Abstract
Over the last decade, the development of stabilised microbubble contrast agents and improvements in available ultrasonic equipment, such as harmonic imaging, have enabled us to display microbubble enhancements on a greyscale with optimal contrast and spatial resolution. Recent technological advances made contrast harmonic technology available for endoscopic ultrasound (EUS) for the first time in 2008. Thus, the evaluation of microcirculation is now feasible with EUS, prompting the evolution of contrast-enhanced EUS from vascular imaging to images of the perfused tissue. Although the relevant experience is still preliminary, several reports have highlighted contrast-enhanced harmonic EUS (CH-EUS) as a promising noninvasive method to visualise and characterise lesions and to differentiate benign from malignant focal lesions. Even if histology remains the gold standard, the combination of CH-EUS and EUS fine needle aspiration (EUS-FNA) can not only render EUS more accurate but may also assist physicians in making decisions when EUS-FNA is inconclusive, increasing the yield of EUS-FNA by guiding the puncture with simultaneous imaging of the vascularity. The development of CH-EUS has also opened up exciting possibilities in other research areas, including monitoring responses to anticancer chemotherapy or to ethanol-induced pancreatic tissue ablation, anticancer therapies based on ultrasound-triggered drug and gene delivery, and therapeutic adjuvants by contrast ultrasound-induced apoptosis. Contrast harmonic imaging is gaining popularity because of its efficacy, simplicity and non-invasive nature, and many expectations are currently resting on this technique. If its potential is confirmed in the near future, contrast harmonic imaging will become a standard practice in EUS.
Keywords: Microbubbles, Contrast agents, Contrast-enhanced harmonic endoscopic ultrasound, Pancreatic tumour, Gastrointestinal submucosal tumour
Core tip: The recent development of stabilised contrast agents and enhancements to the endoscopic ultrasound equipment have opened an avenue for the real-time imaging of parenchymal microvascularisation without Doppler-related artefacts. Preliminary experience suggests that contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) is superior to other imaging techniques, such as EUS or computed tomography, in the differential diagnosis of pancreatic masses and other gastrointestinal diseases. In this paper, we describe the basic principles and technical aspects of performing this new technique and analyse the clinical value of CH-EUS and its potential applications in the near future.
INTRODUCTION
The dynamic observation of parenchymal enhancement during computed tomography (CT) or magnetic resonance imaging (MRI) examinations allows for the evaluation of abdominal organ perfusion. The patterns of enhancement may predict diagnoses of focal lesions and other digestive disorders. Concerning transabdominal ultrasonography (US), attempts have been made for years to depict the microcirculation with Doppler ultrasound to achieve a better characterisation of several pathological processes. Doppler is useful for evaluating large blood vessels with fast-flowing blood, including the major visceral vessels of the abdomen (portal vein, hepatic artery¡), but unfortunately, this modality is not sensitive enough to detect slow and low-volume flow at the level of perfusion[1]. Microbubble contrast agents increase the intensity of backscattered ultrasounds and were used initially to improve the Doppler detectability of the blood flow in small vessels[2]. However, contrast-enhanced Doppler US has several disadvantages. First, it may produce blooming artefacts (over-painting), which refer to the widened appearance of a vessel when compared with the results of fundamental B-mode imaging[3,4]. Second, Doppler US is sensitive to motion artefacts. Tissue movement can produce stronger Doppler signals than the signals from the contrast, resulting in a flash artefact[3,4]. Therefore, contrast harmonic imaging (HI) has been introduced to overcome this problem by suppressing the background tissue signals[5].
The past decade has witnessed great activity towards the development of stabilised microbubble contrast agents. During the same time period, there have also been enhancements of the available ultrasound equipment, such as HI-specific software and broad-band transducers, which have dramatically improved the ability of US to explore microcirculation and given rise to the evolution of contrast-enhanced transabdominal US for uses ranging from vascular imaging to images of the perfused tissue. Additionally, endoscopic ultrasonography (EUS) has become the imaging modality with the highest spatial resolution since its introduction in the 1980s. However, because of its low acoustic power and limited bandwidth, contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) has not been feasible until five years ago, when technological advances made contrast harmonic technology available for the first time for EUS. Although experience is still limited, several reports have highlighted this technique as a promising noninvasive method, not only for characterising lesions but also for better assessing tumour staging and resectability. In this article, we aim to review the physical principles of contrast harmonic imaging (CHI), technical issues of CH-EUS, clinical applications and future research areas.
WHAT IS HI?
HI is a relatively new ultrasonic imaging method that produces images based on non-linear acoustic effects of ultrasound interactions with tissues or microbubble contrast agents. “Linear” and “non-linear” are engineering terms that describe the response of a system to an applied echo-signal. The scattered signals from a linear system have the same frequency as the transmitted pulse. However, a non-linear response contains both the original insonated frequency and signals containing multiples of the transmitted frequency, known as harmonics. Unlike conventional US, in which images are formed from scattered echoes of the insonating frequency (fundamental frequency), harmonic US uses harmonic signals. More specifically, HI picks up twice the transmission frequency (second harmonic) from the received signals[6-10].
CHI: BASIC PRINCIPLES
Ultrasound contrast agents: Physicochemical overview
The major differences in acoustic impedance between the gas microbubbles and the surrounding blood or tissue make microbubbles the most effective scatterers for ultrasound contrast agents (UCAs)[6]. The general composition of a bubble is a gas core stabilised by a shell. Current UCAs consist of microbubbles with a 2-5 micron diameter capable of transpulmonary passage for systemic enhancement after intravenous injection. A bubble of this size is unstable in an aqueous system, and therefore, it must be encapsulated by a stabilising shell to achieve long lasting persistence[11,12].
The type of gas and the composition of the shell confer different physicochemical properties and different behaviours in the ultrasound field. However, the physical principles of the interaction between the microbubble and the incident ultrasound wave are basically the same. An acoustic wave generated by an ultrasound system consists of alternating high and low pressures. A microbubble exposed to an ultrasound wave alternately compresses under the positive pressure and expands under the negative pressure. In ultrasound imaging, the mechanical index (MI) is displayed on the monitor to indicate the acoustic power; this index can be used to characterise the response of microbubbles to an applied acoustic wave[6,8-10]. The MI of an ultrasound wave is defined as the peak negative pressure amplitude estimated in situ divided by the square root of the frequency. Depending on this ultrasound parameter, three different phenomena may occur, as follows[13]: (1) scattering increase. At very low MI (< 0.1), the bubbles oscillate in a symmetrical manner and a stable linear scattering is produced; (2) harmonic production. At low/medium MI (0.1-0.6), an asymmetrical bubble oscillation occurs, during which the microbubbles expand more than they contract because of their higher resistance to compression. This asymmetry is what constitutes the harmonic emissions; and (3) bubble collapse. At high MI (> 0.6), a transient non-linear scattering is generated, followed by microbubble destruction.
Contrast ultrasound imaging using the first generation of air-filled UCAs, such as Levovist®, requires destruction of the microbubble, thereby limiting the imaging in real time[4]. As CH-EUS was not possible with these UCAs because the transducer of the echoendoscope is too small to produce sufficient acoustic power to break the microbubbles, Doppler EUS has been used[14]. In the last years, a new class of more stabilised and longer lasting UCAs has been manufactured; these UCAs use a heavy gas (perfluorocarbon) with a lower diffusibility and blood solubility that is encapsulated into a resistant and flexible lipid shell. These properties make it possible to use a low acoustic power, avoiding the destruction of microbubbles for a longer period and resulting in continuous real-time assessment[15-17]. Moreover, a low MI allows for effective tissue signal suppression because the non-linear response from tissue is minimal with low acoustic power[4]. Among these second-generation UCAs, only three (SonoVue®, Sonazoid® and Definity®) have been used for CH-EUS. Their main physicochemical characteristics and dosages are shown in Table 1. After an intravenous bolus injection, they present a pure intravascular distribution, which means that they do not diffuse into the extravascular space, unlike the currently approved contrast agents for CT or MRI. This behaviour allows for real-time scanning with higher temporal resolution than other imaging modalities are capable of generating[18]. Gases are not metabolised in the human body, and several minutes after their administration, they are eliminated by the lungs in exhaled air. The stabilising shells are filtered by the kidney and eliminated by the liver. The most remarkable difference between these UCAs is that Sonazoid is a tissue-specific agent; as such, after a preliminary vascular phase, it presents a late hepatosplenic phase. This effect has been shown to be due to the trapping of microbubbles by Kupffer cells[19].
Table 1.
