Abstract
Pancreatic adenocarcinoma remains a most deadly malignancy, with an overall 5-year survival of 5%. A subset of patients will be diagnosed with potentially resectable disease, and while complete surgical resection provides the only chance at cure, data from trials of postoperative chemoradiation and/or chemotherapy demonstrate a modest survival advantage over those patients who undergo resection alone. As such, most practitioners believe that completion of multimodality therapy is the optimal treatment. However, the sequence of surgery, chemotherapy and radiation therapy is frequently debated, as patients may benefit from a neoadjuvant approach by initiating chemotherapy and/or chemoradiation prior to resection. Here we review the rationale for neoadjuvant therapy, which includes a higher rate of completion of multimodality therapy, minimizing the risk of unnecessary surgical resection for patients who develop early metastatic disease, improved surgical outcomes and the potential for longer overall survival. However, there are no prospective, randomized studies of the neoadjuvant approach compared to a surgery-first strategy; the established and ongoing investigations of neoadjuvant therapy for pancreatic cancer are discussed in detail. Lastly, as the future of therapeutic regimens is likely to entail patient-specific genetic and molecular analyses, and the treatment that is best applied based on those data, a review of clinically relevant biomarkers in pancreatic cancer is also presented.
Keywords: Pancreatic cancer, Neoadjuvant therapy, Chemotherapy, Chemoradiation, Biomarkers
Core tip: The sequence of multi-modality therapy for pancreatic cancer continues to be debated, though many pancreatic cancer specialists are increasingly utilizing neoadjuvant chemoradiation prior to surgical resection. This manuscript details the rationale for neoadjuvant therapy, the data that supports its use, and the potential of biomarker use for personalizing care in pancreatic cancer.
INTRODUCTION
The diagnosis of pancreatic cancer portends a very poor prognosis. Worldwide, approximately 300000 new cases of pancreatic cancer are diagnosed annually, while in the United States, pancreatic adenocarcinoma remains the fourth leading cancer-related cause of death in both men and women[1]. The American Cancer Society estimates that approximately 45000 patients will be diagnosed with pancreatic cancer in 2013 while over 37000 patients will succumb to this disease by year’s end[2]. The average American’s lifetime risk for developing pancreatic cancer is 1%-2% and, unlike most other malignancies, the incidence of pancreatic cancer has been slowly increasing over the last decade. Pertinent risk factors for developing pancreatic cancer include chronic pancreatitis, smoking, diabetes mellitus, significant family history, and certain genetic disorders such as cystic fibrosis, hereditary pancreatitis, Peutz-Jeghers syndrome, and Lynch syndrome[3]. Despite more sophisticated imaging modalities including high-resolution computed tomography scanning and endoscopic ultrasound, the overall 5-year survival for all patients with pancreatic cancer approaches 5%. This abysmal statistic is underscored by the fact that many patients present late in their disease process, with four out of five patients initially presenting with unresectable tumor burden; one-third of these patients will have locally-advanced disease deemed unresectable while the remaining two-thirds will have evidence of stage IV distant metastases found on staging work-up[4].
Complete resection of pancreatic cancer with negative surgical margins is obligatory for long-term survival, and, while surgery remains the only curative therapy for pancreatic cancer, only 15% of patients undergoing resection are actually cured of their disease long-term[5]. The remaining 85% will eventually develop locoregional recurrence and/or distant metastases. Frequently, pancreatic cancer recurs systemically while sparing the surgical site, suggesting systemic disease was present at the time of diagnosis and initial resection. As such, both chemoradiation and chemotherapy have been utilized as adjuncts to surgical resection in an attempt to minimize locoregional and distant recurrence, respectively.
Two major drivers of poor survival in patients with pancreatic cancer are delayed detection and lack of effective chemotherapy. Unlike many other gastrointestinal malignancies, pancreatic cancer tends to remain asymptomatic and undiagnosed until significant locoregional or distant disease is present. While early detection is rare, patients diagnosed with tumors limited to the pancreas without nodal involvement who undergo resection can experience a median survival of 33 mo with 1-, 3-, and 5-year survivals of 80%, 49%, and 41%, respectively[6]. Unfortunately, current screening modalities are neither sensitive nor specific enough to identify and diagnose these tumors at such an early stage, limiting the number of patients who undergo meaningful and curative surgical resection. Furthermore, current chemotherapy regimens are only marginally effective in extending survival (a fact that has not changed in over 30 years)[7-12]. Based on these challenges, recent attention has turned to novel therapeutic agents and delivery sequences in an attempt to improve survival within this vulnerable patient population.
LESSONS FROM THE PAST - ADJUVANT THERAPY TRIALS
Several prospective randomized trials investigating the benefit of adjuvant therapy following surgical resection paved the way for current practice patterns. Early studies utilized fluorouracil (5-FU) as the backbone of chemoradiation and/or chemotherapy regimens. In 1985, the Gastrointestinal Tumor Study Group (GITSG) demonstrated a nearly 2-fold improvement in median survival and a 3-fold improvement in 2-year survival rates of patients who received systemic 5-FU and 5-FU based chemoradiation (40 Gy) following resection[7]. However, due to the small sample sizes (n = 22 for observation, n = 21 for treatment), as well as the historically low survival within the control group, many have remained critical of these early findings. Fourteen years later, the European Organisation of Research and Treatment of Cancer (EORTC) published a study comparing split course 5-FU-based chemoradiation vs observation following resection with curative intent of combined pancreatic and periampullary tumors[13]. Despite the modest improvement in survival (17.1 mo vs 12.6 mo) in patients who received adjuvant therapy, the study was underpowered to achieve statistical significance in the subset of patients with pancreatic tumors, and these authors concluded that chemoradiation was not beneficial in this setting.
In an effort to further clarify the role of adjuvant therapy, the European Study Group for Pancreatic Cancer (ESPAC) investigated the efficacy of both chemotherapy and chemoradiation in patients undergoing surgical resection[9]. Utilizing a 2 × 2 factorial design, patients were randomized to either observation, 5-FU based chemotherapy, 5-FU based chemoradiation, or both after undergoing curative resection. This study demonstrated a significant survival benefit for patients who received systemic 5-FU based chemotherapy, while chemoradiation conferred a negative prognosis, leading to a European standard of care that does not include radiation therapy in the multimodal treatment of pancreatic cancer. Due to criticisms regarding the randomization scheme, the relatively low-dose of radiation administered (a hypofractionated dose of 20 Gy vs the traditional 50.4 Gy), as well as the lack of quality control of the radiotherapy administered, the negative prognostic effect of chemoradiotherapy was generally not accepted within the United States.
For many years, 5-FU was the only efficacious chemotherapeutic agent available in the treatment of pancreatic cancer. One promising alternative, gemcitabine, was introduced in the 1990s, and initial phase 1 studies demonstrated a reasonable safety profile with low rates of significant toxicity[14]. Burris et al[15] noted that patients with locally advanced pancreatic cancer treated with gemcitabine demonstrated a significant clinical benefit and modest improvement in survival compared to treatment with the traditional 5-FU regimen. That same year, the phase 3 study CONKO-001 began accruing. This trial sought to compare adjuvant single-agent gemcitabine vs observation in patients following curative-intent resection of pancreatic cancer. Almost a decade later, the initial results of the CONKO-001 trial demonstrated a significantly prolonged disease-free survival in the gemcitabine arm[8]. An updated analysis published this year with a median follow-up of 136 mo confirmed the disease-free survival advantage of gemcitabine compared to observation (13.4 mo vs 6.7 mo, P < 0.001). Beyond that, patients treated with adjuvant gemcitabine also had a significantly improved overall survival (OS). Five- and ten-year OS rates between the gemcitabine and observation groups were 20.7% vs 10.4% and 12.2% vs 7.7%, respectively[16]. In a more recent phase III trial, the US Intergroup/RTOG9704 study investigated the impact of adding gemcitabine to a 5-FU based chemoradiation and chemotherapy regimen following resection[17]. This study demonstrated a modest clinical (but not statistically significant) improvement in median and 5 year overall survivals. These adjuvant trials, and other studies substantiating the activity of gemcitabine in the setting of advanced and metastatic disease, have established this drug as a standard first-line therapy postoperatively[18].
RATIONALE FOR NEOADJUVANT THERAPY
While chemoradiation and systemic chemotherapy have been shown to improve survival in patients undergoing surgical resection of pancreatic cancer, the sequencing of these treatment modalities remains a topic of continued research and debate. Specifically, with emerging data that chemotherapy and/or chemoradiation prior to surgical extirpation can be associated with superior patient outcomes, the notion of neoadjuvant therapy followed by resection has gained traction among many pancreatic cancer specialists. As the neoadjuvant strategy evolves, the question of which patients should undergo neoadjuvant therapy persists. Should all patients with potentially resectable tumors receive chemoradiation prior to surgery, or only those with borderline resectable or locally advanced tumors? And which patients are likely to benefit most from a neoadjuvant regimen? Currently, we do not have strong evidence-based answers to these questions. However, supporters of a neoadjuvant approach point to its ability to select a patient population that will ultimately and maximally benefit from completion of multimodality therapy. In general, initially treating patients with a neoadjuvant approach will inherently “select-in” ideal candidates for surgery while “selecting-out” poor operative candidates or those with distant disease[19]. A more detailed rationale for the neoadjuvant approach is outlined here (Table 1).
Table 1.
