerization domain receptors; TLR: Toll-like receptors; CDAA: Choline-deficient, L-amino acid-defined; MCD: Methionine-choline deficient.
In rats with HFD-induced NAFLD, we showed that VSL#3 markedly reduced the oxidative damage, protein nitrosylation, and tissue TNFα level, and increased the expression of peroxisome proliferator-activated receptor (PPARα), which indicates that it can control inflammatory and oxidative damage[64]. The treatment also significantly reduced serum and liver triglyceride concentrations, which were associated with a reduction in body but liver fat mass, thus suggesting that this probiotic could reduce dietary fat absorption. Our data confirmed previous findings[65] that VSL#3 improved IR and natural killer cell-depletion. The effects of VSL#3 on IR were also corroborated in other NAFLD animal models, in which the probiotic did not affect hepatic steatosis and had variable effects on the inflammatory component of NASH[66,67]. Improved liver fibrosis was primarily due to a reduction in the accumulation of collagen and alpha-smooth muscle actin, likely via PPARγ transactivation/upregulation[66].
We identified ten studies in which several genera of Lactobacilli alone or mixed with another genus were used in mammal or avian NAFLD animal models. As shown in Table 2, Lactobacilli decreased liver fat deposition, serum levels of total cholesterol, triglycerides and uric acid, and IR[68]. Interestingly, they also prevented fructose-induced steatosis by markedly attenuating the TLR4 signaling pathway and increasing PPARγ activity[69]. A combination of Lactobacilli and Enterococci probiotics reduced by at least 50% the incidence of steatohepatitis in choline-deficient diet-induced NAFLD by modulating apoptosis and anti-inflammatory activity[70]. In a low-protein-diet avian NAFLD model, PrimaLac 454 (a mixture of two Lactobacilli, an Enterococcus, and a Bifidobacterium) produced a significant reduction in the histological grade of steatosis and in cell ballooning scores[71].
In a study comparing two probiotics (Lactobacillus acidophilus and Bifidobacterium longum) in rats with HFD-induced NAFLD, Bifidobacterium longum was superior in attenuating liver fat accumulation. The lack of changes in intestinal permeability in treated mice was attributed to the effect of peptidoglycan-polysaccharide polymers rather than to endotoxin-induced stimulation of TNF-α release[72]. This concept is supported by a human study in which levels of antibodies to peptidoglycan-polysaccharide polymers significantly decreased after administration of Lactobacillus GG in pediatric NAFLD[73] (see below).
In rats with liver damage due to ischemia/reperfusion (I/R) that were fed a standard or steatogenic (MCD) diet, Lactobacillus paracasei attenuated I/R-related damage, whereas the effect was less pronounced in MCD-fed rats[74]. Lactobacillus plantarum A7 reduced lipid levels and/or IR in rats receiving high-cholesterol diets[75].
A very recent study showed that a synbiotic composed of Lactobacillus paracasei B21060 plus arabinogalactan and fructooligosaccharides, delayed NAFLD progression in a HFD rat model. The synbiotic improved liver inflammatory markers and many aspects of IR, such as fasting response, hormonal homeostasis, and glycemic control[76].
Other probiotics: A butyrate-producing probiotic (MIYAIRI 588) reduced the lipid deposition and significantly improved the triglyceride content, IR, serum endotoxin levels, and hepatic inflammatory indexes in a rat model of choline-deficient diet-induced NAFLD[77]. This observation indirectly supports the proposed role of butyrate in the maintenance of intestinal barrier integrity[12]. Finally, in vitro studies showed that Lactobacilli also exert direct anti-inflammatory activity against target (HepG2) liver cells previously exposed to LPS by inducing IL10 and suppressor of cytokine signaling 1 and PPAR alpha via NOD-NF-κB and TLR4 cross regulation[78].