Types of microbubbles used in contrast-enhanced harmonic endoscopic ultrasound
| Microbubble | Company | Shell | Gas | Size (μm) | Storage | Preparation | Approval |
| Definity | Bristol-Myers Squibb Medical imaging Inc (New York) | Lipids | Octofluoropropane | 1.1-3.3 | Refrigerate 2 °C-8 °C | Activate through Vialmix agitation | Worldwide |
| Sonovue | Bracco Diagnostics (Princeton, NJ) | Lipids | Sulfurhexafluoride | 2-3 | No special storage conditions | Reconstitute with 5 mL saline | Europe, China, South Korea, Hong Kong, India, Singapore |
| Sonazoid | GE Healthcare (Piscataway, NJ) | Lipids | Perfluorobutane | 1-2 | - | Reconstitute with 2 mL water | Japan |
UCAs have demonstrated an excellent safety profile, as any adverse reactions are rare and usually of mild severity. They have no specific renal, cerebral or liver toxicity, and their embolic potential is of no clinical significance[18-21]. Severe anaphylactic reactions are rare (< 0.002%)[21]. There are several subgroups of patients who will substantially benefit from their use, including patients with renal impairment and patients with CT or MR contrast allergies. Furthermore, their use is not associated with any ionising radiation[1]. Nevertheless, serious cardiopulmonary reactions, including fatalities, have been reported in echocardiographic applications, and thus, microbubbles must be avoided in patients with severe cardiopulmonary diseases. Interactions between ultrasounds and microbubbles also produce biological effects, such as cavitation, haemolysis and cell death, in in vitro experiments, although generally when high ultrasound powers are used, unlike the low MIs applied in human diagnostic procedures[18-21]. However, this may constitute a potential safety concern when UCAs are used in tissues where microvascular damage could have severe consequences, such as the eye or the brain[18,21].
CHI modalities
Because tissues respond in mainly linear ways to incident ultrasound, the use of the non-linear properties of microbubbles in CHI offers the possibility of separating the response of the bubble from that of the surrounding tissue, thus enabling the evaluation of the microcirculation[22].
Contrast harmonic power doppler imaging
As previously mentioned, tissue movements can produce stronger Doppler signals than those from the contrast, resulting in a flash artefact. The combination of power Doppler with secondary harmonic filtering may reduce these motion artefacts[23] (Figure 1). To increase the sensitivity to the UCA, the tissue response must be reduced due to the spectral overlap between the fundamental (f0) and second harmonic (2 × f0) components of the received spectrum. This can be achieved by transmitting narrow-band signals that, in return, deteriorate the resolution of the image[8-10,22-24] (Figure 2). In addition, contrast harmonic power Doppler images will always be obscured by a residual harmonic component of the tissue response[24]. Although it was originally assumed that ultrasound propagation through the tissue was completely linear, in fact, tissues are not linearly elastic, leading to a propagation distortion that results in the generation of harmonics[10,22-25].
Figure 1.

Schematic view of the frequency range used for filtering the second harmonic.
Figure 2.
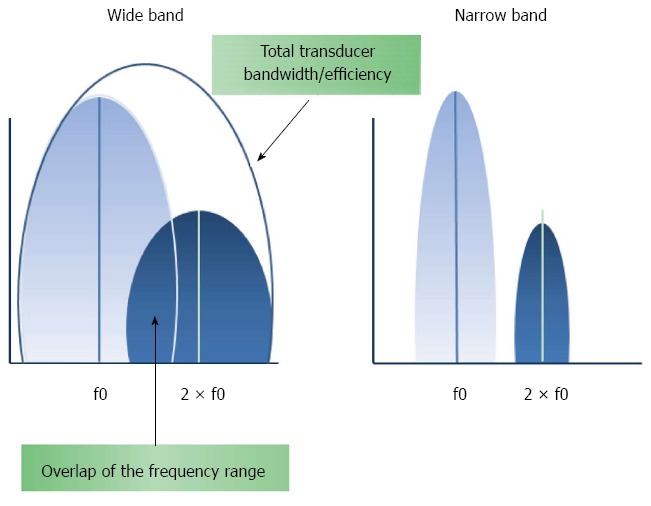
Effects of narrowing the band pulse.
Contrast enhanced HI in greyscale
Both tissue and UCA have a second harmonic frequency spectrum. The need to distinguish between them to improve image contrast led to the development of contrast-specific imaging techniques. These techniques display microbubble enhancement in greyscale, and their main advantage over harmonic power Doppler imaging is that they can work over the entire bandwidth of the received echo signal[26]. The harmonic signal is separated from the fundamental one, not by filtering, but rather by sending two trains of pulses out of phase with each other, followed by adding the values of the returning echoes. As a consequence, the signal bandwidth is preserved with increased spatial resolution[27]. Two of these contrast-specific imaging techniques have been implemented for contrast-enhanced HI in EUS: the dynamic contrast harmonic imaging (dCHI) used by Hitachi platforms and the extended pure harmonic detection (ExPHD) for Aloka systems.
dCHI: A sequence of two ultrasound waves of alternating phases (the second wave is the inverted replica of the first) is sent out. The image is then processed based on the sum of both pulses. In a linear medium, the responses to the first and second waves are equal waves with an opposite form, and the sum of the two responses is zero. A non-linear system does not reflect back identical inverted waveforms; because the waves do not cancel each other completely, the harmonic addition results in an image of non-linear scatterers[1,4,5] (Figure 3). Although this pulse-inversion technique more intensively depicts signals from microbubbles than those from the tissues, it is not able to completely filter out signals from the tissue, and non-linear propagation effects still limit the maximal obtainable agent-to-tissue ratio[23,24].
Figure 3.
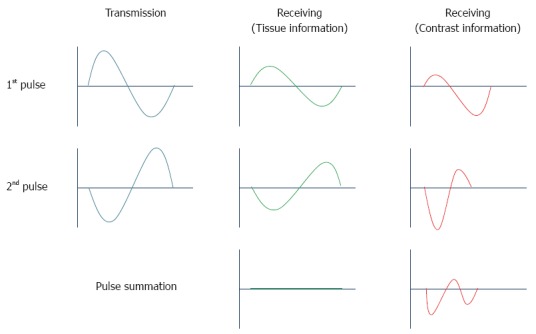
Principle of phase inversion mode.
ExPHD: This technique depicts the presence of a contrast agent by differentiating between the harmonics generated by the tissue from the harmonics generated by the agent. Signals are changed between the two transmissions in the area with contrast and in the area without contrast. However, microbubbles produce not only stronger second harmonic signals but also greater phase variations (phase shifts) than tissue. ExPHD technology can be used to detect phase shifts in the received signals and synthesises them with the signals of the second harmonics to reinforce the second harmonics (Figure 4). This processing results in an enhanced imaging of the signals from the contrasts[14,28,29].
Figure 4.
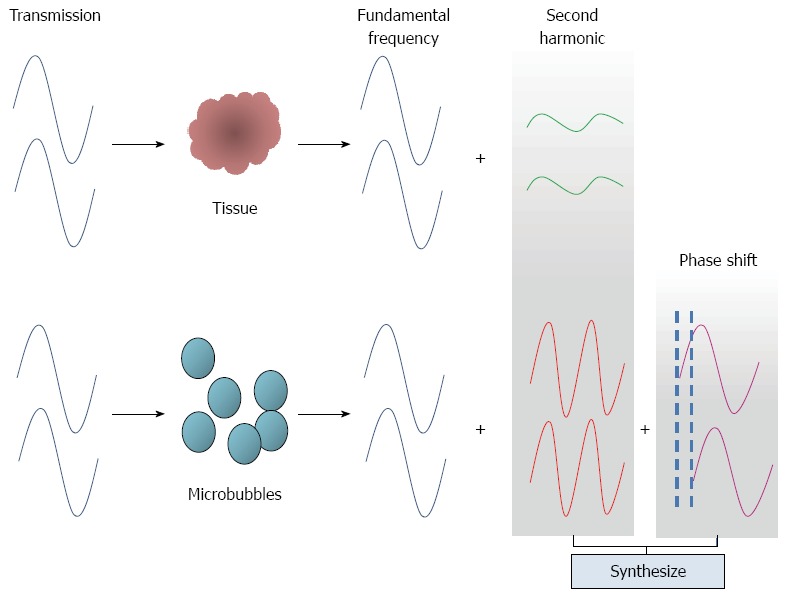
Principle of extended pure harmonic technique.
In addition to these contrast-specific techniques, one of the most prominent components in HI is the transducer technology. The reflection frequency range of the second harmonic components is wide compared with the fundamental frequency (Figure 2). Therefore, a large transducer bandwidth is necessary for these harmonic techniques because they require that the central frequency of the received response be set to twice the central frequency of the transmitted pulse to cover the total range of the fundamental and second harmonic spectra[8,30].