Rationale for patient selection for neoadjuvant therapy in pancreatic cancer
| Rationale in support of neoadjuvant therapy |
| Increasing the likelihood of margin-negative resection[4,20-25] |
| Increasing the likelihood of completion of multimodality therapy[5,26-29] |
| Increasing efficacy of radiotherapy[4,24] |
| Minimizing pancreatic leak (without increasing complications)[19,30-34] |
| Determination of indeterminant lesions[29,35] |
| Declaration of distant metastases[4,29] |
| Decreasing “open-and-close” rates[19] |
| Allowing a patient’s functional status to declare itself[36] |
| Improved cost-effectiveness[37] |
Improving margin negative resection rates
Neoadjuvant radiotherapy results in the killing of cancer cells at the periphery of the tumor. By sterilizing the microscopic edge of the tumor, patients may experience improved negative margin resection rates and therefore a reduction in local recurrence[20,21]. Because surgical resection leads to a significant disruption of the native blood supply within the pancreatic tissue and neighboring retroperitoneal nodal basin, chemotherapeutic agents delivered as pro-drugs which rely on the production of active metabolites for cytotoxicity and/or sensitization of the tissue prior to radiation may not be delivered as effectively to the site of cancer in post-operative tissue without an intact blood supply[22]. A study published in Annals of Surgery in 2008 investigated the tissue-level response in patients with resectable pancreatic tumors undergoing neoadjuvant chemotherapy[23]. In this prospective phase II trial, patients received 4 bi-weekly cycles of gemcitabine and cisplatin prior to restaging and surgical resection. Following therapy, cytopathologic and histological responses to the chemotherapy regimen were noted in 83% and 54% of patients, respectively. A significant reduction in tumor metabolism as determined by fluorodeoxyglucose uptake was also identified as compared to baseline, and this finding correlated with histological Ki-67 expression. Similarly, external beam radiotherapy requires well-perfused and well-oxygenated tissue to exert its maximal ionizing damage. Relative tissue hypoxia has been shown to confer radiation resistance, especially in an adjuvant setting[4,24]. By providing radiotherapy to unadulterated and well-oxygenated tissue, radiation therapy proves more potent and efficacious. Furthermore, receipt of radiotherapy preoperatively allows for delivery of a smaller dose in a more directed radiation field and avoids administering radiation to a freshly reconstructed bowel anastomosis[4].
Increasing resectability
While pre-operative locoregional and systemic therapy has the potential to improve rates of margin-negative resection, neoadjuvant therapy can also increase the number of operable candidates by converting an initially unresectable tumor to a resectable one. A meta-analysis by Gillen et al[25] demonstrated that approximately one-third of patients who were deemed unresectable at initial staging may undergo neoadjuvant therapy and convert to operable candidates while maintaining similar survival estimates as those initially deemed resectable. With the estimated 45000 new cases diagnosed annually it the United States, this translates to a chance at a curative resection for 15000 patients who would have otherwise died of their disease burden.
Completion of multimodality therapy
The use of multimodality therapy (surgical resection, chemotherapy and radiation therapy) is being increasingly recognized as the optimal approach to treating patients with pancreatic cancer[26]. However, the efficacy of multimodality therapy, whether given pre- or postoperatively, is contingent upon completion of the ascribed regimen. Given the inherent morbidity and associated post-operative complications of pancreatic surgery, an estimated 25%-50% of patients will experience a delay or never initiate adjuvant therapy[5,27,28]. A recent study from The University of Texas MD Anderson Cancer Center evaluated the rates of completion of multimodality therapy in those who underwent neoadjuvant therapy vs those who initially underwent surgical resection[29]. Eighty-three percent of patients who underwent neoadjuvant therapy received their entire multimodality regimen vs just 58% of patients who underwent surgical resection first. Consider also that 100% of patients who underwent resection - the modality of pancreatic cancer treatment proven to matter the most - received the maximal benefit of their multimodality therapy. While there was no significant difference in major postoperative complications between the two treatment strategies, those patients who experienced major postoperative complications were less likely to complete their adjuvant multimodality therapy. Not surprisingly, those patients who completed multimodality therapy demonstrated a significant survival advantage compared to those who did not (36 mo vs 11 mo, P < 0.001) regardless of timing (Figure 1). This study demonstrates that patients who undergo neoadjuvant therapy are more likely to not only initiate but also complete their course of non-surgical treatment, and therefore experience its full therapeutic potential.
Figure 1.
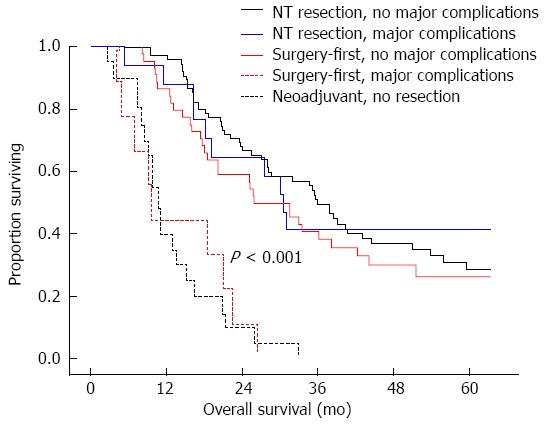
Survival in patients with pancreatic cancer who undergo neoadjuvant therapy vs a surgery-first approach. Survival curves for patients who complete multimodality therapy, inclusive of chemoradiation and curative-intent resection, incorporating major postoperative complications or those who never undergo resection.
Minimizing pancreatic leak (without increasing complications)
As directed radiation can result in significant glandular fibrosis, the radiation therapy itself may allow for a pancreatic anastomosis with a lower leak rate as a result of a firmer, fibrotic pancreas[30-32]. Numerous studies evaluating intra- and post-operative complications following neoadjuvant chemoradiation have noted relative reductions in pancreatic anastomotic leak rates in patients treated preoperatively, with pancreatic fistula rates in the single digits[19,33]. Though some studies have demonstrated that neoadjuvant therapy is associated with higher intraoperative blood loss, vascular reconstruction rates, and longer operative time, no documented studies have demonstrated increased rates of postoperative complications in patients following neoadjuvant therapy[31,34].
Indeterminant lesions, declaration of distant metastases, and decreasing "open-and-close" rates
Roughly half of the patients who are newly diagnosed with pancreatic adenocarcinoma present with distant metastases at the time of initial staging[4]. Additionally, a significant number of patients without evidence of distant metastases on initial staging are found to have unanticipated metastases at the time of surgery (though this is partially dependent upon the interval duration between imaging and operation); these patients likely harbored micrometastatic tumor cells at the time of initial staging[35]. As such, neoadjuvant therapy may help to identify this subset of patients and prevent “open-and-close” operations. Still yet, some patients may demonstrate no evidence of distant disease on initial staging or at the time of surgery, yet develop radiographic evidence of distance metastases within months of resection. Undergoing neoadjuvant treatment in this scenario provides time for micrometastases to declare themselves radiographically, prior to operation. A recent study by Tzeng et al[29] outlined these possibilities (Figure 2). In those patients who demonstrate distant disease at the completion of neoadjuvant therapy, the identification of aggressive tumor biology spares them the morbidity of an otherwise futile surgery. An additional cohort of patients may have indeterminate lesions on initial staging. Neoadjuvant treatment allows for these potentially metastatic lesions to enlarge, shrink, or remain unchanged-thus providing additional diagnostic information for otherwise potentially resectable disease prior to undergoing surgery.
Figure 2.
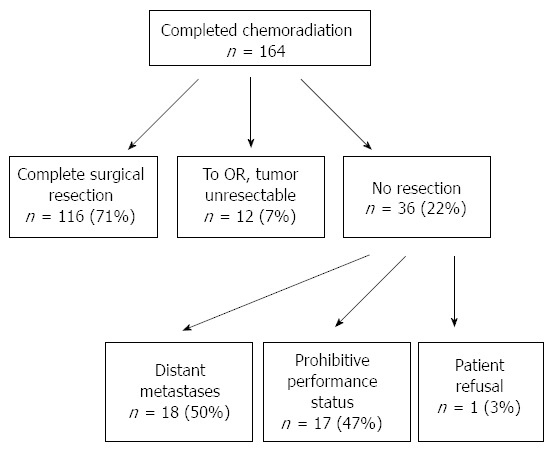
Outcomes in patients who undergo neoadjuvant therapy. Of those patients with potentially resectable pancreatic tumors who undergo chemoradiation, 71% went on to undergo complete surgical resection. Reasons for not undergoing complete resection included declaration of distant metastases, prohibitive performance status, anatomically unresectable locally-advanced tumor, and patient refusal.
Temporal assessment of functional status
In addition to those patients with anatomically borderline pancreatic tumors, the group from MD Anderson describes another cohort of patients who may have a borderline functional status. This group, termed Borderline Resectable Type C, are those patients with resectable tumors who present as sub-optimal surgical candidates given their extreme age, poor functional status, significant weight loss and/or malnutrition, or debilitating medical comorbidities[36]. Utilizing the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Database, they noted that approximately one-third of patients undergoing elective pancreaticoduodenectomies met their criteria for Borderline Type C[36]. Moreover, this cohort was more likely to experience major postoperative complications and death compared to medically-optimized patients[28]. One may suggest that neoadjuvant therapy in these frail patients could provide a window for medical optimization prior to surgery, or at the very least a chance for a debilitated functional status to declare itself prior to undergoing the morbidity of a pancreatic resection.