Collectively, these studies conducted in animal models indicate that probiotics may play a role in NAFLD treatment. It is not always clear how probiotics modulate the various aspects of the inflammatory, oxidative, and metabolic pathomechanisms underlying both the origin and progression of NAFLD. It is conceivable that they might correct the IM imbalance in obese individuals or they may act as direct modulators of intestinal barrier integrity by producing bacteria-derived molecules (“host-bacterial cross talk”)[79]. Accordingly, it might be useful, in future studies, to integrate studies on the effectiveness of a single probiotic or probiotic cocktails with small intestinal and colonic colonization data.
Human studies
Based on the results of cellular and animal models, probiotics have long been an attractive potential therapeutic tool for human NAFLD. Unexpectedly, we retrieved only ten such studies [seven randomized controlled trials (RCTs)]. Indeed, the 2007 Cochrane meta-analysis, performed to elucidate the beneficial and harmful effects of probiotics in NAFLD or NASH, did not yield clear outcomes due to a lack of RCTs[80]. The only two pilot non-randomized studies identified at that time showed that VSL#3 or Lactobacilli plus a prebiotic and vitamin mixture (Bio-Flora) were well tolerated, and that they improved conventional liver function tests and reduced the levels of markers of lipid peroxidation and/or TNF-α. In particular, a 2-mo supplementation with BioFlora decreased the levels of liver enzymes in 10 biopsied adults affected by NASH. One month after washout, both ALT and gamma glutamyltransferase improved significantly. The treatment also induced a reduction in oxidative stress markers [malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE)][81]. A second study included in the Cochrane meta-analysis assessed the treatment effects of the probiotic VSL#3 in patients with different categories of adult chronic liver diseases, including 22 NAFLD patients for whom treatment significantly improved the plasma levels of MDA and 4-HNE (precise data were not reported)[82].
Subsequent to that publication[80] and to an ESPGHAN meta-analysis[83], Solga et al[84] published a preliminary study warning about the possible deleterious effect of VSL#3 on hepatic steatosis. However, as shown in Table 3, five RCTs appeared a few years after this study, none of which recorded such a harmful effect. In the first RCT, conducted by our group in a pediatric NAFLD population, a multivariate analysis revealed a significant decrease in ALT values (average variation vs placebo, P = 0.03), with normalization in most (80%) cases, after Lactobacillus GG treatment, irrespective of changes in the BMI Z score and visceral fat. In addition, the levels of anti-peptidoglycan-polysaccharide antibodies, which are indirect indicators of small intestinal bacterial overgrowth, decreased significantly, thereby suggesting a possible improvement of intestinal dysbiosis and/or gut barrier leakage. TNFα and bright liver parameters on ultrasound remained unmodified, as reported in earlier studies[73].
Table 3.
Studies with probiotics in human non-alcoholic fatty liver disease
| Ref. | Probiotic(s) | Study design | Month | Main results | ALT or AST | GGT | US/MRI/LH |
| Wong et al[87] | Lepicol: 10 g/d10 adult NASH ctrls10 adult NASH patients | RCT | 6 | Significantly reduced ASTChanged intrahepatic triglyceride content (IHTG) | Pr vs plac: ALT NS decrease AST: -13 ± 31 vs 23 ± 32, P = 0.021 | NR | Reduced IHTG at SMR (P = 0.034) |