The microcirculation of the abdominal organs was evaluated by contrast harmonic sonography with an experimental EUS probe in the surgically opened abdomens of 12 dogs in 2005[31]. Soon after, phase inversion software was adapted to try CH-EUS and was preliminary used in six patients[32]. In 2008, for the first time, CH-EUS was introduced into clinical practice as a consequence of the manufacturing of a prototype echoendoscope with a dedicated broadband transducer[29].
PERFORMING CH-EUS: CRITICAL POINTS
There are several important aspects that need to be observed when performing CH-EUS. A large-gauge intravenous catheter of 16-18 G must be employed for the injection to avoid breaking the microbubbles. A three-way stop-cock should be connected to the syringe with the contrast in line with the catheter, and an additional syringe should contain a saline solution at an angle of 90º. Because the high pressure during the injection may burst the bubbles, the contrast must be administered slowly and followed by a saline flush to avoid microbubble persistence in the vein[33,34].
After exploring the area of interest in fundamental B-mode, a dual screen is displayed, showing the CH-EUS image and conventional B-mode image at the same time. This is useful to confirm the appropriate scanning area. The setting will be optimised, and a low MI (typically below 0.4) should be selected. However, fine adjustments of the MI and insonation mode (intermittent vs real-time modes) are advisable, mainly depending on the target. A relatively lower MI (0.25-0.30) is suitable for lesions located near the transducer (2 cm or less), but a frequency decrease (5 MHz) and therefore a higher MI (0.35-0.40) may be needed to explore targets farther away from the transducer (1.5-3 cm). Due to its higher sensitivity, a higher MI may also be useful to ascertain the presence of a microvessel in very hypovascular lesions. Intermittent transmissions of the ultrasound beam are used to allow enough bubbles to flow into the small parenchymal vessels before they are imaged and destroyed by the fresh blood inflow during the non-transmission period. Therefore, intermittent contrast harmonic images are obtained infrequently (lower image rate) at selectable time intervals (0.2 s - interval delay) with a higher acoustic power and higher MI (0.35-0.40), able to destroy bubbles in the area of interest. This results in a stronger signal intensity and longer enhancement, thereby optimising the exploration of microvascular perfusion. With a continuous insonation at a lower MI (0.25-0.30), images are obtained at a higher rate, allowing for real-time observations; thus, flow is observed mostly in larger vessels with a higher flow velocity. After setting the parameters in the ultrasound platform and while keeping the interest area within the field of vision, the contrast is injected, and dynamic observation is then performed[33].
It can take 10-20 s to observe the contrast agent’s arrival, after which the arterial phase starts. This arterial phase lasts approximately 30-45 s, during which time the enhancement increases progressively. Most abdominal organs have only an arterial blood supply, the exception being the liver, which also has a portal venous supply that leads to two different inflow phases (arterial and portal) with different arrival times. After the arterial phase, there is a progressive washout of the contrast, and the venous phase persists from 30 to 120 s (Figure 5). Video recording of the most relevant sequences for subsequent review is recommended[21,33].
Figure 5.
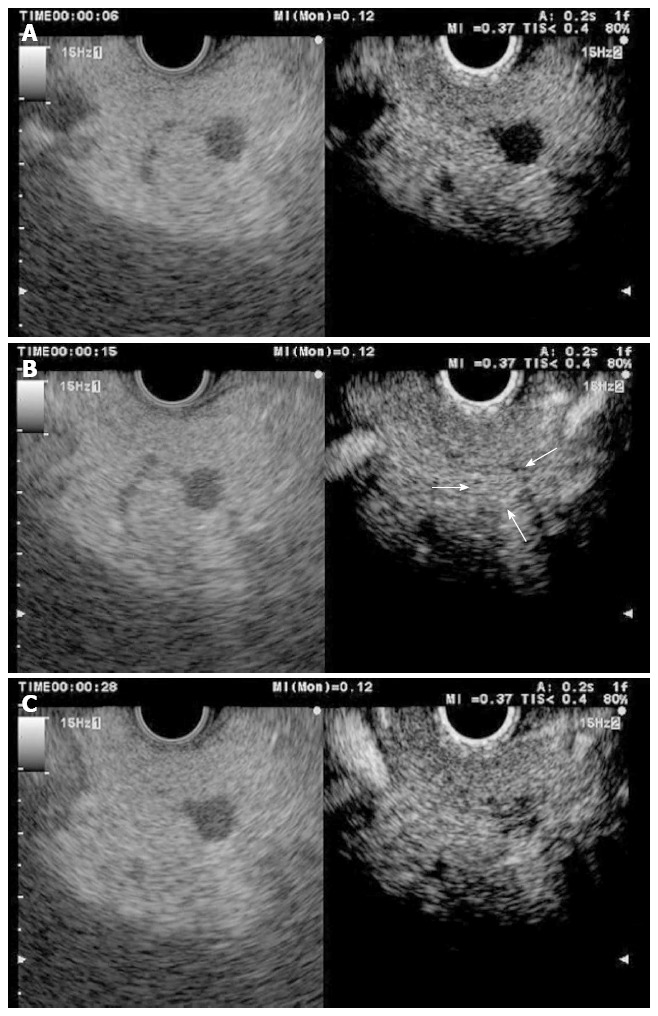
Pancreatic neuroendocrine tumour (left side B mode, right side CH mode). A: No tumour enhancement is observed 6 s after injecting the contrast; B: 15 s after the injection, the contrast uptake is maximal; C: 30 s after the injection, the enhancement begins to decrease.
CLINICAL APPLICATIONS OF CH-EUS
The first experience with CH-EUS using a new linear prototype echoendoscope was reported by Kitano et al[29]. Initially, they carried out experiments in vitro on a phantom model to determine the optimal MI. The maximum changes in echointensity were observed with a MI of 0.35-0.40. Then, they performed in vivo experiments on dogs to examine the microcirculation of the canine digestive organs and, finally, on two patients, one with pancreatic cancer and another with a gastrointestinal stromal tumour. CH-EUS showed no vessels in the pancreatic tumour, although diffuse fine vessels were detected inside the stromal tumour. These results suggest the possible role of CH-EUS in the investigation of certain pathological digestive conditions. In light of these encouraging findings, the same authors conducted a second study to evaluate the potential of CH-EUS in different clinical applications[35]. After performing CH-EUS on 77 patients with a normal pancreas, a MI of 0.4 was established as the most appropriate value to observe the pancreatic perfusion. Then, a heterogeneous group of 27 patients with different pancreatic, gallbladder and gastrointestinal diseases underwent CH-EUS and contrast-enhanced Doppler EUS. Although no firm conclusions could be drawn because of the small number of patients, the capacity of CH-EUS to successfully visualise the microcirculation of several digestive diseases was demonstrated, and its utility in differential diagnoses was also suggested. Following this preliminary experience, several studies have confirmed the potential of this technique, mainly in the field of pancreatic pathology, although recently other indications have also been studied.
Solid pancreatic masses
The characterisation of pancreatic tumours remains a challenge with important diagnostic and therapeutic implications. Despite recent advances introduced in diagnostic imaging, there is no ideal diagnostic procedure for the differentiation of pancreatic tumours. Histology obtained by EUS-FNA is the gold standard. Nevertheless, needle aspiration can produce false results because of sampling errors.
Ductal adenocarcinomas are generally hypovascular because of their markedly desmoplastic changes. In contrast, endocrine tumours have abundant arterial vascularisation, whereas the vascularity of chronic pancreatitis is usually equivalent to that in normal parenchyma[36]. These particularities have been exploited, and characteristic enhancement patterns of pancreatic masses using CH transabdominal ultrasonography (CH-US) have been recently described[36,37]. Adenocarcinomas have been found to be hypoenhanced after contrast injection. Conversely, neuroendocrine tumours (NET) were mostly hyperenhanced. Pancreatitis-associated masses have been reported to have similar enhancements to those of the normal pancreatic parenchyma, depending upon the inflammation and fibrosis. The main limitation of CH-US is the restricted image resolution and poor visualisation of deep areas due to intervening abdominal gas and fat. These drawbacks are overcome by CH-EUS; due to the proximity of the probe to the pancreatic gland, EUS yields the highest spatial resolution images, surpassing those achieved by US, CT and MRI[38].