Cost-effectiveness
In addition to data supporting neoadjuvant therapy from a patient outcomes perspective, there are data to suggest that neoadjuvant therapy is more cost-effective -from a societal perspective - than a surgery first approach. A recent study by Abbott et al[37] utilized data from the National Surgical Quality Improvement program, the American College of Surgeons National Cancer Data Base, and a prospectively-maintained database of patients undergoing neoadjuvant therapy at MD Anderson to construct an analytic model investigating the costs and survival for patients undergoing various treatment strategies. The authors concluded that receipt of neoadjuvant chemoradiation for pancreatic cancer yielded an improved survival (reported in quality-adjusted life months) as well as a significant cost savings of approximately $10000 per patient-case compared to those undergoing a surgery-first approach. In our current healthcare climate, which stresses improved quality under continued fiscal constraints, treatment strategies that achieve optimal outcomes at reduced costs will be increasingly expected.
Limitations of neoadjuvant therapy
There are, of course, limitations to pursuing neoadjuvant therapy in all patients. Firstly, patients with initially resectable tumor burdens may experience local progression of their disease while receiving neoadjuvant therapy. Though very rare (2%-3%) in these patients, the absence of distant metastases with concomitant local advancement may result in an unresectable tumor. In these patients, a surgery-first approach may have benefitted them, though with an aggressive tumor biology - marked by progression on chemotherapy - it is difficult to make any strong conclusions about outcomes in this small cohort of patients. Secondly, unlike data on adjuvant therapy regimens, large randomized prospective phase III trials investigating the efficacy of neoadjuvant regimens are lacking. To date, no phase III trials directly comparing neoadjuvant therapies to adjuvant therapies have been published; as such, we are currently forced to extrapolate our knowledge from smaller phase I/II investigations.
NEOADJUVANT THERAPY TRIALS
Two recently published reviews by Lowy[5] and Abbott et al[38] extensively discuss the landmark neoadjuvant trials published through 2008. Here, we will review a select few of these studies and proceed to focus on recent investigations published within the last 5 years (Table 2).
Table 2.
Recently published prospective neoadjuvant trials of multimodal therapy for pancreatic cancer n (%)
| Ref. | Initial staging |
Regimen |
Resection | Survival | Notes | |
| Chemoradiation | Chemotherapy | |||||
| Palmer et al[106], 2007 | Resectable (n = 50) | N/A | Gem vs Gem + Cis | Overall: 27 (54); Gem: 9 (38), Gem + Cis: and 18 (70) | Gem: 42%, Gem + Cis: 62% (1 yr survival) | Randomized Phase II; No difference in surgical complcations |
| Le Scodan et al[41], 2008 | Resectable (n = 41) | 50 Gy + 5-FU + Cis | N/A | 26 (63) | Overall: 9.4 mo, R: 11.7 mo (2 yr survival 32%) UR: 5.7 mo | Phase II; 67.5% were successfully treated with entire radiation dose and ≥ 75% chemotherapy dose; 81% achieved an R0 resection |
| Heinrich et al[107], 2008 | Resectable (n = 28) | N/A | Gem + Cis | 26 (93) | Overall: 26.5 mo; R: 19.1 mo | Phase II; 80% achieved an R0 resection |
| Evans et al[19], 2008 | Resectable (n = 86) | 30 Gy + Gem | N/A | 64 (74) | Overall: 22.7 mo; R: 34 mo, UR: 7 mo (P < 0.001) | Phase II; 27% 5 yr OS, 36% vs 0% for resected vs unresected |
| Varadhachary et al[33], 2008 | Resectable (n = 90) | 30 Gy + Gem + Cis | Gem + Cis | 52 (66) | Overall: 17.4 mo; R: 31 mo, UR: 10.5 mo | Phase II |
| Turrini et al[108], 2010 | Resectable (n = 34) | 45 Gy + Docetaxel | N/A | 17 (50) | R: 32 mo | Phase II; 10% R0 resection; 5 yr survival resected 41% |
| Landry et al[109], 2010 | Borderline | Arm A: 50.4 Gy + Gem (n = 10) | Arm B: Gem + Cis + 5-FU then 50.4 Gy + 5-FU (n = 11) | A: 3 (30) B: 2 (22) | R: 26.3 mo A: 19.4 mo B: 13.4 mo | Phase II; early termination due to poor accrual |
| Sahora et al[45], 2011 | Unresectable (n = 18), borderline (n = 15) | N/A | Gem + Oxaliplatin | 13 (39) | R: 22 mo UR: 12 mo (P = 0.046) | Phase II; 69% R0 resection |
| Sahora et al[46], 2011 | Borderline (n = 12), Unresectable (n = 13) | N/A | Gem + Docetaxel | 8 (32) | R: 16 mo, UR: 12 mo | Phase II; 87% R0 resection |
| Pipas et al[43], 2012 | Resectable (n = 4), Borderline (n = 23), Unresectable (n = 6) | 54 Gy + Cetuximab + Gem | N/A | 25 (76) | R: 24.3 mo | Phase II; 92% R0 resection |
| Wo et al[51], 2013 | Resectable (n = 10) | Short-course photon RT (3 Gy × 10, 5 Gy × 5 qod, 5 Gy × 5 qd) + Capecitabine | N/A | N/A | N/A | Phase I; closed early due to intraoperative complications (fibrosis) |
| Kim et al[44], 2013 | Resectable (n = 23), Borderline (n = 39), Unresectable (n = 6) | 30 Gy + Gem + Oxaliplatin | N/A | 43 (63) | Overall: 18.2 mo; R:27.1 mo, UR: 10.9 | Phase II, multi-institutional |
| Shinoto et al[52], 2013 | Resectable (n = 26) | 30.0-36.8 Gy E of Carbon-ion radiotherapy (CIRT) | N/A | 21 (81) | Overall: 42%, R: 52% (5 yr survival) | Phase I; short course radiation |
Gem: Gemcitabine, Cis: Cisplatin; 5-FU: Fluorouracil; Gy: Gray; R: Resected patients; UR: Unresected patients; N/A: Not available.
The first major study investigating the effects of chemoradiation in the neoadjuvant setting was published in 1993 by Yeung et al[39]. In this Phase II study, patients with biopsy-proven pancreatic (n = 26) or duodenal cancer (n = 5) were treated with 50.4 Gy of radiation and concurrent tissue-sensitizing 5-FU and mitomycin C. Due to progression of disease in one-third of the study population, patients with pancreatic adenocarcinoma achieved a resection rate of 38%. In those resected patients, the authors reported a reduction in the rate of positive surgical margins and regional lymph node involvement in patients who underwent neoadjuvant therapy. The 5-year survival rates were 58% and 0% for those patients who underwent resection vs those unresected, respectively.
Since that initial study, further investigations into the role of neoadjuvant therapy prior to attempted resection have demonstrated improved resection rates with variability in effects on median survival. With various radiation regimens (30.0 to 50.4 Gy), combinations of chemotherapeutic agents (e.g., 5-FU, mitomycin C, paclitaxel, and cisplatin) and dosing, resection rates ranging from 45%[27] to 85%[40] were associated with median survival durations of two years or less. A recent Phase II study from France published in 2008 enrolled 41 patients with localized, potentially resectable pancreatic adenocarcinoma and treated them with 50 Gy of radiation combined with 5-FU and cisplatin[41]. Twenty-six patients (63%) went on to curative resection, and 81% had an R0 resection. Despite this, the 2-year overall survival rate in those resected patients was 32%.
The introduction of gemcitabine-based chemoradiation into neoadjuvant treatment regimens showed more promise for patients with pancreatic adenocarcinoma. In part due to gemcitabine’s potent radiosensitizing effect compared to alternative chemotherapeutic agents[42], its use in the neoadjuvant setting has led to significantly longer median survival rates compared to 5-FU, cisplatin, and mitomycin C based chemoradiation regimens, often with an improved side-effect profile. In a recent report from MD Anderson, patients with potentially resectable stage I/II pancreatic adenocarcinoma received gemcitabine-based chemoradiation with rapid-fractionation external beam radiation therapy (30 Gy)[19]. Of the 74% patients who underwent successful resection, the median survival was 34 mo (compared to 7 mo for the 26% of patients who did not undergo resection). This study concluded that even with a rapid-fractionation protocol of gemcitabine-based chemoradiation, similar (if not improved) survival outcomes can be achieved in patients who receive the standard-fractionation external beam radiation therapy dose of 50.4 Gy. That same year, the same group failed to demonstrate any benefit of adding gemcitabine and cisplatin to preoperative gemcitabine-based radiation therapy beyond that achieved by neoadjuvant gemcitabine-based radiation alone[33]. However, despite no statistical survival benefit, this trial demonstrated an excellent overall survival of over 30 mo in those undergoing resection - an improvement over many prior trials.
In reviewing these survival data, recognizing the results based on intent-to-treat analyses is critical. Regimen crossover, treatment-related toxicity, debilitating performance status, progression of disease while receiving therapy, patient refusal, and patient death are several factors that prevent patients from receiving the full extent of their therapy. When analyzing all patients with initially potentially resectable pancreatic cancer from these two studies - combining those who underwent resection and those patients who did not - an overall survival of 22.7 and 17.4 mo, respectively, was noted. Based on these intent-to-treat analyses, neoadjuvant therapy followed by resection of the pancreatic tumor yields comparable survival with patients randomized to undergo resection followed by adjuvant therapy. At the very least, neoadjuvant therapy does not result in inferior survival, while sparing a significant number of patients the morbidity of a futile PD. Thus, neoadjuvant therapy should be considered as a favorable therapeutic approach to patients with potentially resectable pancreatic cancer.