| Vajro et al[73] | Lactobacillus GG: 12 billion CFU/d 10 pediatric obese ctrls 10 pediatric obese patients | RCT | 2 | Significantly reduced aminotransferases and anti-peptidoglycan-polysaccharide Abs. TNF-α stable | Pr vs plac: Decreased ALT 70.3 ± 34.76 vs 40.1 ± 22.37, P = 0.03 | Normal | Unchanged |
| Loguercio et al[81] | Bio-Flora: 4 tablets/d 10 adult NASH patients | Open label | 2 | Decreased ALT and GGT | Decreased ALT -64.5% ± 26.5%, P < 0.01 | -55 ± 31, P < 0.01 | NR |
| Loguercio et al[82] | VSL#3: 450 billion/d 22 patients | Open label | NR | Significantly improved plasma MDA and 4-HNE (data not shown) | NR | NR | NR |
| Solga et al[84] | VSL#3: 450 billion/d 4 adult NAFLD patients | Open label | 4 | Significantly increased ultrasound liver fat NS different glycosylated Hb; TNF-α, IL-6 | Unchanged | NR | Increased liver fat at MRS |
| Aller et al[85] | Lactobacillus bulgaricus streptoc. | RCT | 3 | Significantly reduced aminotransferases | Decreased in Pr:ALT: 67.7 ± 25.1 vs 60.4 ± 30.4, P < 0.05AST: 41.3 ± 15.5 UI/L vs 35.6 ± 10.4 UI/L, P < 0.05 | 118 ± 63 vs 107 ± 60 (P < 0.05) | NR |
| Thermophiles: 500 million CFU/d | |||||||
| 14 adult NAFLD ctrls | |||||||
| 14 adult NAFLD patients | |||||||
| Malaguarnera et al[86] | Bifidobacterium longum and Fos: 2.5 g/d + vit B1, B2 , B6, B12 + life style 34 adult NASH ctrls 32 adult NASH patients | RCT | 4 | Improved fibrosis scores in 70% of patients. Reduced HOMA-IR, LDL cholesterol , CRP, TNF-α, AST | Pr vs plac: ALT NS decrease AST -69.6 vs -45.9, P = 0.05 | NR | Decreased US bright liver -42% vs -11%, P < 0.001 |
| Shavakhi et al[91] | Proxetin: 2 tablets/d | RCT | 6 | Significantly reduced ALT in Metformin/ | Pr vs plac:ALT Decrease45.2 ± 32.5 vs 112.5 ± 69,P < 0.001 | NR | Reduced US grade in M/Pr, P < 0.01 |
| Metformin: 500 mg/d | Probiotic (M/Pr) vs M/ | ||||||
| 36 adult NASH ctrls | placebo (M/Plac) | ||||||
| 34 adult NASH patients | M reduced BMI enhanced by Pr | ||||||
| Eslamparast et al[89] | Proxetin 2 tablets/d26 adult NASH ctrls26 adult NASH patients | RCT | 6 | Significantly and persistently reduced ALT Significantly reduced AST, HOMA-IR, GGT, CRP, TNF-α, and NF-κB p65 | Pr vs placDecreased ALT (wk) 28 -25.1 vs -7.3, P < 0.001 | Reduced Pr vs plac-15.8 vs -5.21, P < 0.01 | Significant improvement elastography and fibrosis score |
| Alisi et al[90] | VSL#3 | RCT | 4 | Significantly increased GLP-1 and aGLP-1 Sign. decreased BMI | Pr vs plac ALT unchanged | NR | Significantly improved ultrasound fatty liver score |
Proxetin: Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophilus + FOS 350 mg; Lepicol: Lactobacillus plantarum, Lactobacillus delbrueckii, Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium bifidum (Each sachet contains 10 g of probiotic cultures) + FOS; VSL#3: Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. bulgaricus; BioFlora: Lactobacilli (acidophilus; lactis; casei; brevis; salivarum; rhamnosus; plantarum; bulgaricus), iron, vitamin C, B6, D3, B2, B12, folic acid and zinc oxide, + FOS; FOS: Fructooligosaccharides; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyl transpeptidase; MRI: Magnetic resonance imaging; LH: Liver histology; BMI: Body mass index; NR: Not reported; Pl: Placebo; Pr: Probiotic; NS: Not significant; Sign: Significantly; TNF: Tumor necrosis factor; IL: Interleukin.