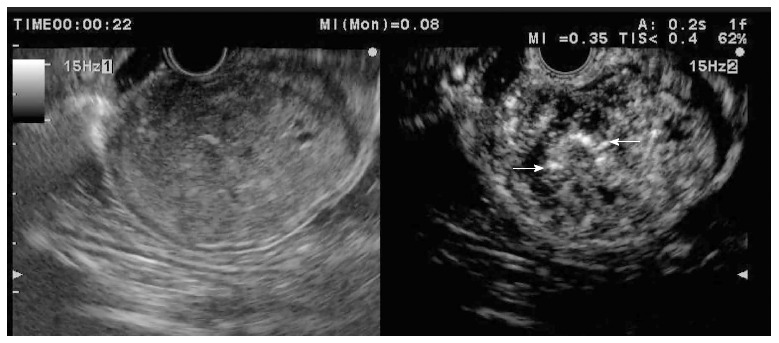
Recently, three groups have prospectively evaluated the performance of CH-EUS in characterising solid pancreatic masses after SonoVue or Sonazoid injection with a new broadband-transducer echoendoscope in 35, 90 and 277 patients, respectively[39-41]. Adenocarcinoma was diagnosed as an inhomogeneous hypoenhanced mass (Figure 6) with high sensitivity (89%-95%) and specificity (64%-89%). A hyperenhanced pattern of the mass on CH-EUS was 88% to 94% predictive of a lesion other than adenocarcinoma in the two first series, whereas a hypervascular enhancement diagnosed NET (Figure 7) with a sensitivity and specificity of 79% and 99%, respectively, in the last series. Although one study using contrast-enhanced Doppler EUS showed the heterogeneous texture as the most significant factor for malignancy in pancreatic NET, at present, no experience has been reported with CH-EUS for this indication[42].
Figure 6.
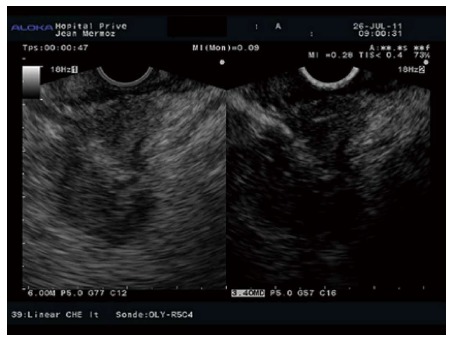
Hypoenhanced pattern in a pancreatic adenocarcinoma.
Figure 7.
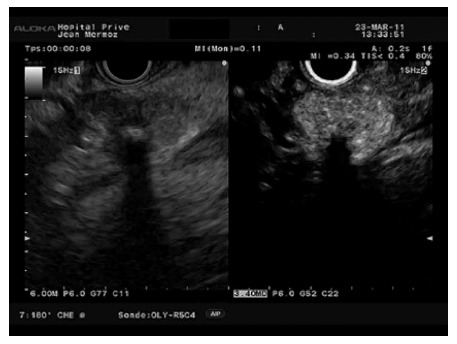
Hyperenhanced pattern in a pancreatic neuroendocrine tumour.
Preliminary experience by several groups has suggested that CH-EUS is superior to CT and that contrast harmonic technology can improve the diagnostic accuracy of conventional EUS. Kitano et al[41] found that CH-EUS was significantly more accurate than multidetector CT in diagnosing small ductal carcinomas (P = 0.034). In this study, EUS detected 12 lesions smaller than 2 cm that were missed by CT (10 adenocarcinomas and 2 NETs). CH-EUS revealed that 7 adenocarcinomas and 2 NETs displayed hypoenhancement and hyperenhancement, respectively. The same author reported that six adenocarcinomas without clear margins when explored by EUS were clearly outlined with CH-EUS. Biliary stents and chronic pancreatitis are well-known limitations of EUS. It has been suggested by Fusaroli et al[40] that CH-EUS may play an important role in overcoming these limitations. The authors were able to diagnose 7 adenocarcinomas in seven patients whose pancreas had been visualised inadequately owing to biliary stents or diffuse chronic pancreatitis.
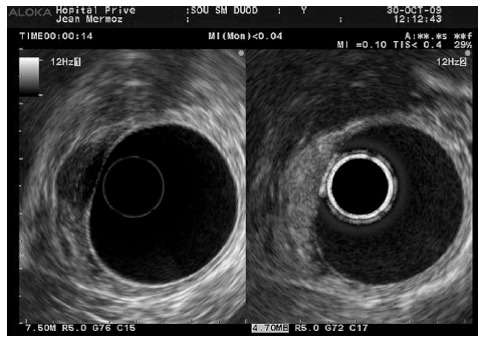
One of the most challenging issues facing current imaging techniques is the differential diagnosis between pancreatic cancer and mass-forming chronic pancreatitis. On CH-US, mass-related chronic pancreatitis [Figure 8, hypoechogenic aspect in B mode (left image) and diffuse enhancement in contrast mode (right image)] have been described to show a similar enhancement during the early phase and a similar wash-out rate to that of the surrounding parenchyma[43]. Nevertheless, there is an inverse relation between the echo intensity and the length of the inflammatory process, most likely related to an increase of the fibrosis. Therefore, the sonographic features of pancreatic cancer and chronic pancreatitis can be very similar and may even coexist within the same patient. Initially, Kitano et al[35] observed a homogeneous isovascular pattern in all three patients with chronic pancreatitis in their first series of CH-EUS. However, 1 out of 7 and 9 out of 13 chronic pancreatitis-related masses in the series by Napoléon and Fusaroli[39,40], respectively, showed hypoenhancement at CH-EUS. Later, in a larger study by Kitano et al[41], 6 out of 46 inflammatory pseudotumours exhibited a non-enhanced pattern, whereas another 4 exhibited a hypoenhanced pattern. According to these findings, a qualitative hypoenhanced pattern after contrast injection could not differentiate between a benign mass of chronic pancreatitis and adenocarcinoma in another study on 30 patients[43]. In this group, Seicean et al[43] used an index of contrast uptake ratio based on greyscale histograms to perform a quantitative analysis. The uptake ratio index was significantly lower for adenocarcinoma than for chronic pancreatitis, with a sensitivity of 80% and a specificity of 91%. More recently, another quantitative approach analysing time intensity curves (TICs) has been evaluated twice[43,44]. Using this new modality, Matsubara et al[44] revealed that the echo-intensity reduction rate from its peak at 1 min was the greatest in pancreatic cancer, followed by mass-forming pancreatitis, autoimmune pancreatitis, and NET (P < 0.05). CH-EUS in combination with TIC increased the sensitivity, specificity and accuracy to 96%, 93%, and 95%, respectively. Gheonea et al[45] confirmed statistically significant differences in TIC values (P < 0.001) when comparing pseudotumoural chronic pancreatitis and pancreatic cancer. In addition, a third study by Imazu et al[46] also suggested TIC analysis as a useful tool in discriminating between adenocarcinoma and autoimmune pancreatitis.
Figure 8.
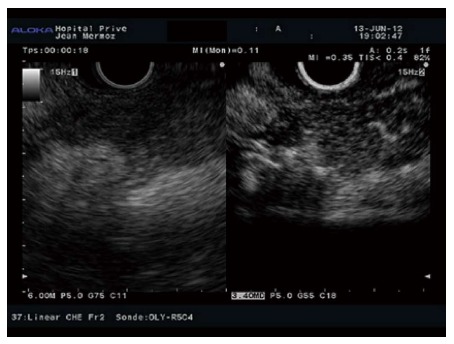
Autoimmune pancreatitis with hypoechogenic aspect in B mode (left image) and diffuse enhancement in contrast mode (right image).
Although EUS-FNA is the gold standard to characterise solid pancreatic masses detected by EUS, due to its low (average rate of 70%) negative predictive value (NPV), a negative result cannot exclude malignancy[39]. Whether CH-EUS is superior to EUS-FNA remains unclear because two studies have reported discordant results[39,41]. Nevertheless, CH-EUS appears to at least act as a helpful complementary tool for EUS-FNA. Napoleon et al[39] suggested that CH-EUS may be helpful in assisting physicians to make a decision between surgery and follow-up in the case of a negative result after EUS-FNA. They observed that 4 out of 5 adenocarcinomas with false-negative EUS-FNA findings were hypoenhanced at CH-EUS. Additionally, CH-EUS revealed that all ductal carcinomas with false-negative EUS-FNA results had hypoenhancement in the study by Kitano et al[41] The combination of CH-EUS and EUS-FNA increased the sensitivity of EUS-FNA for identifying adenocarcinoma in this series from 92% to 100%. In addition, CH-EUS may be useful in increasing the yield of EUS-FNA. Usually, multiple passes of a FNA needle are required to obtain adequate samples from pancreatic tumours because of the frequent presence of fibrosis and necrosis with no viable cells. Simultaneous imaging of microcirculation by CH-EUS could help avoid false negative results due to bloody samples [in hyperintense areas, (Figure 9) or due to necrosis or fibrosis (in areas with no signal)]. However, this possibility has not been extensively evaluated. Kitano et al[47] performed FNA guided by CH-EUS on 39 patients with pancreatic tumours. No viable cells were found in areas with a enhancement, whereas adequate samples were obtained from 80% of sites with a heterogeneous vascular pattern and in all cases from areas with a homogeneous pattern. Malignant cells were found in 73% and 61% of sites with heterogeneous and homogeneous enhancement, respectively.