Gemcitabine has also been studied in the setting of borderline and unresectable tumors. Pipas et al[43] recently investigated a cetuximab/gemcitabine/intensity-modulated radiotherapy combination in an initial cohort consisting of patients with resectable (n = 4), borderline resectable (n = 23), and unresectable (n = 6) tumors. In total, following neoadjuvant therapy, 25 patients (76%) underwent curative-intent resection. Ninety-two percent of resected patients had a negative surgical margin, and two experienced complete pathologic responses to the regimen. The median survival of those patients who underwent resection was 24.3 mo, comparable to historical controls of patients initially deemed resectable upon presentation. A similar finding was recently published by Kim et al[44], evaluating gemcitabine, oxaliplatin, and 30 Gy of radiation in patients with localized and locally-advanced pancreatic adenocarcinoma. Two-thirds of the 68 enrolled patients initially presented with either borderline or unresectable tumors. However, 63% of patients went on to curative resection, and the median survival of patients undergoing resection was 27.1 mo.
Others have investigated neoadjuvant chemotherapy without concurrent radiation therapy. Sahora et al[45] have reported two phase II trials evaluating gemcitabine-based neoadjuvant chemotherapy alone in patients with borderline or unresectable tumors. One study used a NeoGemOx protocol consisting of gemcitabine and oxaliplatin given as IV infusions once weekly for 6-9 wk[45]. Notably, 18 patients presented with disease deemed unresectable at inclusion, while the remaining 15 patients had borderline resectable tumors. Following treatment with the NeoGemOx regimen, 13 patients, or 39% of those without clearly resectable tumors proceeded to undergo resection with curative intent. The median overall survival of this cohort was 22 mo, statistically longer than those without significant tumor regression. The second study utilized gemcitabine and docetaxel-based neoadjuvant chemotherapy (NeoGemTax) in a similar patient population[46]. Of the total 25 eligible patients, 13 had unresectable disease and 12 had borderline resectable disease at the time of inclusion. Although a similar percentage of patients (32%) experienced tumor regression to allow an attempt at curative resection as compared to the NeoGemOx regimen, the median survival of those patients downstaged with the NeoGemTax did not significantly differ from those patients whose tumors failed to regress (16.3 mo vs 12.2 mo). Despite a lack a radiotherapy in either study, R0 resection rates for the NeoGemOx and NeoGemTax regimens were 69% (9/13 resected patients) and 87% (7/8 patients), respectively. It is important to keep in mind, however, the relatively low sample sizes within these phase II trials (n = 33 and n = 25) as well as the overall resection rates (13/33, 39%; and 8/25, 32%) before concluding that radiation has a limited role in the neoadjuvant setting.
THERAPIES OF THE FUTURE
Ongoing trials
In an attempt to better define optimal treatment sequences, the NEOPAC study (NCT01521702) is an ongoing multicenter prospective randomized phase III trial which aims to determine the efficacy of neoadjuvant chemotherapy vs adjuvant chemotherapy in patients with pancreatic head cancer[47]. This trial is currently recruiting patients with biopsy-proven resectable cancer who will be randomized to one of two arms: (1) neoadjuvant chemotherapy consisting of gemcitabine and oxaliplatin followed by surgery and adjuvant gemcitabine; or (2) initial surgical resection followed by adjuvant gemcitabine, with the primary endpoint of progression-free survival. No patients are randomized to receive concurrent radiotherapy, as this study aims to investigate the efficacy of chemotherapy only.
The Interdisciplinary Study Group of Gastrointestinal Tumours of the German Cancer Aid is also currently performing a multicenter randomized phase II trial comparing neoadjuvant therapy with adjuvant therapy in patients undergoing resection of their pancreatic tumor[48]. Patients with potentially resectable tumors are randomized to A) neoadjuvant chemoradiation with concurrent gemcitabine and cisplatin followed by surgical resection or B) immediate resection. Given its repeated demonstration of therapeutic benefit, post-operative adjuvant chemotherapy will be administered for 6 mo to patients in both arms of the study.
The combination chemotherapeutic regimen of 5-FU, leucovorin, irinotecan, and oxaliplatin, referred to collectively as FOLFIRINOX, is a newer treatment option for patients with pancreatic cancer. A recent phase III study published in the New England Journal of Medicine found this regimen to be superior to the gemcitabine in patients with metastatic pancreatic cancer, with prolonged median overall survival (11.1 mo vs 6.8 mo) and disease-free survival (6.4 mo vs 3.3 mo)[11]. Given the relative success of this novel regimen in the metastatic setting, the Alliance for Clinical Trials in Oncology is spearheading a multicenter single-arm pilot study (Alliance A021101) to evaluate FOLFIRINOX as a neoadjuvant regimen for patients with borderline resectable tumors at initial presentation, utilizing FOLFIRINOX followed by 50.4 Gy of capecitabine-based chemoradiotherapy prior to surgery[49]. Primary outcomes are focused on survival and toxicity of this regimen. This will be the first multicenter trial specifically evaluating FOLFIRINOX in the neoadjuvant setting for patients with borderline resectable disease.
For those patients with locally advanced unresectable pancreatic cancer, the RECLAP trial is a phase I study investigating the safety and efficacy of super-selective intra-arterial delivery of chemotherapy to the tumor bed via an indwelling subcutaneous port[50]. Outcomes of interest include toxicity, disease-free and overall survival, and conversion from unresectable to potentially resectable tumors.
Wo et al[51] recently published a small phase I study of patients with resectable pancreatic cancer who underwent neoadjuvant accelerated short-course photon chemoradiation therapy with concurrent capecitabine. Patients received photon radiotherapy at escalating doses of 3Gy × 10 d, 5 Gy × 5 d administered every other day, and 5 Gy × 5 consecutive days. Unfortunately, this radiation protocol resulted in significant intraoperative morbidity associated with radiation-induced fibrosis of the surgical field and forced the study to close early. Shinoto et al[52] investigated the toxicity and efficacy of carbon-ion radiotherapy (CIRT) as a short-course neoadjuvant treatment in patients with resectable tumors. The dose of CIRT was sequentially increased by 5% increments from 30 to 36.8 Gy equivalents with resection 2 to 4 wk after the completion of CIRT. None of the resected patients experienced local recurrence, and 5-year survival rates for those resected was 52% - a rather promising finding when compared to historical rates.
Finally, the NEOPANC trial is a single-arm prospective phase I/II study investigating neoadjuvant short course intensity-modulated radiation therapy in combination with surgery and intraoperative radiation therapy of 15 Gy for the treatment of resectable pancreatic cancer[53]. The authors hypothesize that neoadjuvant and intraoperative radiation administration will allow for dose escalation, reduced toxicity, and improved patient tolerance. The primary outcomes include 1 year local recurrence as well as feasibility of delivering such a regimen, with secondary endpoints of overall and disease-free survival, toxicity, and associated morbidity and mortality.
Additional therapeutic options
The novel agent S-1, an oral fluoropyrimidine analogue, has shown great therapeutic success in numerous Japanese studies. S-1 consists of a combination of three drugs: the 5-FU prodrug tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP; an inhibitor of dihydropyrimidine dehydrogenase enzyme activity), and potassium oxonate (OXO; an inhibitor of 5-FU phosphorylation in the gastrointestinal tract, thereby reducing side effects[54]. This drug mimics the anticancer agent 5-FU by intercalating itself into actively-synthesizing strands of DNA and causing the rapidly dividing cells to undergo apoptosis, and this formulation has clear advantages over 5-FU. The bioavailability of single-agent 5-FU administered orally is minimal due to the high activity of dihydropyrimidine dehydrogenase within the enterocytes, which lead to premature metabolism. CDHP and OXO within S-1 act to prevent the premature metabolism of the prodrug until it has successfully been absorbed and delivered to its target cells. The oral administration is therefore not only more convenient than the IV form of 5-FU, but also allows for predictable absorption and pharmacokinetic properties[54].
Given its widespread success in numerous phase II trials, S-1 was studied in a phase III trial in patients with locally advanced or metastatic disease[55]. This trial was powered to demonstrate non-inferiority of S-1 to gemcitabine, and 834 chemotherapy-naïve patients were randomly assigned to receive S-1, gemcitabine, or both. The noninferiority of S-1 to gemcitabine was demonstrated, but gemcitabine plus S-1 was not superior to gemcitabine alone, providing an argument for the use of S-1 as single-agent therapy in the setting of advanced pancreatic cancer. From these data, a study was designed to evaluate the efficacy of S-1 in the neoadjuvant setting. Tajima et al[56] retrospectively evaluated neoadjuvant gemcitabine plus oral S-1 in patients with potentially resectable pancreatic cancer. Of the 13 patients who received the neoadjuvant treatment, no patients demonstrated disease-progression or distant metastases prior to resection. The investigators found a trend towards improved 3-year survival (55.6% for the neoadjuvant treatment group vs 29.6% in the resection group), but this pilot study was underpowered to detect a significant difference. A larger phase I study of the gemcitabine plus S-1 neoadjuvant regimen is currently underway. While most trials involving S-1 have been conducted in Japan, additional trials are being developed across Europe and the United States.