Subsequent to our pediatric data, a study on the effect of probiotic treatment (Lactobacillus bulgaricus and Streptococcus thermophilus) in adults with histologically proven NAFLD confirmed a significant reduction in liver enzymes[85]. In agreement with our study, anthropometric parameters and cardiovascular risk factors remained unchanged in both the treated and control groups. Treatment with Bifidobacterium longum plus the prebiotic FOS induced a significant improvement in serum inflammatory, metabolic, and liver enzyme parameters. End-of-study repeat liver biopsies showed improved fibrosis scores in 70% of patients and a decrease in the NASH activity index[86]. Administration of Lepicol (a 5 probiotics mixture) in histologically proven adult NAFLD patients resulted in a significant decrease in their intrahepatic triglyceride (IHTG) content, as measured by proton-magnetic resonance spectroscopy (P = 0.034), and a reduction in their serum aspartate aminotransferase level[87]. In another paper, the same authors reported IM colonization data before and after treatment compared to a healthy control population[38]. Improvement in IHTG was associated with a reduction in the abundance of Firmicutes and an increase in Bacteroidetes. This was accompanied by corresponding changes at the class, order, and genus levels. In contrast, bacterial biodiversity did not differ between NASH patients and controls, and did not change with probiotic treatment[38].
The clinical studies reported above have very recently been reviewed in a meta-analysis which confirmed that probiotic treatment reduces levels of aminotransferases, total-cholesterol, and TNF-alpha, and improves IR in NAFLD patients[88]. However, these results should be viewed in the light of the modest number of patients included in each study and the short time frame of treatment (median 3.75 mo)[88]. Two new pilot RCTs studies published after the meta-analysis confirmed the previous results. In the first pilot RCT, the effectiveness of synbiotic supplementation (Primalac) plus lifestyle changes vs lifestyle changes alone was studied in adult NAFLD[89]. The primary outcome (ALT reduction) was attained after only 14 wk of treatment and was maintained until completion of the study. Inflammatory parameters (CRP, TNF-α, and NF-κB p65) were also significantly reduced. In the second pilot RCT that enrolled 22 children with biopsy-proven NAFLD, administration of VSL#3 for 4 mo significantly improved BMI and ultrasonographic fatty liver parameters[90]. Moreover, there was an increase in the levels of glucagon-like peptide 1 and of its activated form, an enterochromaffin cell product that promotes insulin sensitivity. ALT values were in normal range at baseline and did not change during the study.
Lastly, a comparison of the effects of metformin ± probiotics (MetPr) in 32 adult NAFLD patients showed a more significant decrease in aminotransferase, cholesterol, triglyceride levels, and BMI levels in the MetPr group than in the Met placebo group[91]. Notably, probiotics enhanced the effect of metformin in reducing the BMI.
Collectively, these clinical studies reinforce experimental observations of the possible therapeutic role of probiotics in NAFLD treatment. In general, it appears that probiotics act on different targets (i.e., they modify the gut microbiota composition; reduce intestinal permeability and the translocation of bacterial products in portal circulation; and modulate the liver inflammation pathways and collagen deposition). However, various aspects remain unclear; it is still not known how different probiotics act on specific targets, and only a few studies have compared the effects of a single probiotic vs another. Moreover, most of the studies performed with a mixture of probiotics were also associated with one or more prebiotics, which exert an independent effect on NAFLD (e.g., increasing the level of Bifidobacterium and Lactobacillus spp[83,92,93]).
Because of the variety of pathomechanisms underlying NAFLD (IR, oxidative stress, and gut-liver axis malfunction), a multi-targeted therapeutic approach that includes add-on IM modulation by probiotics[91,94,95] seems more reasonable than a single treatment approach. Nonetheless, as the data available are not yet sufficient to recommend any individual pharmacological treatment for human NAFLD[96,97], it appears that patients clearly unable to lose weight or change sedentary habits might benefit the most from tailored treatments targeting multiple pathomechanisms.