Figure 9.
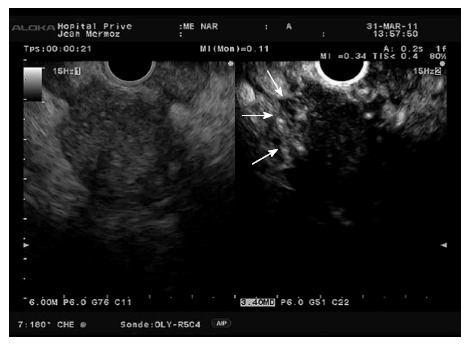
Hypoenhanced pancreatic adenocarcinoma with hyperenhanced peripheral foci (arrows) corresponding to inflammatory changes.
Cystic pancreatic tumours
Cystic pancreatic tumours encompass a wide variety of lesions with similar sonographic features but different biological behaviours, and their accurate preoperative diagnosis remains difficult. During CH-US, the vascularised structures of cystic lesions become progressively echogenic, whereas the intracystic content (blood clots, debris and mucin) remains completely invisible. For this reason, CH-EUS can improve the characterisation of pseudocysts and the differential diagnosis between benign and malignant intraductal papillary mucinous neoplasia (IPMN). The presence of mural nodules is one of the most important criteria for making decisions about surgical intervention for IPMN[48]. The performance of CT and EUS to discriminate between mural nodules and mucous clots is not satisfactory[49]. The use of CH-EUS on IPMN was initially reported twice at the DDW in 2008 and 2009[50,51]. Some reports performed CH-EUS on 75 patients with IPMN. All of the operated cases among 28 patients showing enhancement corresponded to adenoma or more advanced lesions. Six of these patients with invasive carcinoma displayed the coexistence of hypervascular papillary growth areas with hypovascular invasive regions. In another series of 19 IMPN, Kitano et al[51] found enhancements on CH-EUS in 17 patients (89%). This figure was significantly greater than the 37% observed using multidetector CT. These results have been confirmed in a prospective study by Yamashita et al[52] who performed CH-EUS on 17 consecutive patients who had an IPMN with mural lesions. The sensitivity, specificity, positive predictive value (PPV), NPV and accuracy of CH-EUS for mural nodule detections were 100%, 80%, 92%, 100%, and 94%, respectively. In contrast, 5 (3 of them larger than 10 mm) of the 12 mural nodules were not detected by multidetector CT.
Gastrointestinal submucosal tumours
Gastrointestinal submucosal lesions include a wide variety of benign and malignant tumours. The differential diagnosis is performed based on the information gathered after observing the wall layer from which the tumour arises, the echogenicity, the echo pattern and internal features. However, these factors are sometimes insufficient, such as in the differential diagnosis between leiomyomas and gastrointestinal stromal tumours (GIST) and especially in comparison between benign and malignant GIST. Although EUS-FNA is widely accepted for diagnosing submucosal tumours, it has several limitations. EUS-FNA and EUS-Trucut biopsy show only a moderate diagnostic yield[53]. Among several prognostic factors for GIST, when invasion of the surrounding structures or metastases are absent, one of the most important factors is the mitotic count of histological samples. Unfortunately, specimens of EUS-FNA from these tumours are often insufficient for counting mitosis[53].
Only three studies have evaluated the role of CH-EUS in characterising submucosal tumours[54-56]. In the first, Sakamoto et al[54] assessed whether CH-EUS can predict the malignant risk of GIST. All 16 high-risk GIST patients had irregular vessels and heterogeneous enhancement, whereas only five of the 13 low-risk GIST patients exhibited this feature. CH-EUS predicted malignant GIST with a sensitivity, specificity and accuracy of 100%, 63%, and 83%, respectively, in comparison with the 63% sensitivity, 92% specificity, and 83% accuracy of EUS-FNA for diagnosing high-grade malignant GIST. The authors suggested that CH-EUS may play an important role in predicting the malignancy risk of GIST. However, there is no agreement about the ability of CH-EUS to distinguish GIST from other spindle cell tumours, as opposite results have also been reported. When CH-EUS was used to assess the vascularity of 17 esophagogastric submucosal tumours, Kannengiesser et al[55] observed that the eight lesions with hyperenhancement were all histologically identified as GIST, whereas the nine hypoenhanced lesions corresponded to four lipomas and five leiomyomas. They concluded that CH-EUS can discriminate GIST from truly benign submucosal lesions with good accuracy. Accordingly, out of 51 submucosal tumours Fusaroli et al[56] found that GIST and NET were prevalently hyperenhanced (Figures 10 and 11), whereas leiomyomas (Figure 12) and lipomas were hypoenhanced, reflecting a significant difference between the contrast uptake of GIST and leiomyomas (P = 0.0007). In contrast, in the study by Sakamoto et al[54] low-grade malignant GIST and all benign spindle cell neoplasms, including 6 leiomyomas and 1 schwannoma, presented regular vessels and homogeneous enhancement at CH-EUS, indicating that EUS-FNA is necessary for the histological differentiation of spindle cell tumours. Further research is required to confirm the role of CH-EUS in differentiating submucosal tumours and in predicting GIST malignancy.
Figure 10.
Gastric gastrointestinal stromal tumour displayed as hyperenhanced submucosal lesion with some macrovessels (arrows) inside the tumour.
Figure 11.
Duodenal carcinoid tumour: Hyperenhancement after contrast injection.
Figure 12.
Leiomyoma of the gastric cardias showing a hypoenhanced pattern.
Other indications
The use of CH-EUS is still in the beginning stages; as such, its anecdotal applications in several areas of potential interest have not yet been completely explored to determine whether CH-EUS could play an important role.
Biliary tract diseases
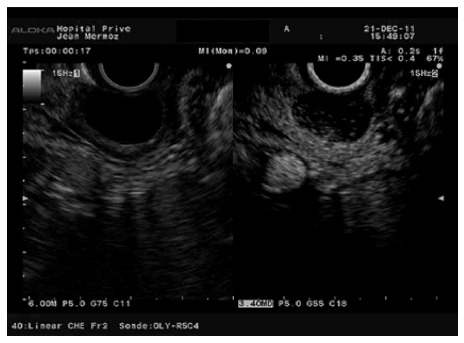
A recent report about CH-EUS in one patient with biliary papillomatosis of the extrahepatic bile duct confirmed that CH-EUS can differentiate sludge from true lesions by demonstrating vascularity[57]. Sludge and non-shadowing stones can be excluded in the presence of arterial enhancement (Figure 13). Nevertheless, the ability of CH-EUS to differentiate between the different polypoid lesions of the gallbladder is less evident and has only been evaluated twice[58,59]. Differentiating gallbladder carcinoma from adenomyomatosis can be easy in fundamental B-mode EUS, but discriminating between gallbladder adenomas and cholesterol polyps based on EUS remains still challenging because they have similar echogenicity and morphology. A differential diagnosis between adenomas and cholesterol polyps is important because adenomas can transform into malignant lesions, whereas cholesterol polyps are benign lesions that do not need to be followed up or resected. Park et al[58] observed that homogeneous and heterogeneous enhancement was shown mostly by adenomas and cholesterol polyps, respectively. The homogeneous pattern corresponds histologically to the microvessels uniformly distributed between glands in the connective tissue, whereas the heterogeneous enhancement may be explained by the loose distribution of microvessels in cholesterol polyps. However, the sensitivity and specificity of CH-EUS were only 75% and 67%, respectively. The authors hypothesised that a quantitative perfusion analysis may be necessary to increase the diagnostic accuracy of CH-EUS in this indication. However, due to their lower risk of malignancy, gallbladder polyps smaller than 10 mm are generally managed by observation, leaving the major pitfall to be dealing with larger gallbladder polyps. Choi et al[59] evaluated the performance of CH-EUS in assessing the malignant potential of gallbladder polyps larger than 10 mm. In this study, the sensitivity and specificity of CH-EUS in diagnosing malignant polyps were 94% and 93%, respectively, when considering the presence of irregular vessels and perfusion defects as predictors of malignancy.
Figure 13.