Molecular markers of interest
The armamentarium of chemotherapeutic agents used to treat pancreatic cancer in the neoadjuvant setting, though continually improving, is sub-optimal. Prolonging survival by mere months suggests that pharmacological improvements can-and need to-be made. A topic of great interest and ongoing research in the field of chemotherapeutic optimization centers on evolving from a one-size-fits-all neoadjuvant regimen to a more individualized regimen tailored to a patient’s specific tumor genotype and biology. Identifying novel biomarkers associated with pancreatic cancer may not only allow for the development of individualized treatment regimens, but also may allow for earlier disease detection, and an extraordinary amount of research is being performed to identify an accurate tumor marker or a panel of markers that could aid in the management of this disease[57]. As a testament to the current popularity of biomarker identification within pancreatic adenocarcinoma, a recent analysis by Harsha et al[58] identified over 2500 gene products with evidence of overexpression at the mRNA level, protein level, or both. Here we will review a select few biomarkers, focusing on those with potential prognostic value and those that may prove “actionable” in future therapeutic endeavors (Table 3).
Table 3.
Select clinically valuable pancreatic cancer biomarkers
| Gene product | Target drug | Mechanism | Ref. |
| hENT1 | Gemcitabine | Nucleoside inhibitor, prevents DNA synthesis in cancer cells | [59-64] |
| TS/DPD | 5-FU/S-1 | Suicide inhibitor of TS, prevents DNA synthesis in cancer cells | [65-71] |
| EGFR | Erlotinib | Tyrosine kinase inhibitor, prevents EGFR-mediated cell cycle progression and cellular proliferation | [72-79] |
| CA 19-9 | N/A | N/A | [80-88] |
| SPARC | Nab-paclitaxel | Disruption of microtubule formation during mitosis | [12,89-94] |
| SMAD4 | N/A | Tumor suppression, initiation, and metastasis | [95-99] |
CA 19-9: Carbohydrate antigen 19-9; TS: Thymidylate synthase; hENT1: Human equilibrative nucleoside transporter-1; SPARC: Secreted protein acidic and rich in cysteine; EGFR: Epidermal growth factor receptor.
Human equilibrative nucleoside transporter-1
Gemcitabine is an analog of the nucleoside deoxycitidine. This prodrug is transported into a cell and subsequently phosphorylated to its active forms gemcitabine diphosphate or gemcitabine triphosphate[59]. The active forms of gemcitabine then confer their cytotoxic effects by inserting into synthesizing DNA chains and disrupting further DNA synthesis, and gemcitabine’s cellular uptake is dependent upon the human equilibrative nucleoside transporter-1 (hENT1) protein[60]. As this receptor is traditionally upregulated on the surface of pancreatic adenocarcinoma cells, gemcitabine has proven to be an effective chemotherapeutic option in many patients.
However, despite its therapeutic benefit in patients with both resectable and unresectable pancreatic tumors, not all patients respond to gemcitabine treatment. One potential mechanism for resistance to gemcitabine includes a relative downregulation or mutation of hENT1 receptors which ultimately leads to decreased cellular uptake of the drug[60]. In vitro analyses of pancreatic tumor cell lines have demonstrated that hENT1 protein expression is a significant determinant of gemcitabine activity within pancreatic tumor cells: overexpression of the hENT1 protein on the pancreatic cell surface correlates with increased uptake and activity of gemcitabine, while relative underexpression of hENT1 along the cell surface correlates with gemcitabine resistance[61].
Several studies have sought to test the prognostic potential of hENT1 receptor status in the clinical setting. One study performed immunohistological staining on tumor blocks of gemcitabine-treated pancreatic cancer[62]. These authors noted that in patients with detectable hENT1 protein staining in pancreatic tumor cells, median survival was significantly longer (13 mo vs 4 mo, P = 0.01) than in those patients with less abundant, heterogenous hENT1 staining. Another investigation identified hENT1 protein expression as highly correlative with clinical outcomes of disease-free survival, overall survival, and time to disease progression. Of concern with both these investigations, however, was the heterogenous patient population, including patients with both localized as well as advanced (metastatic) disease. Additionally, as both studies were retrospective analyses, it is difficult to make claims beyond those of a correlative nature.
Farrell et al[63] performed immunohistological staining for hENT1 on a tissue microarray of resected pancreatic tumors as part of the RTOG9704 study. These authors noted that hENT1 receptor expression was predictive of disease-free and overall survival in resected pancreatic cancer for those patients treated with gemcitabine but not 5-FU. Similarly, Kim et al[64] also found associations between low expressions of hENT1 protein and worse overall and disease-free survival in patients with resected pancreatic adenocarcinoma independent of gemcitabine therapy. However, no studies to date have investigated hENT1 expression in a neoadjuvant setting. As such, evaluation of hENT1 receptor status at the time of initial pancreatic tumor biopsy may prove advantageous in predicting eventual pathological and clinical responsiveness to gemcitabine-based chemotherapy.
Thymidylate synthase and dihydropyrimidine dehydrogenase
Thymidylate synthase (TS) is the intracellular enzyme responsible for synthesizing thymidine, a pyrimidine nucleoside required for DNA replication. 5-FU acts as a suicide inhibitor by irreversibly binding and inhibiting TS and thus preventing the production of deoxythymine monophosphate (dTMP)[65]. Without sufficient levels of dTMP, rapidly dividing cells are unable to synthesize DNA and therefore undergo apoptosis. Acting as a pyrimidine analogue, 5-FU is metabolized inside the cell into one of several possible cytotoxic metabolites which are incorporated into the actively synthesizing strands of DNA and RNA.
Both from an efficacy and a toxicity perspective, significant variability exists between patients treated with 5-FU. Such therapeutic unpredictability in response to 5-FU has been linked to the rate-limiting enzyme in 5-FU’s metabolic pathway, known as dihydropyrimidine dehydrogenase (DPD)[66]. Interestingly, an estimated one in ten individuals carries a genetic mutation rendering them unable to metabolize 5-FU to its active metabolite. Laboratory testing for this mutation is available and could be used to identify patients in whom 5-FU may be ineffective (or even toxic). While both TS and DPD have been shown to be upregulated in the setting of pancreatic cancer[67], genetic variations do exist. For example, one Japanese study identified that over half of Japanese pancreatic tissue samples expressed low levels of TS in combination with high levels of DPD[68]. Perhaps not surprisingly, another study from Japan concluded that high DPD mRNA levels within pancreatic tumor sections were associated with high rates of therapeutic response to S-1[69].
Several studies have investigated TS enzyme expression in pancreatic tumors as a prognostic variable. One study reviewed tissue cores from a retrospective series of 132 resected patients[70]. On immunohistological analysis, roughly two-thirds of patients had high intratumoral TS protein expression while the remaining one-third had low expression. The median survival of patients with low TS expression was longer than those with high TS expression, and high TS expression was identified as an independent predictor of mortality on multivariate analysis. Moreover, in the subset of patients who received adjuvant 5-FU, there was a significant survival advantage in patients with high TS protein expression. In contrast, adjuvant 5-FU did not influence survival in patients with low TS expression. From these data, the authors concluded that high TS expression is a poor prognostic marker in patients with resected pancreatic cancer, however these patients do benefit from adjuvant 5-FU therapy. Alternatively, a similar study investigating TS protein expression found conflicting results[71]. Again, TS expression was evaluated via immunohistochemistry in 98 patients following an R0 resection of pancreatic head cancer. These authors noted only 26% of these tumors demonstrated high TS expression, and, in contrast to the prior study, these authors concluded that TS predicted favorable disease-free, cancer-specific, and overall survivals. While the specific prognostic value of TS remains debatable, it is clear that genetic variations in the protein expression of TS and DPD may contribute to variable efficacy of 5-FU-based regimens.
Epidermal growth factor receptor
The epidermal growth factor receptor (EGFR) is a transmembrane receptor responsible for a wide array of downstream signaling pathways involved in both normal cells and those undergoing carcinogenesis[72]. Pancreatic cancer cells are known to overexpress EGFR, and studies have demonstrated correlations between receptor/ligand coexpression and larger tumors, advanced clinical staging, and decreased survival[73,74]. As such, tyrosine kinase inhibitors are a novel class of therapeutic agents developed to act at one of the active binding sites along the receptor to prevent EGFR-mediated cell-cycle progression and cellular proliferation. Erlotinib is one of the most thoroughly investigated agents in the pre-clinical setting, and this tyrosine kinase inhibitor has been shown to act synergistically with gemcitabine to exhibit extended antitumor activity in both in vitro and in vivo models[75-77].
Additionally, erlotinib has been a component of combination therapy. Unfortunately, despite reports of favorable safety and toxicity profiles, few studies have yielded breakthrough improvements in patient survival. Combination therapy of erlotinib and bevacizumab, a monoclonal antibody to vascular endothelial growth factor receptor (VEGF-R), found relatively little improvement in patients with advanced pancreatic cancer who failed previous gemcitabine therapy[78]. However, another recent phase III trial randomly assigned patients with unresectable locally advanced or metastatic pancreatic cancer to receive either gemcitabine or gemcitabine plus erlotinib[79]. One-year survival and disease-free survival were statistically significantly improved in those patients with combination therapy. Overall survival was statistically improved as well, though only by several weeks, which calls into question its clinical significance. EGFR inhibition has also been shown to act synergistically with chemoradiation in promoting antitumor properties[77].