CONCLUSION
In summary, the issues discussed in this review make it increasingly clear that the IM influences gut permeability, systemic inflammation levels, and host metabolism, thereby contributing to obesity and fatty liver disease. Several findings suggest that probiotics affect the IM and that they act by modulating visceral and hepatic fatty deposition via the gut-liver axis. Consequently, they may be proposed as add-on NAFLD treatment complementary to standard dietary and behavior strategies.
Since different probiotic species may exert different effects on the IM, further studies are needed to shed light on the interaction between probiotics and the IM[98]. In particular, a more precise evaluation of the specific gut microbiota composition profiles in lean, obese, and/or NAFLD individuals will probably enable better personalized modulation of the IM by pro-, pre-, and synbiotics. Finally, because fructose appears to be closely related to obesity, hepatic fat accumulation, IR, and gut-liver axis malfunction, the use of pro-and prebiotics to limit the adverse effects of fructose by reducing TLR4 receptor activation is another appealing strategy that warrants further attention[98,99].
ACKNOWLEDGMENTS
We thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for editing the manuscript.
Footnotes
Supported by (in part) FARB-ex 60% 2012 of the University of Salerno grant to Vajro P
P- Reviewer: Marotta F, Servin AL S- Editor: Gou SX L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut-liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314–5324. [PubMed] [Google Scholar]
- 4.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461–468. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li DY, Yang M, Edwards S, Ye SQ. Nonalcoholic fatty liver disease: for better or worse, blame the gut microbiota? JPEN J Parenter Enteral Nutr. 2013;37:787–793. doi: 10.1177/0148607113481623. [DOI] [PubMed] [Google Scholar]
- 6.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218–1229. doi: 10.1016/j.jhep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V, Garg S, Aggarwal S. Probiotics and liver disease. Perm J. 2013;17:62–67. doi: 10.7812/TPP/12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehal WZ. The gut-liver axis: a busy two-way street. Hepatology. 2012;55:1647–1649. doi: 10.1002/hep.25704. [DOI] [PubMed] [Google Scholar]
- 10.Guzman JR, Conlin VS, Jobin C. Diet, microbiome, and the intestinal epithelium: an essential triumvirate? Biomed Res Int. 2013;2013:425146. doi: 10.1155/2013/425146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 14.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 15.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 16.Kinnebrew MA, Pamer EG. Innate immune signaling in defense against intestinal microbes. Immunol Rev. 2012;245:113–131. doi: 10.1111/j.1600-065X.2011.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 18.Liévin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 21.Stecher B, Maier L, Hardt WD. ‚Blooming‘ in the gut: how dysbiosis might contribute to pathogen evolution. Nat Rev Microbiol. 2013;11:277–284. doi: 10.1038/nrmicro2989. [DOI] [PubMed] [Google Scholar]
- 22.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in’t Veld JH. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 23.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 24.Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, Goossens H, Desager KN, Vankerckhoven V. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. 2013;5:10. doi: 10.1186/1757-4749-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira TF, Collado MC, Ferreira CL, Bressan J, Peluzio Mdo C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–647. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Payne AN, Chassard C, Zimmermann M, Müller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes. 2011;1:e12. doi: 10.1038/nutd.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 31.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 33.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes (Lond) 2012;36:817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri JM, Moreno LA, Martin-Matillas M, Campoy C, Martí A, Moleres A, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 2009;33:758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 36.Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, Yeung DK, Law PT, Kwan HS, Yu J, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8:e62885. doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 40.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 41.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–75.e1-3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 43.Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014;42:49–53. doi: 10.1177/0192623313508481. [DOI] [PubMed] [Google Scholar]