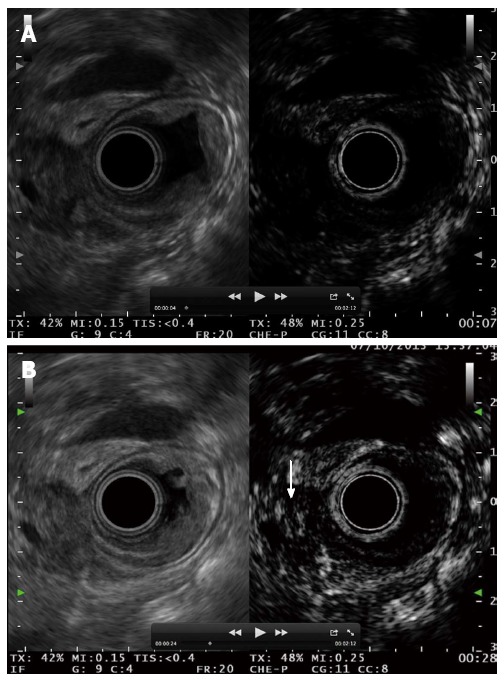
Ampullary tumour with echoic material inside the distal bile duct. A: No enhancement is observed 7 s after contrast injection; B: Bile duct involvement is diagnosed after contrast enhancement 28 s after the injection.
Lymph nodes
The accurate characterisation of lymph nodes is a crucial factor in oncologic staging for the estimation of the prognosis and selection of treatment options. Morphologic echo features are not accurate enough to predict the benign or malignant nature of lymph nodes. Lymph nodes have been explored by this new technique as part of a study investigating intra-abdominal lesions of undetermined origin[60]. A preliminary suspicion of lymphadenopathy can be intuitively determined, but other possibilities must be considered. CH-EUS was used to study 43 undetermined intra-abdominal lesions suspected to be malignant lymph nodes; in fact, 35 lesions were lymph nodes in the final pathological analysis, but the remaining 8 lesions included 3 GIST, 3 abscesses, 1 schwannoma, and 1 paraganglioma. Heterogeneous enhancement was only observed in malignant lesions, and only one malignancy presented a homogeneous pattern. The sensitivity, specificity, PPV, NPV and accuracy of CH-EUS for the differential diagnosis between benign and malignant lesions were 96%, 100%, 100%, 94%, and 98%, respectively. Although cytological diagnosis based on the samples obtained by EUS-FNA is the standard practice, a non-invasive diagnostic tool is also important to guide clinical decision-making, especially when tumours are not accessible for EUS-FNA, when EUS-FNA of the lymph nodes requires traversing the tumour, or when the obtained samples are insufficient.
Tumour staging
In addition to the characterisation of pancreatic tumours, T staging, especially regarding vascular invasion, is crucial to planning the appropriate treatment strategy for pancreaticobiliary neoplasia (Figure 14). EUS errors in the local staging of tumours are often somehow due to peritumoural inflammation, which renders the tumoural margins unclear due to the difficulty of distinguishing between peritumoural inflammatory tissue and hypoechoic tumours. The interface between inflammatory tissue and the tumour was better depicted with CH-EUS by enhancing the peritumoural inflammation in a study by Imazu et al[61]. They compared EUS and CH-EUS in 26 patients before each underwent a surgical resection for pancreaticobiliary tumours. CH-EUS was significantly superior to EUS for T staging, with an overall accuracy of 92% and 69%, respectively (P < 0.05). CH-EUS correctly staged six cases over-staged by EUS. No portal vein involvement was demonstrated in four of the cases by CH-EUS. CH-EUS depicted the portal wall more clearly and therefore was more specific for the diagnosis of portal invasion, compared to EUS (100% vs 83%). Using quantitative CH-EUS, Seicean et al[43] were unable to reproduce these results. The staging of pancreatic adenocarcinoma was not modified based on the application of CH-EUS compared with EUS. Therefore, the role of CH-EUS for tumour staging needs to be clarified in further and larger studies.
Figure 14.
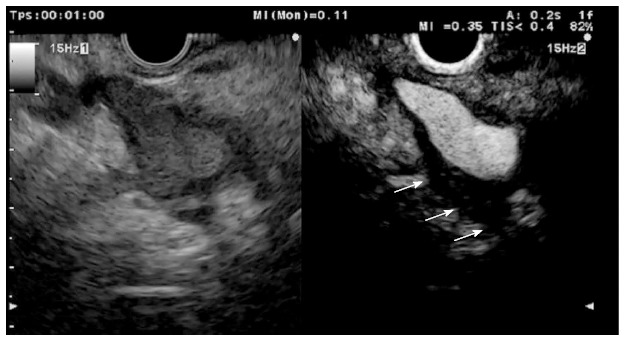
Perivascular infiltration by a pancreatic adenocarcinoma displayed as a hypoechoic wall thickening of the celiac artery (arrows).
Nodules and masses in other organs, such as the suprarenal glands and the spleen, also warrant subsequent assessment. Although histology is the optimal mode of diagnosis for infiltration by lymphoma, it is not ethically feasible to perform systematic splenic biopsies as part of the diagnostic work-up for staging lymphomas due to the higher complication rates of splenic-FNA. Recently, in a study on 100 patients with Hodgkin lymphoma, CH-US correctly identified 30 out 33 patients with nodular infiltration of the spleen, whereas CT and positron emission tomography only detected 13 patients[62]. Nevertheless, splenic sonography is limited by the difficulty of imaging the deep pole and subphrenic regions, as well as the potential for shadows from the left colonic flexure. Therefore, CH-EUS might be a useful mean of avoiding these obstacles. Other potential applications, such as portal hypertension and the assessment of depth invasion in oesophageal and gastric neoplasias, require further investigation.
It is important to know that all of the above indications constitute off-label uses of UCAs. The use of UCAs for abdominal indications is only licensed for liver applications in Europe and the United States. However, the safety and efficacy of UCAs for off-label areas have been demonstrated in several studies by researchers all over the world. Moreover, the publication of the European guidelines on UCAs for non-liver examinations recognises their utility in many other areas, allowing practitioners of ultrasound to move forward with the application of off-label UCAs. The lack of license means patients should be informed and asked to sign an informed consent document. Practitioners must screen all patients for contraindications, and resuscitation equipment should be at hand[21,33].
FUTURE: THERANOSTICS
Significant progress towards the development of microbubbles as theranostics has expanded their applications to a wide variety of biomedical indications, especially in oncology.
Predicting and monitoring therapy response
Understanding prognostic factors before treating patients with anti-tumour agents may be helpful in selecting patients predicted to have an improved survival after chemotherapy. Gemcitabine is currently the chemotherapeutic agent of choice for unresectable pancreatic cancer, although in some patients, the treatment has not been effective. Sofuni et al[63] used CH-US in patients with unresectable advanced pancreatic cancer treated by chemotherapy. They found that patients with abundant intratumour blood flow had a significantly better response, and these changes in intratumour blood flow after treatment were related to prognosis (P = 0.006).
More recently, Yamashita et al[64] performed CH-EUS on 39 consecutively admitted patients with unresectable pancreatic cancer who were scheduled to undergo chemotherapy. They looked for large intratumoural vessels, as opposed to the previous study’s evaluation of enhancement patterns. The hypothesis that tumours with intratumoural vessels are chemosensitive appears to be reasonable because drugs penetrate tumours through vessels. In fact, the authors showed that both progression-free survival and overall survival were significantly longer in patients with intratumoural large vessels (P = 0.037 and P = 0.027, respectively) and that a positive vessel sign was an independent factor associated with longer survival by multivariate analysis (OR = 0.22).
In the past few years, pancreatic cancer treatment by tissue ablation has gradually been accepted. Experiments in pigs have demonstrated that CH-EUS is a successful technique to visualise the altered pancreatic perfusion in the focal areas of pancreatic necrosis after tissue ablation, thus facilitating post-treatment follow-up[65].
Although no study has evaluated the role of CH-EUS in monitoring anti-angiogenic treatments of GIST, an early predicted response with CH-EUS may be possible by analysing the decrease in vascularity before any volume reduction is observed. If confirmed in further studies, CH-EUS may become a safe, highly accurate and cost-effective alternative to CT and PET-CT[66].
Targeted microbubbles
Future challenges include targeted imaging and the use of microbubbles for therapeutic purposes. Only a few studies in these fields have been performed using CH-US, and most of these occurred in an experimental setting.