Carbohydrate antigen 19-9
Carbohydrate antigen 19-9 (CA 19-9) is the most familiar cell-surface protein used in the management of pancreatic cancer[80,81]. It was first discovered in the serum of pancreatic and colon cancer patients in 1981 and has since been identified in other malignant and benign pathologies within the gastrointestinal tract[82]. Initial studies proposed CA 19-9 as a screening tool, but its relatively low sensitivity and specificity prevented its widespread adoption as a screening tool for pancreatic adenocarcinoma[83,84]. Traditionally, the utility of this overexpressed cellular surface protein has been in the assessment of response to chemotherapy and identification of tumor recurrence following resection, but data suggest CA 19-9 may also have a prognostic role[81,85]. A recent study of 324 patients with resectable pancreatic cancer correlated outcomes with various tumor markers, and the investigators demonstrated that a high preoperative CA 19-9 × carcinoembryonic antigen (CEA) index was an independent predictor of survival and strongly correlated with early postoperative mortality[86].
Two recent studies from MD Anderson evaluated CA 19-9 in patients who underwent neoadjuvant therapy prior to surgical resection. The first evaluated the relationship between CA 19-9 and surgical outcomes in patients with borderline resectable disease[87]. Normalization of CA 19-9 following neoadjuvant therapy was associated with longer overall survival in both resected (38 mo vs 26 mo; P = 0.020) and unresected (15 mo vs 11 mo; P = 0.022) patients. Conversely, failure of CA 19-9 to normalize was identified as an independent factor associated with shorter overall survival (HR = 2.13, P = 0.001). The second study evaluated the ability of CA 19-9 to predict completion of multimodality therapy involving neoadjuvant chemoradiation and surgical resection[88]. Although a low pretreatment CA 19-9 had a high positive predictive value of completing neoadjuvant multimodality therapy, it concurrently demonstrated a low negative predictive value. Additionally, the investigators in this study found no association between a drop in CA 19-9 and histopathologic response to neoadjuvant multimodality therapy. From this, the authors discarded the notion of incorporating pretreatment CA 19-9 levels into their decision-making algorithm.
Secreted protein acidic and rich in cysteine
Beyond cell surface proteins and secreted molecules, increasing preclinical evidence is demonstrating the role of tumor microenvironments in the initiation, migration, basement membrane invasion, angiogenesis, and potential metastasis of pancreatic cancer[58]. One protein found in this microenvironment, the cell-surface molecule secreted protein acidic and rich in cysteine, or SPARC, is one of the most heavily researched stromal proteins[89]. Healthy pancreatic tissue typically stains faintly positive for the SPARC protein within acinar cells, islet cells, and fibroblasts within the pancreatic extracellular matrix. While normal ductal cells rarely stain positive, Guweidhi et al[90] noted a 31-fold increase in SPARC protein staining within pancreatic adenocarcinoma compared to normal tissue. (Interestingly, they also noted a 16-fold increase in SPARC protein expression in chronic pancreatitis.) One study identified positive immunohistological SPARC staining in 84% of retrospectively reviewed pancreatic adenocarcinoma tissue samples[91]. SPARC mRNA overexpression has been associated with both disease progression and poor prognosis in resected pancreatic tumors[92,93].
A recent phase III multi-institutional study published demonstrated a significant survival benefit in patients with stage-IV pancreatic cancer who received nab-paxlitaxel in combination with gemcitabine vs gemcitabine monotherapy[12]. Nab-paclitaxel’s antitumor effects are found in the disruption of microtubule formation and disassembly during cellular mitosis, and this particular formulation exploits paclitaxel bound to albumin. This structure not only improves the side effect profile but also favors accumulation within tumor cells by the binding of albumin to SPARC. By binding to SPARC within the extracellular matrix, nab-paclitaxel successfully disrupts the organization of the tumor cells and induces a marked alteration in the tumor architecture, resulting in increased tumor softening and permeability[94]. These findings could prove beneficial from both chemotherapeutic delivery and surgical resection perspectives.
SMAD4
Another stromal-based protein gaining popularity in pancreatic cancer is SMAD4, a member of the Smad family. SMAD4 activity is muted in pancreatic cancer, and its specificity for pancreatic cancer makes it one of the most heavily investigated tumor markers[95]. This family of proteins signals through the transforming growth factor-beta (TGF-β) receptor, a major receptor in the pathogenesis of pancreatic cancer[96]. This transcription factor pathway typically regulates cellular proliferation, differentiation, and apoptosis, and has been shown to act in tumor suppression, as well as tumor initiation and progression depending on the stage of carcinogenesis as well as cell type[97]. SMAD4 has also been implicated in promoting tumor metastasis in pancreatic cancer[98].
SMAD4 has been shown to be mutated in up to 50% of all pancreatic adenocarcinomas. Despite its clear association with the diagnosis, its prognostic role remains less distinct. Though some investigations suggest functional SMAD4 loss predicts a poor prognosis, other studies failed to demonstrate a relationship between SMAD4 mRNA expression and patient survival[99]. Due to the complexity of TGF-β signaling in pancreatic cancer, further investigations are needed to identify potential novel targeted therapies involving SMAD4.
Non-coding RNA
Beyond the classical protein products discussed previously, the field of epigenetics has begun to play an increasingly important role in the identification oncologic tumor markers and treatment optimization[100]. Unlike typical RNA molecules which code for functional proteins, non-coding RNA molecules themselves function in various methods to influence transcriptional and post-transcriptional regulation of gene expression[101]. In particular, one recent study suggested that the microenvironment of a tumor may stimulate a microRNA gene family that induces tumor resistance to therapy and promotes tumor cell invasion and metastasis[102]. Individual microRNA molecules are proving to have important treatment and prognostic value, as is found in a non-coding RNA named HOTAIR, which is upregulated in pancreatic tissue and has demonstrated pro-oncogenic function and an association with more aggressive tumor biology[103]. Preis et al[104] identified a consistent and significant overexpression of a microRNA named miR-10b in pancreatic cancer cells compared to benign tissue, while decreased expression of miR-10b was correlated with improved response to multimodality neoadjuvant therapy, likelihood of resection, delayed time to metastasis, and increased rate of survival. The field of research in epigenetics will likely support further studies on the identification of biomarkers diagnostic and therapeutic for pancreatic cancer.
While these select few biomarkers have been presented individually, it is likely they will prove most prognostic and therapeutically efficacious when analyzed as biomarker arrays instead of individual proteins. Indeed, patients harboring multiple mismatch repair gene polymorphisms have been associated with significantly worse survival compared to those patients with fewer (or no) mutations[105]. In the near future, gene expression analyses will likely play a significant role in the management of cancer patients, allowing for accurate prognostic information gleaned from the tissue at the time of initial biopsy.
CONCLUSION
In summary, patients with pancreatic adenocarcinoma who undergo surgical resection coupled with chemotherapy and/or chemoradiation have the best opportunity for long-term survival. However, the multitude and variety of chemotherapeutic options demonstrate that no current regimen in our armamentarium is clearly superior to others. Variations in tumor biology, the presence or absence of molecular markers, a patient’s functional status, and tolerability of potential side effects of current chemotherapeutic and radiation regimens make a simple and single universal therapeutic treatment modality difficult to advocate. In the meantime, there are a number of reasons to believe a neoadjuvant approach may be the best available strategy at this time, capitalizing on the critical concept of patient selection. Furthermore, molecular biomarkers such as hENT1, SPARC, and SMAD4 have gained recent popularity for their apparent predictive and prognostic abilities, and both epigenetic profiling and the identification of various oncologic microRNA molecules are likely to contribute to the field of pancreatic cancer treatment.
Footnotes
P- Reviewer: Crea F, Fujino Y, Miyagawa S, Sakamoto Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.World Cancer Research Fund International. Pancreatic Cancer. 2013. Available from: http://www.wcrf.org/cancer_statistics/data_specific_cancers/pancreatic_cancer_statistics.php.
- 2.American Cancer Society. Key Statistics about Pancreatic Cancer. 2013. Available from: http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-key-statistics.