- 44.Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45 Suppl:S120–S127. doi: 10.1097/MCG.0b013e31822fecfe. [DOI] [PubMed] [Google Scholar]
- 45.Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, Grieco A, Alisi A, Nobili V. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556–560. doi: 10.1016/j.dld.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 47.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kesar V, Odin JA. Toll-like receptors and liver disease. Liver Int. 2014;34:184–196. doi: 10.1111/liv.12315. [DOI] [PubMed] [Google Scholar]
- 49.Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:637–644. doi: 10.1038/nrgastro.2013.146. [DOI] [PubMed] [Google Scholar]
- 50.Cario E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 2008;1 Suppl 1:S62–S66. doi: 10.1038/mi.2008.47. [DOI] [PubMed] [Google Scholar]
- 51.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Requena T, Shahar DR, Kleiveland CR, Martínez-Cuesta MC, Peláez C, Lea T. Interactions between gut microbiota, food and the obese host. Rends Food Sci Tech. 2013;34:44–53. [Google Scholar]
- 53.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouwehand A, Forssten S, Lehtinen M, Galbraith E, Davis E. Probiotic lactic acid bacteria vs bacilli: pros and cons. Agro Food Ind Hi Tec. 2013;24:13–18. [Google Scholar]
- 55.Govender M, Choonara YE, Kumar P, du Toit LC, van Vuuren S, Pillay V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech. 2014;15:29–43. doi: 10.1208/s12249-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 59.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 60.Parassol N, Freitas M, Thoreux K, Dalmasso G, Bourdet-Sicard R, Rampal P. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res Microbiol. 2005;156:256–262. doi: 10.1016/j.resmic.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–G626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 62.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 64.Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905–911. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 65.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mencarelli A, Cipriani S, Renga B, Bruno A, D’Amore C, Distrutti E, Fiorucci S. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7:e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhathena J, Martoni C, Kulamarva A, Tomaro-Duchesneau C, Malhotra M, Paul A, Urbanska AM, Prakash S. Oral probiotic microcapsule formulation ameliorates non-alcoholic fatty liver disease in Bio F1B Golden Syrian hamsters. PLoS One. 2013;8:e58394. doi: 10.1371/journal.pone.0058394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagnerberger S, Spruss A, Kanuri G, Stahl C, Schröder M, Vetter W, Bischoff SC, Bergheim I. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. J Nutr Biochem. 2013;24:531–538. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Karahan N, Işler M, Koyu A, Karahan AG, Başyığıt Kiliç G, Cırış IM, Sütçü R, Onaran I, Cam H, Keskın M. Effects of probiotics on methionine choline deficient diet-induced steatohepatitis in rats. Turk J Gastroenterol. 2012;23:110–121. doi: 10.4318/tjg.2012.0330. [DOI] [PubMed] [Google Scholar]
- 71.Yalçin SS, Güçer Ş, Yalçin S, Onbaşilar İ, Kale G, Coşkun T. Effects of probiotic (Primalac 454) on nonalcoholic fatty liver disease in broilers. Revue Méd Vét. 2011;7:371–376. [Google Scholar]
- 72.Xu RY, Wan YP, Fang QY, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012;50:72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, Caropreso M, Vallone G, Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 74.Nardone G, Compare D, Liguori E, Di Mauro V, Rocco A, Barone M, Napoli A, Lapi D, Iovene MR, Colantuoni A. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G669–G676. doi: 10.1152/ajpgi.00188.2010. [DOI] [PubMed] [Google Scholar]