Targeted ultrasound imaging: Antibodies, peptides and other targeting moieties may be bound to microbubbles to target sites of inflammation, angiogenesis and cancer to obtain more disease-specific information, to better characterise tumours and to more sensitively monitor therapeutic responses[67]. Although targeted imaging is becoming especially popular in monitoring the response to anticancer treatments in the last few years, few preclinical studies have been carried out. In one of these studies, Korpanty and co-workers[68] used molecular ultrasound targeting the VEGF in an orthotopic model of pancreatic cancer during anti-angiogenetic treatment. They found a decreasing marker density after therapy that correlated with the observed treatment effects.
Therapeutic applications of microbubbles: The applications of therapeutic ultrasounds can be classified into those that enhance the delivery of therapeutic agents and those based on the direct effects of ultrasound energy deposition[69].
Ultrasound-triggered drug and gene delivery: Bioactive substances (genes, drugs, proteins, etc.) can be attached to or incorporated into microbubble shells. Microbubbles can be destroyed by applying high ultrasound energies. This leads to increased capillary and cell membrane permeability in the immediate vicinity, thus facilitating tissue and cell penetration by loaded bioactive substances[70].
Usually, the major limitation of systemic chemotherapy is the off-targeted side-effects. Focusing the ultrasound field at the target tissues and organs improves not only the efficacy but also the selectivity of the treatment; thus, the undesirable side effects in healthy tissues may be reduced[71]. Recently, Tinkov et al[72] demonstrated the significantly decreased tumour growth of a pancreas carcinoma model in rats due to ultrasound-targeted microbubble destruction following the administration of a novel doxorubicin-loaded, phospholipid-based microbubble formulation.
Similarly, microbubble UCAs have the potential to dramatically improve gene therapies by enhancing the delivery of plasmid DNA products to malignant tissues[73].
Microbubbles as therapeutic agents: The therapeutic potential of microbubbles is mainly based on damage to the cell wall. Microbubble oscillation in a sonicated medium generates heat as a result of friction with surrounding structures and their decompression. The release of heat to the surrounding tissues causes local damage[74]. Although thermal injury is the best-known application of therapeutic ultrasound, another mechanism of cell damage is acoustic cavitation. Acoustic cavitation consists of fast microbubble growth and expansion, leading to their ultimate collapse under the influence of high powered ultrasound waves in a fluid medium[69]. The collapse of the bubbles near the blood vessel walls results in irreversible damage to intact cells and a non-destructive increase in membrane permeability[74].
In conclusion, CHI has been available for EUS over the last five years, and preliminary experience has shown CH-EUS to be a successful imaging technique for the characterisation of several digestive disorders, especially pancreatic tumours, which have been more extensively studied. More recently, the potential of CH-EUS to improve EUS-FNA accuracy and the local staging of pancreaticobiliary cancers has also been suggested. Other possible applications have been proposed, such as the prediction and monitoring of tumour responses to chemotherapy drugs, follow-up after tumoural tissue ablation, and targeted delivery of gene and drug-based therapies. Prospective and larger studies are needed to confirm these promising results, to standardise the microvascularity patterns and to investigate unexplored potential indications.
Footnotes
P- Reviewer: Conzo G, Tsimogiannis K S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol. 2009;193:55–60. doi: 10.2214/AJR.09.2553. [DOI] [PubMed] [Google Scholar]
- 2.Kang ST, Yeh CK. Ultrasound microbubble contrast agents for diagnostic and therapeutic applications: current status and future design. Chang Gung Med J. 2012;35:125–139. doi: 10.4103/2319-4170.106159. [DOI] [PubMed] [Google Scholar]
- 3.Kitano M, Kudo M, Sakamoto H, Komaki T. Endoscopic ultrasonography and contrast-enhanced endoscopic ultrasonography. Pancreatology. 2011;11 Suppl 2:28–33. doi: 10.1159/000323493. [DOI] [PubMed] [Google Scholar]
- 4.Săftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy. 2012;44:612–617. doi: 10.1055/s-0032-1308909. [DOI] [PubMed] [Google Scholar]
- 5.Unnikrishnan S, Klibanov AL. Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. AJR Am J Roentgenol. 2012;199:292–299. doi: 10.2214/AJR.12.8826. [DOI] [PubMed] [Google Scholar]
- 6.Lencioni R, Cioni D, Bartolozzi C. Tissue harmonic and contrast-specific imaging: back to gray scale in ultrasound. Eur Radiol. 2002;12:151–165. doi: 10.1007/s003300101022. [DOI] [PubMed] [Google Scholar]
- 7.Desser TS, Jeffrey RB. Tissue harmonic imaging techniques: physical principles and clinical applications. Semin Ultrasound CT MR. 2001;22:1–10. doi: 10.1016/s0887-2171(01)90014-9. [DOI] [PubMed] [Google Scholar]
- 8.Kollmann C. New sonographic techniques for harmonic imaging--underlying physical principles. Eur J Radiol. 2007;64:164–172. doi: 10.1016/j.ejrad.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Leen E. Ultrasound contrast harmonic imaging of abdominal organs. Semin Ultrasound CT MR. 2001;22:11–24. doi: 10.1016/s0887-2171(01)90015-0. [DOI] [PubMed] [Google Scholar]
- 10.Averkiou M, Powers J, Skyba D, Bruce M, Jensen S. Ultrasound contrast imaging research. Ultrasound Q. 2003;19:27–37. doi: 10.1097/00013644-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Raisinghani A, DeMaria AN. Physical principles of microbubble ultrasound contrast agents. Am J Cardiol. 2002;90:3J–7J. doi: 10.1016/s0002-9149(02)02858-8. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove D. Ultrasound contrast agent: An overview. Eur J Radiol. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: what is available, how do they work, and are they effective? Gastrointest Endosc. 2009;69:S71–S77. doi: 10.1016/j.gie.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasound: contrast enhancement. Gastrointest Endosc Clin N Am. 2012;22:349–58, xi. doi: 10.1016/j.giec.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Schneider M. SonoVue, a new ultrasound contrast agent. Eur Radiol. 1999;9 Suppl 3:S347–S348. doi: 10.1007/pl00014071. [DOI] [PubMed] [Google Scholar]
- 16.Bokor D. Diagnostic efficacy of SonoVue. Am J Cardiol. 2000;86:19G–24G. doi: 10.1016/s0002-9149(00)00985-1. [DOI] [PubMed] [Google Scholar]
- 17.Sontum PC. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol. 2008;34:824–833. doi: 10.1016/j.ultrasmedbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 19.Baert AL, Sartor K. Contrast media in ultrasonography. In: Quaia E, editor. Basic principles and clinical applications. 1st ed. Berlin: Springer; 2005. pp. 3–14. [Google Scholar]
- 20.ter Haar GR. Ultrasonic contrast agents: safety considerations reviewed. Eur J Radiol. 2002;41:217–221. doi: 10.1016/s0720-048x(01)00456-9. [DOI] [PubMed] [Google Scholar]
- 21.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 22.Bauer A, Hauff P, Lazenby J, von Behren P, Zomack M, Reinhardt M, Schlief R. Wideband harmonic imaging: a novel contrast ultrasound imaging technique. Eur Radiol. 1999;9 Suppl 3:S364–S367. doi: 10.1007/pl00014075. [DOI] [PubMed] [Google Scholar]
- 23.de Jong N, Frinking PJ, Bouakaz A, Ten Cate FJ. Detection procedures of ultrasound contrast agents. Ultrasonics. 2000;38:87–92. doi: 10.1016/s0041-624x(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 24.Frinking PJ, Bouakaz A, Kirkhorn J, Ten Cate FJ, de Jong N. Ultrasound contrast imaging: current and new potential methods. Ultrasound Med Biol. 2000;26:965–975. doi: 10.1016/s0301-5629(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 25.Azmin M, Harfield C, Ahmad Z, Edirisinghe M, Stride E. How do microbubbles and ultrasound interact? Basic physical, dynamic and engineering principles. Curr Pharm Des. 2012;18:2118–2134. doi: 10.2174/138161212800099955. [DOI] [PubMed] [Google Scholar]
- 26.Napoléon B. L’échoendoscopie de contraste. Acta Endosc. 2010;40:31–34. [Google Scholar]
- 27.Dalla Palma L, Bertolotto M. Introduction to ultrasound contrast agents: physics overview. Eur Radiol. 1999;9 Suppl 3:S338–S342. doi: 10.1007/pl00014069. [DOI] [PubMed] [Google Scholar]
- 28.Kudo M. Contrast harmonic imaging in the diagnosis and treatment of hepatic tumors. Tokyo: Springer; 2003. pp. 31–68. [Google Scholar]