- 3.Zavoral M, Minarikova P, Zavada F, Salek C, Minarik M. Molecular biology of pancreatic cancer. World J Gastroenterol. 2011;17:2897–2908. doi: 10.3748/wjg.v17.i24.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner TB, Scott-Brown M. The role of radiotherapy in multimodal treatment of pancreatic carcinoma. Radiat Oncol. 2010;5:64. doi: 10.1186/1748-717X-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowy AM. Neoadjuvant therapy for pancreatic cancer. J Gastrointest Surg. 2008;12:1600–1608. doi: 10.1007/s11605-008-0482-2. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 10.Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Rich TA, Willett CG. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, Arnaud JP, Gonzalez DG, de Wit LT, Hennipman A, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–82; discussion 782-4. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aapro MS, Martin C, Hatty S. Gemcitabine--a safety review. Anticancer Drugs. 1998;9:191–201. doi: 10.1097/00001813-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 16.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 17.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 19.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 20.Greer SE, Pipas JM, Sutton JE, Zaki BI, Tsapakos M, Colacchio TA, Gibson JJ, Wiener DC, Ripple GH, Barth RJ. Effect of neoadjuvant therapy on local recurrence after resection of pancreatic adenocarcinoma. J Am Coll Surg. 2008;206:451–457. doi: 10.1016/j.jamcollsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich S, Schäfer M, Weber A, Hany TF, Bhure U, Pestalozzi BC, Clavien PA. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 24.Palta M, Willett C, Czito B. Role of radiation therapy in patients with resectable pancreatic cancer. Oncology (Williston Park) 2011;25:715–21, 727. [PubMed] [Google Scholar]
- 25.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, Talamonti MS. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 27.Spitz FR, Abbruzzese JL, Lee JE, Pisters PW, Lowy AM, Fenoglio CJ, Cleary KR, Janjan NA, Goswitz MS, Rich TA, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 28.Aloia TA, Lee JE, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, Abbruzzese JL, Crane CH, Evans DB, Pisters PW. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–355. doi: 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Tzeng CW, Tran Cao HS, Lee JE, Pisters PW, Varadhachary GR, Wolff RA, Abbruzzese JL, Crane CH, Evans DB, Wang H, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16–24; discussion 24-5. doi: 10.1007/s11605-013-2412-1. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee D, Katz MH, Rashid A, Estrella JS, Wang H, Varadhachary GR, Wolff RA, Lee JE, Pisters PW, Abbruzzese JL, et al. Pancreatic intraepithelial neoplasia and histological changes in non-neoplastic pancreas associated with neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma. Histopathology. 2013;63:841–851. doi: 10.1111/his.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SW, Tzeng CW, Johnston WC, Cassera MA, Newell PH, Hammill CW, Wolf RF, Aloia TA, Hansen PD. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) HPB (Oxford) 2014;16:350–356. doi: 10.1111/hpb.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi H, Ogawa H, Ohigashi H, Gotoh K, Yamada T, Ohue M, Miyashiro I, Noura S, Kishi K, Motoori M, et al. Preoperative chemoradiation reduces the risk of pancreatic fistula after distal pancreatectomy for pancreatic adenocarcinoma. Surgery. 2011;150:547–556. doi: 10.1016/j.surg.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Abdalla E, Wang H, Staerkel GA, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 34.Lind PA, Isaksson B, Almström M, Johnsson A, Albiin N, Byström P, Permert J. Efficacy of preoperative radiochemotherapy in patients with locally advanced pancreatic carcinoma. Acta Oncol. 2008;47:413–420. doi: 10.1080/02841860701592384. [DOI] [PubMed] [Google Scholar]
- 35.Glant JA, Waters JA, House MG, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD, Schmidt CM. Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery. 2011;150:607–616. doi: 10.1016/j.surg.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 36.Tzeng CW, Katz MH, Fleming JB, Lee JE, Pisters PW, Holmes HM, Varadhachary GR, Wolff RA, Abbruzzese JL, Vauthey JN, et al. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg. 2014;18:146–55; discussion 155-6. doi: 10.1007/s11605-013-2371-6. [DOI] [PubMed] [Google Scholar]
- 37.Abbott DE, Tzeng CW, Merkow RP, Cantor SB, Chang GJ, Katz MH, Bentrem DJ, Bilimoria KY, Crane CH, Varadhachary GR, et al. The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol. 2013;20 Suppl 3:S500–S508. doi: 10.1245/s10434-013-2882-0. [DOI] [PubMed] [Google Scholar]
- 38.Abbott DE, Baker MS, Talamonti MS. Neoadjuvant therapy for pancreatic cancer: a current review. J Surg Oncol. 2010;101:315–320. doi: 10.1002/jso.21469. [DOI] [PubMed] [Google Scholar]
- 39.Yeung RS, Weese JL, Hoffman JP, Solin LJ, Paul AR, Engstrom PF, Litwin S, Kowalyshyn MJ, Eisenberg BL. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer. 1993;72:2124–2133. doi: 10.1002/1097-0142(19931001)72:7<2124::aid-cncr2820720711>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 40.Talamonti MS, Small W, Mulcahy MF, Wayne JD, Attaluri V, Colletti LM, Zalupski MM, Hoffman JP, Freedman GM, Kinsella TJ, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150–158. doi: 10.1245/ASO.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 41.Le Scodan R, Mornex F, Partensky C, Mercier C, Valette PJ, Ychou M, Roy P, Scoazec JY. Histopathological response to preoperative chemoradiation for resectable pancreatic adenocarcinoma: the French Phase II FFCD 9704-SFRO Trial. Am J Clin Oncol. 2008;31:545–552. doi: 10.1097/COC.0b013e318172d5c5. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence TS, Davis MA, Hough A, Rehemtulla A. The role of apoptosis in 2’,2’-difluoro-2’-deoxycytidine (gemcitabine)-mediated radiosensitization. Clin Cancer Res. 2001;7:314–319. [PubMed] [Google Scholar]
- 43.Pipas JM, Zaki BI, McGowan MM, Tsapakos MJ, Ripple GH, Suriawinata AA, Tsongalis GJ, Colacchio TA, Gordon SR, Sutton JE, et al. Neoadjuvant cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with pancreatic adenocarcinoma. Ann Oncol. 2012;23:2820–2827. doi: 10.1093/annonc/mds109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim EJ, Ben-Josef E, Herman JM, Bekaii-Saab T, Dawson LA, Griffith KA, Francis IR, Greenson JK, Simeone DM, Lawrence TS, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013;119:2692–2700. doi: 10.1002/cncr.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahora K, Kuehrer I, Eisenhut A, Akan B, Koellblinger C, Goetzinger P, Teleky B, Jakesz R, Peck-Radosavljevic M, Ba’ssalamah A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149:311–320. doi: 10.1016/j.surg.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 46.Sahora K, Kuehrer I, Schindl M, Koelblinger C, Goetzinger P, Gnant M. NeoGemTax: gemcitabine and docetaxel as neoadjuvant treatment for locally advanced nonmetastasized pancreatic cancer. World J Surg. 2011;35:1580–1589. doi: 10.1007/s00268-011-1113-8. [DOI] [PubMed] [Google Scholar]
- 47.Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR, Raoul JL, Bachellier P, Dufour P, Moehler M, et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study) BMC Cancer. 2011;11:346. doi: 10.1186/1471-2407-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunner TB, Grabenbauer GG, Meyer T, Golcher H, Sauer R, Hohenberger W. Primary resection versus neoadjuvant chemoradiation followed by resection for locally resectable or potentially resectable pancreatic carcinoma without distant metastasis. A multi-centre prospectively randomised phase II-study of the Interdisciplinary Working Group Gastrointestinal Tumours (AIO, ARO, and CAO) BMC Cancer. 2007;7:41. doi: 10.1186/1471-2407-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis JL, Pandalai P, Ripley RT, Langan RC, Steinberg SM, Walker M, Toomey MA, Levy E, Avital I. Regional chemotherapy in locally advanced pancreatic cancer: RECLAP trial. Trials. 2011;12:129. doi: 10.1186/1745-6215-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wo JY, Mamon HJ, Ferrone CR, Ryan DP, Blaszkowsky LS, Kwak EL, Tseng YD, Napolitano BN, Ancukiewicz M, Swanson RS, et al. Phase I study of neoadjuvant accelerated short course radiation therapy with photons and capecitabine for resectable pancreatic cancer. Radiother Oncol. 2014;110:160–164. doi: 10.1016/j.radonc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 52.Shinoto M, Yamada S, Yasuda S, Imada H, Shioyama Y, Honda H, Kamada T, Tsujii H, Saisho H. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer. 2013;119:45–51. doi: 10.1002/cncr.27723. [DOI] [PubMed] [Google Scholar]
- 53.Roeder F, Timke C, Saleh-Ebrahimi L, Schneider L, Hackert T, Hartwig W, Kopp-Schneider A, Hensley FW, Buechler MW, Debus J, et al. Clinical phase I/II trial to investigate neoadjuvant intensity-modulated short term radiation therapy (5 × 5 Gy) and intraoperative radiation therapy (15 Gy) in patients with primarily resectable pancreatic cancer - NEOPANC. BMC Cancer. 2012;12:112. doi: 10.1186/1471-2407-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schöffski P. The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs. 2004;15:85–106. doi: 10.1097/00001813-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 56.Tajima H, Ohta T, Kitagawa H, Okamoto K, Sakai S, Makino I, Kinoshita J, Furukawa H, Nakamura K, Hayashi H, et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. 2012;3:787–792. doi: 10.3892/etm.2012.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ballehaninna UK, Chamberlain RS. Biomarkers for pancreatic cancer: promising new markers and options beyond CA 19-9. Tumour Biol. 2013;34:3279–3292. doi: 10.1007/s13277-013-1033-3. [DOI] [PubMed] [Google Scholar]
- 58.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 60.Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 61.Mori R, Ishikawa T, Ichikawa Y, Taniguchi K, Matsuyama R, Ueda M, Fujii Y, Endo I, Togo S, Danenberg PV, et al. Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol Rep. 2007;17:1201–1205. [PubMed] [Google Scholar]