- 75.Fazeli H, Moshtaghian J, Mirlohi M, Shirzadi M. Reduction in serum lipid parameters by incorporation of a native strain of Lactobacillus Plantarum A7 in Mice. Iran J Diab Lipid Disorders. 2010;9:1–7. [Google Scholar]
- 76.Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, Russo R, D’Agostino G, Di Costanzo M, Canani RB, et al. Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem. 2014;25:81–90. doi: 10.1016/j.jnutbio.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiua YH, Linb SL, Oua CC, Lue YC, Huanga HY, Lina MY. Anti-inflammatory effect of lactobacilli bacteria on HepG2 cells is through cross-regulation of TLR4 and NOD2 signalling. J Funct Foods. 2013;5:820–828. Available from: http://www.sciencedirect.com/science/article/pii/S1756464613000467. [Google Scholar]
- 79.Konishi H, Fujiya M, Kohgo Y. Traffic control of bacteria-derived molecules: a new system of host-bacterial crosstalk. Int J Cell Biol. 2013;2013:757148. doi: 10.1155/2013/757148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lirussi F, Mastropasqua E, Orando S, Orlando R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007;(1):CD005165. doi: 10.1002/14651858.CD005165.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loguercio C, De Simone T, Federico A, Terracciano F, Tuccillo C, Di Chicco M, Cartenì M. Gut-liver axis: a new point of attack to treat chronic liver damage? Am J Gastroenterol. 2002;97:2144–2146. doi: 10.1111/j.1572-0241.2002.05942.x. [DOI] [PubMed] [Google Scholar]
- 82.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, Del Vecchio Blanco C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 83.Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587–596. doi: 10.1097/MPG.0b013e31818e04d1. [DOI] [PubMed] [Google Scholar]
- 84.Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol. 2008;42:1117–1119. doi: 10.1097/MCG.0b013e31816d920c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 86.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 87.Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, Chan HL. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–262. [PubMed] [Google Scholar]
- 88.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535–542. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]
- 90.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int J Prev Med. 2013;4:531–537. [PMC free article] [PubMed] [Google Scholar]
- 92.Parnell JA, Raman M, Rioux KP, Reimer RA. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012;32:701–711. doi: 10.1111/j.1478-3231.2011.02730.x. [DOI] [PubMed] [Google Scholar]
- 93.Kleessen B, Schwarz S, Boehm A, Fuhrmann H, Richter A, Henle T, Krueger M. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br J Nutr. 2007;98:540–549. doi: 10.1017/S0007114507730751. [DOI] [PubMed] [Google Scholar]
- 94.Vajro P, Lenta S, Pignata C, Salerno M, D’Aniello R, De Micco I, Paolella G, Parenti G. Therapeutic options in pediatric non alcoholic fatty liver disease: current status and future directions. Ital J Pediatr. 2012;38:55. doi: 10.1186/1824-7288-38-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vajro P, Paolella G, Poeta M, Pizza C, Sangermano M, Massa G. Pediatric non alcoholic fatty liver disease: more on novel treatment targets. BMC Pediatr. 2013;13:109. doi: 10.1186/1471-2431-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Younossi ZM, Reyes MJ, Mishra A, Mehta R, Henry L. Systematic review with meta-analysis: non-alcoholic steatohepatitis - a case for personalised treatment based on pathogenic targets. Aliment Pharmacol Ther. 2014;39:3–14. doi: 10.1111/apt.12543. [DOI] [PubMed] [Google Scholar]
- 97.Volynets V, Machann J, Küper MA, Maier IB, Spruss A, Königsrainer A, Bischoff SC, Bergheim I. A moderate weight reduction through dietary intervention decreases hepatic fat content in patients with non-alcoholic fatty liver disease (NAFLD): a pilot study. Eur J Nutr. 2013;52:527–535. doi: 10.1007/s00394-012-0355-z. [DOI] [PubMed] [Google Scholar]
- 98.Vajro P, Poeta M, Pierri L, Pizza C, D’Aniello R, Sangermano M, Massa M, Paolella G. Probiotics to Treat Visceral Obesity and Related Liver Disease. In: Watson RR, editor. Editor “Nutrition in the Prevention and Treatment of Abdominal Obesity”. London: Academic Press; 2014. pp. 363–380. [Google Scholar]
- 99.Ong JP, Younossi ZM. Editorial: probiotics in NASH - more studies are needed. Aliment Pharmacol Ther. 2014;40:211–212. doi: 10.1111/apt.12812. [DOI] [PubMed] [Google Scholar]