- 29.Kitano M, Kudo M, Sakamoto H, Nakatani T, Maekawa K, Mizuguchi N, Ito Y, Miki M, Matsui U, von Schrenk T. Preliminary study of contrast-enhanced harmonic endosonography with second generation contrast agents. J Med Ultrasonics. 2008;35:11–18. doi: 10.1007/s10396-007-0167-6. [DOI] [PubMed] [Google Scholar]
- 30.Whittingham TA. Broadband transducers. Eur Radiol. 1999;9 Suppl 3:S298–S303. doi: 10.1007/pl00014060. [DOI] [PubMed] [Google Scholar]
- 31.Digestive Disease Week, American Society for Gastrointestinal Endoscopy meeting abstracts. May 2005, Chicago, Illinois, USA. Gastrointest Endosc. 2005;61:AB77–A309. [PubMed] [Google Scholar]
- 32.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol. 2005;43:1219–1223. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 33.Barr RG. Off-label use of ultrasound contrast agents for abdominal imaging in the United States. J Ultrasound Med. 2013;32:7–12. doi: 10.7863/jum.2013.32.1.7. [DOI] [PubMed] [Google Scholar]
- 34.Kudo M. Contrast harmonic imaging in the diagnosis and treatment of hepatic tumors. Tokyo: Springer; 2003. pp. 31–88. [Google Scholar]
- 35.Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 36.D’Onofrio M, Zamboni G, Faccioli N, Capelli P, Pozzi Mucelli R. Ultrasonography of the pancreas. 4. Contrast-enhanced imaging. Abdom Imaging. 2007;32:171–181. doi: 10.1007/s00261-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 37.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Nakaoka R, Kawasaki T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varadarajulu S, Eloubeidi MA. The role of endoscopic ultrasonography in the evaluation of pancreatico-biliary cancer. Surg Clin North Am. 2010;90:251–263. doi: 10.1016/j.suc.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564–570. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 40.Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–34.e1-2. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, et al. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–310. doi: 10.1038/ajg.2011.354. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Nakamura M, Miyahara R, Hayashi K, et al. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951–959. doi: 10.1016/j.gie.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Seicean A, Badea R, Stan-Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571–576. doi: 10.1055/s-0029-1245833. [DOI] [PubMed] [Google Scholar]
- 44.Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073–1079. doi: 10.1097/MPA.0b013e31821f57b7. [DOI] [PubMed] [Google Scholar]
- 45.Gheonea DI, Streba CT, Ciurea T, Săftoiu A. Quantitative low mechanical index contrast-enhanced endoscopic ultrasound for the differential diagnosis of chronic pseudotumoral pancreatitis and pancreatic cancer. BMC Gastroenterol. 2013;13:2. doi: 10.1186/1471-230X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imazu H, Kanazawa K, Mori N, Ikeda K, Kakutani H, Sumiyama K, Hino S, Ang TL, Omar S, Tajiri H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand J Gastroenterol. 2012;47:853–860. doi: 10.3109/00365521.2012.679686. [DOI] [PubMed] [Google Scholar]
- 47.Kitano M, Sakamoto H, Komaki T, Kudo M. FNA guided by contrast enhanced harmonic EUS in pancreatic tumors. Gastrointest Endosc. 2009;69:AB328. [Google Scholar]
- 48.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Zhong N, Zhang L, Takahashi N, Shalmiyev V, Canto MI, Clain JE, Deutsch JC, DeWitt J, Eloubeidi MA, Gleeson FC, et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol. 2012;10:192–198, 198.e1-2. doi: 10.1016/j.cgh.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 50.Abstracts of Digestive Disease Week, May 17-22, 2008 and the ASGE (American Society for Gastrointestinal Endoscopy) Postgraduate Course, May 21-22, 2008. San Diego, California, USA. Gastrointest Endosc. 2008;67:AB57–A349. [PubMed] [Google Scholar]
- 51.Kitano M, Takagi T, Sakamoto H, Das K, Komaki T, Noda K, Yamao K, Kudo M. Dynamic imaging by contrast enhanced harmonic EUS with long-lasting contrast: Role in diagnosis of pancreatic tumors. Gastrointest Endosc. 2009;69:AB235. [Google Scholar]
- 52.Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61–68. doi: 10.7863/jum.2013.32.1.61. [DOI] [PubMed] [Google Scholar]
- 53.Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, Heresbach D, Pujol B, Fernández-Esparrach G, Vazquez-Sequeiros E, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 54.Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, Dote K, Kudo M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–237. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Kannengiesser K, Mahlke R, Petersen F, Peters A, Ross M, Kucharzik T, Maaser C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515–1520. doi: 10.3109/00365521.2012.729082. [DOI] [PubMed] [Google Scholar]
- 56.Fusaroli P, D’Ercole MC, Geroni L, Caletti G. Contrast enhanced-endoscopic ultrasonography (Ch-EUS) in the differential diagnosis of gastrointestinal submucosal tumors (SMTs) Gastrointest Endosc. 2013;77 Suppl:Mo1481. [Google Scholar]
- 57.Cui XW, Ignee A, Braden B, Woenckhaus M, Dietrich CF. Biliary papillomatosis and new ultrasound imaging modalities. Z Gastroenterol. 2012;50:226–231. doi: 10.1055/s-0031-1281967. [DOI] [PubMed] [Google Scholar]
- 58.Park CH, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Kim H, Hwang HK, Lee WJ, Song SY. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414–1421. doi: 10.1007/s00464-012-2620-x. [DOI] [PubMed] [Google Scholar]
- 59.Choi JH, Seo DW, Choi JH, Park do H, Lee SS, Lee SK, Kim MH. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos) Gastrointest Endosc. 2013;78:484–493. doi: 10.1016/j.gie.2013.03.1328. [DOI] [PubMed] [Google Scholar]
- 60.Xia Y, Kitano M, Kudo M, Imai H, Kamata K, Sakamoto H, Komaki T. Characterization of intra-abdominal lesions of undetermined origin by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2010;72:637–642. doi: 10.1016/j.gie.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 61.Imazu H, Uchiyama Y, Matsunaga K, Ikeda K, Kakutani H, Sasaki Y, Sumiyama K, Ang TL, Omar S, Tajiri H. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010;45:732–738. doi: 10.3109/00365521003690269. [DOI] [PubMed] [Google Scholar]
- 62.Picardi M, Soricelli A, Pane F, Zeppa P, Nicolai E, De Laurentiis M, Grimaldi F, Rotoli B. Contrast-enhanced harmonic compound US of the spleen to increase staging accuracy in patients with Hodgkin lymphoma: a prospective study. Radiology. 2009;251:574–582. doi: 10.1148/radiol.2512081293. [DOI] [PubMed] [Google Scholar]
- 63.Sofuni A, Itoi T, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Usefulness of contrast-enhanced ultrasonography in determining treatment efficacy and outcome after pancreatic cancer chemotherapy. World J Gastroenterol. 2008;14:7183–7191. doi: 10.3748/wjg.14.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Tumor vessel depiction with contrast-enhanced endoscopic ultrasonography predicts efficacy of chemotherapy in pancreatic cancer. Pancreas. 2013;42:990–995. doi: 10.1097/MPA.0b013e31827fe94c. [DOI] [PubMed] [Google Scholar]
- 65.Giday SA, Magno P, Gabrielson KL, Buscaglia JM, Canto MI, Ko CW, Clarke JO, Kalloo AN, Jagannath SB, Shin EJ, et al. The utility of contrast-enhanced endoscopic ultrasound in monitoring ethanol-induced pancreatic tissue ablation: a pilot study in a porcine model. Endoscopy. 2007;39:525–529. doi: 10.1055/s-2007-966391. [DOI] [PubMed] [Google Scholar]
- 66.Dietrich CF, Jenssen C, Hocke M, Cui XW, Woenckhaus M, Ignee A. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques - a pictorial essay. Z Gastroenterol. 2012;50:457–467. doi: 10.1055/s-0031-1282076. [DOI] [PubMed] [Google Scholar]
- 67.Kiessling F, Bzyl J, Fokong S, Siepmann M, Schmitz G, Palmowski M. Targeted ultrasound imaging of cancer: an emerging technology on its way to clinics. Curr Pharm Des. 2012;18:2184–2199. doi: 10.2174/138161212800099900. [DOI] [PubMed] [Google Scholar]
- 68.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 69.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther. 2013;7:375–388. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G, Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release. 2010;148:368–372. doi: 10.1016/j.jconrel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics. 2012;2:1208–1222. doi: 10.7150/thno.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alzaraa A, Gravante G, Chung WY, Al-Leswas D, Bruno M, Dennison AR, Lloyd DM. Targeted microbubbles in the experimental and clinical setting. Am J Surg. 2012;204:355–366. doi: 10.1016/j.amjsurg.2011.10.024. [DOI] [PubMed] [Google Scholar]