- 62.Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956–6961. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 63.Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 64.Kim R, Tan A, Lai KK, Jiang J, Wang Y, Rybicki LA, Liu X. Prognostic roles of human equilibrative transporter 1 (hENT-1) and ribonucleoside reductase subunit M1 (RRM1) in resected pancreatic cancer. Cancer. 2011;117:3126–3134. doi: 10.1002/cncr.25883. [DOI] [PubMed] [Google Scholar]
- 65.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 66.Lee A, Ezzeldin H, Fourie J, Diasio R. Dihydropyrimidine dehydrogenase deficiency: impact of pharmacogenetics on 5-fluorouracil therapy. Clin Adv Hematol Oncol. 2004;2:527–532. [PubMed] [Google Scholar]
- 67.Shimoda M, Sawada T, Kubota K. Thymidylate synthase and dihydropyrimidine dehydrogenase are upregulated in pancreatic and biliary tract cancers. Pathobiology. 2009;76:193–198. doi: 10.1159/000218335. [DOI] [PubMed] [Google Scholar]
- 68.Fukui Y, Oka T, Nagayama S, Danenberg PV, Danenberg KD, Fukushima M. Thymidylate synthase, dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase mRNA and protein expression levels in solid tumors in large scale population analysis. Int J Mol Med. 2008;22:709–716. [PubMed] [Google Scholar]
- 69.Kuramochi H, Hayashi K, Uchida K, Nakajima G, Hatori T, Danenberg KD, Danenberg PV, Yamamoto M. High intratumoral dihydropyrimidine dehydrogenase mRNA levels in pancreatic cancer associated with a high rate of response to S-1. Cancer Chemother Pharmacol. 2008;63:85–89. doi: 10.1007/s00280-008-0714-x. [DOI] [PubMed] [Google Scholar]
- 70.Hu YC, Komorowski RA, Graewin S, Hostetter G, Kallioniemi OP, Pitt HA, Ahrendt SA. Thymidylate synthase expression predicts the response to 5-fluorouracil-based adjuvant therapy in pancreatic cancer. Clin Cancer Res. 2003;9:4165–4171. [PubMed] [Google Scholar]
- 71.van der Zee JA, van Eijck CH, Hop WC, van Dekken H, Dicheva BM, Seynhaeve AL, Koning GA, Eggermont AM, Ten Hagen TL. Expression and prognostic significance of thymidylate synthase (TS) in pancreatic head and periampullary cancer. Eur J Surg Oncol. 2012;38:1058–1064. doi: 10.1016/j.ejso.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 2001;19:32S–40S. [PubMed] [Google Scholar]
- 73.Xiong HQ, Abbruzzese JL. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002;29:31–37. doi: 10.1053/sonc.2002.35645. [DOI] [PubMed] [Google Scholar]
- 74.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Beger HG, Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–569. [PubMed] [Google Scholar]
- 75.Bartholomeusz C, Yamasaki F, Saso H, Kurisu K, Hortobagyi GN, Ueno NT. Gemcitabine Overcomes Erlotinib Resistance in EGFR-Overexpressing Cancer Cells through Downregulation of Akt. J Cancer. 2011;2:435–442. doi: 10.7150/jca.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iwai T, Moriya Y, Shirane M, Fujimoto-Ouchi K, Mori K. Continuous inhibition of epidermal growth factor receptor phosphorylation by erlotinib enhances antitumor activity of chemotherapy in erlotinib-resistant tumor xenografts. Oncol Rep. 2012;27:923–928. doi: 10.3892/or.2011.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianco C, Giovannetti E, Ciardiello F, Mey V, Nannizzi S, Tortora G, Troiani T, Pasqualetti F, Eckhardt G, de Liguoro M, et al. Synergistic antitumor activity of ZD6474, an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, with gemcitabine and ionizing radiation against pancreatic cancer. Clin Cancer Res. 2006;12:7099–7107. doi: 10.1158/1078-0432.CCR-06-0833. [DOI] [PubMed] [Google Scholar]
- 78.Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, Schillinger B, Scott J, Hwang J, Tempero MA. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051–1057. doi: 10.1007/s00280-010-1257-5. [DOI] [PubMed] [Google Scholar]
- 79.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 80.Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501–5503. [PubMed] [Google Scholar]
- 81.Montgomery RC, Hoffman JP, Riley LB, Rogatko A, Ridge JA, Eisenberg BL. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997;4:551–556. doi: 10.1007/BF02305535. [DOI] [PubMed] [Google Scholar]
- 82.Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 83.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 84.Hawes RH, Xiong Q, Waxman I, Chang KJ, Evans DB, Abbruzzese JL. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol. 2000;95:17–31. doi: 10.1111/j.1572-0241.2000.01699.x. [DOI] [PubMed] [Google Scholar]
- 85.Distler M, Pilarsky E, Kersting S, Grützmann R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas - a retrospective tumor marker prognostic study. Int J Surg. 2013;11:1067–1072. doi: 10.1016/j.ijsu.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Kanda M, Fujii T, Takami H, Suenaga M, Inokawa Y, Yamada S, Nakayama G, Sugimoto H, Koike M, Nomoto S, et al. The combination of the serum carbohydrate antigen 19-9 and carcinoembryonic antigen is a simple and accurate predictor of mortality in pancreatic cancer patients. Surg Today. 2014;44:1692–1701. doi: 10.1007/s00595-013-0752-9. [DOI] [PubMed] [Google Scholar]
- 87.Tzeng CW, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H, Crane CH, Wolff RA, Varadhachary GR, Pisters PW, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430–438. doi: 10.1111/hpb.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katz MH, Varadhachary GR, Fleming JB, Wolff RA, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Sun CC, Wang H, et al. Serum CA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol. 2010;17:1794–1801. doi: 10.1245/s10434-010-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neuzillet C, Tijeras-Raballand A, Cros J, Faivre S, Hammel P, Raymond E. Stromal expression of SPARC in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32:585–602. doi: 10.1007/s10555-013-9439-3. [DOI] [PubMed] [Google Scholar]
- 90.Guweidhi A, Kleeff J, Adwan H, Giese NA, Wente MN, Giese T, Büchler MW, Berger MR, Friess H. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg. 2005;242:224–234. doi: 10.1097/01.sla.0000171866.45848.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 92.Miyoshi K, Sato N, Ohuchida K, Mizumoto K, Tanaka M. SPARC mRNA expression as a prognostic marker for pancreatic adenocarcinoma patients. Anticancer Res. 2010;30:867–871. [PubMed] [Google Scholar]
- 93.Prenzel KL, Warnecke-Eberz U, Xi H, Brabender J, Baldus SE, Bollschweiler E, Gutschow CA, Hölscher AH, Schneider PM. Significant overexpression of SPARC/osteonectin mRNA in pancreatic cancer compared to cancer of the papilla of Vater. Oncol Rep. 2006;15:1397–1401. [PubMed] [Google Scholar]
- 94.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dempe S, Stroh-Dege AY, Schwarz E, Rommelaere J, Dinsart C. SMAD4: a predictive marker of PDAC cell permissiveness for oncolytic infection with parvovirus H-1PV. Int J Cancer. 2010;126:2914–2927. doi: 10.1002/ijc.24992. [DOI] [PubMed] [Google Scholar]
- 96.Singh P, Wig JD, Srinivasan R. The Smad family and its role in pancreatic cancer. Indian J Cancer. 2010;48:351–360. doi: 10.4103/0019-509X.84939. [DOI] [PubMed] [Google Scholar]
- 97.Wang P, Chen Z, Meng ZQ, Fan J, Luo JM, Liang W, Lin JH, Zhou ZH, Chen H, Wang K, et al. Dual role of Ski in pancreatic cancer cells: tumor-promoting versus metastasis-suppressive function. Carcinogenesis. 2009;30:1497–1506. doi: 10.1093/carcin/bgp154. [DOI] [PubMed] [Google Scholar]
- 98.Malkoski SP, Wang XJ. Two sides of the story? Smad4 loss in pancreatic cancer versus head-and-neck cancer. FEBS Lett. 2012;586:1984–1992. doi: 10.1016/j.febslet.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh P, Wig JD, Srinivasan R, Radotra BD. A comprehensive examination of Smad4, Smad6 and Smad7 mRNA expression in pancreatic ductal adenocarcinoma. Indian J Cancer. 2011;48:170–174. doi: 10.4103/0019-509X.82876. [DOI] [PubMed] [Google Scholar]
- 100.Pai P, Rachagani S, Are C, Batra SK. Prospects of miRNA-based therapy for pancreatic cancer. Curr Drug Targets. 2013;14:1101–1109. doi: 10.2174/13894501113149990181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 102.Bryant JL, Britson J, Balko JM, Willian M, Timmons R, Frolov A, Black EP. A microRNA gene expression signature predicts response to erlotinib in epithelial cancer cell lines and targets EMT. Br J Cancer. 2012;106:148–156. doi: 10.1038/bjc.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Preis M, Gardner TB, Gordon SR, Pipas JM, Mackenzie TA, Klein EE, Longnecker DS, Gutmann EJ, Sempere LF, Korc M. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:5812–5821. doi: 10.1158/1078-0432.CCR-11-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dong X, Jiao L, Li Y, Evans DB, Wang H, Hess KR, Abbruzzese JL, Li D. Significant associations of mismatch repair gene polymorphisms with clinical outcome of pancreatic cancer. J Clin Oncol. 2009;27:1592–1599. doi: 10.1200/JCO.2008.20.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palmer DH, Stocken DD, Hewitt H, Markham CE, Hassan AB, Johnson PJ, Buckels JA, Bramhall SR. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–2096. doi: 10.1245/s10434-007-9384-x. [DOI] [PubMed] [Google Scholar]
- 107.Heinrich S, Pestalozzi BC, Schäfer M, Weber A, Bauerfeind P, Knuth A, Clavien PA. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:2526–2531. doi: 10.1200/JCO.2007.15.5556. [DOI] [PubMed] [Google Scholar]
- 108.Turrini O, Ychou M, Moureau-Zabotto L, Rouanet P, Giovannini M, Moutardier V, Azria D, Delpero JR, Viret F. Neoadjuvant docetaxel-based chemoradiation for resectable adenocarcinoma of the pancreas: New neoadjuvant regimen was safe and provided an interesting pathologic response. Eur J Surg Oncol. 2010;36:987–992. doi: 10.1016/j.ejso.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Landry J, Catalano PJ, Staley C, Harris W, Hoffman J, Talamonti M, Xu N, Cooper H, Benson AB. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101:587–592. doi: 10.1002/jso.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]


