Abstract
AIM: To evaluate protective effects of Chunggan extract (CGX), a traditional herbal formula, under 4 wk of alcohol consumption-induced liver injury.
METHODS: Male Sprague-Dawley Rats were orally administered 30% ethanol daily for 4 wk with or without CGX. The pharmaceutical properties were assessed through liver enzymes, histopathology, fibrogenic cytokines, and alcohol metabolism in hepatic tissues as well as by in vitro experiment using HSC-T6 cells.
RESULTS: Four weeks of alcohol consumption notably increased liver enzymes and malondialdehyde levels in serum and hepatic tissue. CGX not only prevented the collagen deposition determined by histopathology and hydroxyproline content, but also normalized transforming growth factor-beta, platelet-derived growth factor-beta and connective tissue growth factor at the gene expression and protein levels in liver tissue. Moreover, CGX treatment also significantly normalized the abnormal changes in gene expression profiles of extracellular matrix proteins, matrix metalloproteinase and their inhibitors, alcohol metabolism, and inflammatory reactions. In the acetaldehyde-stimulated HSC-T6 cells, CGX considerably inhibited collagen production and normalized fibrogenic cytokines in both gene expression and protein levels.
CONCLUSION: The present study evidenced that CGX has hepatoprotective properties via modulation of fibrogenic cytokines and alcohol metabolism in alcoholic liver injury.
Keywords: Alcohol abuse, Liver injury, Traditional Chinese Medicine, Fibrogenic cytokines, Hepato stellate cell
Core tip: We observed that the protective effect of Chunggan extract (CGX) on alcohol induced rat model of hepatic injury. In this study, 4 wk of alcohol consumptions markedly induced hepatic injury. Treatment with CGX significantly reverses and ameliorates pro-fibrogenic cytokines including transforming growth factor-β, platelet-derived growth factor-BB, and connective tissue growth factor. We also revealed the significant effects of alcohol metabolism related molecules by CGX treatment. The pharmacological actions were supported by in vitro assay that acetaldehyde stimulated HSC-T6 cell activation was normalized by CGX. Collectively our results suggest that CGX will be applicable to treat patients with alcoholic liver injury through amelioration of fibrotic changes and alcohol metabolisms.
INTRODUCTION
Various types of liver diseases can be induced by diverse causes, such as hepatitis virus types A, B, and C; chemicals; toxic drugs; metabolic disorders; and alcohol abuse[1]. Among them the alcohol abuse is the most common cause of hepatic disorders worldwide, in the United States, and in north-western Europe, with mortality rates of 5% to 6%[2]. Liver diseases show a wide spectrum of pathological cascades, from steatostasis to hepatofibrosis and hepatocellular carcinomas. And then, these cascades represent major concerns within the field of hepatology worldwide[3]. Especially, in the case of chronic alcohol abuse-related liver injuries are approximately attributed to 10%-15% of all cases of fibrotic change[4].
In alcohol metabolism, several mediators are directly or indirectly associated to hepatic injury. Alcohol is metabolised mainly by cytochrome P450 2E1 (CYP2E1) in hepatocytes and it does not damage, if the amount ingested is not in excessive quantity. Under the circumstance of an excessive amount of alcohol ingestion, however, CYP2E1 not only generates reactive oxygen species (ROS), including the superoxide anion radical and hydrogen peroxide, but also produces highly reactive conjugated adducts[5]. These oxidative stressors can lead to attack the normal liver cells. In addition, acetaldehyde, an intermediate of the alcohol metabolism process, accumulates in the hepatic tissues and acts as a free radical that readily damages normal hepatic tissues during extreme amount or long-term alcohol consumption[6].
Particularly the production of pro-inflammatory cytokines, including tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1β, also causes liver injuries by oxidative stress. These pathological states lead to hepatostellate cell (HSC) activation[5,7,8]. The activated HSCs rapidly release large amounts of pro-fibrogenic cytokines, such as transforming growth factor-beta (TGF-β), platelet-derived growth factor-beta (PDGF-β), and connective tissue growth factor (CTGF); they then form extracellular matrix (ECM) including collagen types I, III, and IV in hepatic tissues[9,10]. Therefore, the main goal in the treatment of alcohol consumption-derived hepatic injury is to relieve or inhibit HSC activation[11].
Chunggan extract (CGX) is a commercially available with modification of a traditional herbal medicine comprising 13 herbs intended to “cleaning the liver”. CGX has been used in South Korea since 2001 as a remedy for patients with chronic liver disorders, such as alcoholic liver injury[12]. We have previously presented scientific evidences for the pharmaceutical effects and antioxidant properties of CGX in various animal models, mainly with various chemotoxins[13-15]. Although we already proved the pharmacological properties of CGX in above models, the anti-fibrosis effects and its pharmacological actions on chronic alcohol-induced liver injury have not yet been determined.
In this study, we investigated the hepato protective properties of CGX and its possible mechanisms of action in a 4-wk alcohol consumption rat model.
MATERIALS AND METHODS
Preparation of CGX
CGX consists of 13 kinds of different types of the Traditional Chinese Medicine (TCM) derived materials (herbal plants and animal based materials), including 5 g each of Artemisia Capillaris Herba, Trionycis Carapax, Raphani Semen; 3 g each of Atractylodis Rhizoma Alba, Hoelen, Alismatis Rhizoma, Atractylodis Rhizoma, Salviae Miltiorrhizae Radix; 2 g each of Polyporus, Aurantii Immature Fructus, Amomi Fructus, and 1 g of Glycyrrhizae Radix and Aucklandiae Radix (Table 1).
Table 1.
Herbal prescription of Chunggan extract
| Herbal name | Scientific name | Relative amounts (g) |
| Artemisiae Capillaris Herba | Artemisia capillaries Thunberg | 5 |
| Trionycis Carapax | Trionyx sinensis Wiegmann | 5 |
| Raphani Semen | Raphanus sativus Linne | 5 |
| Atractylodis Rhizoma Alba | Atractylodes macrocephala Koidz | 3 |
| Hoelen | Poria cocos Wolf | 3 |
| Alismatis Rhizoma | Alisma orientalis (Sam.) Juzepczuk | 3 |
| Atractylodis Rhizoma | Atractylodes chinensis Koidzumi | 3 |
| Salviae Miltiorrhizae Radix | Salvia miltiorrhiza Bunge | 3 |
| Polyporus | Polyporus umbellatus Fries | 2 |
| Aurantii Immaturus Fructus | Poncirus trifoliate Rafin | 2 |
| Amomi Fructus | Amomum villosum Lour | 2 |
| Glycyrrhizae Radix | Glycyrrhiza uralensis Fisch. | 1 |
| Aucklandiae Radix | Aucklandia lappa Decne. | 1 |
All of the materials in CGX were identified by a herbalogy professor of Oriental Medicine Collage in Daejeon University. The commercially available CGX was manufactured by Kyoungbang Pharmacy (Incheon, South Korea) which company approved good manufacturing practice (GMP) from the Korea Food and Drug Administration, according to over-the-counter Korean monographs. Briefly, 120 kg of the 13 kinds of mixture was boiled in 1200 L distilled water for 4 h at 100 °C, filtered using a 300-mesh filter (50 μm), condensed, and lyophilised. The CGX extract satisfied the herb, heavy metals, general bacteria, fungi, and specific pathogens criteria, as determined by a confirmation test for each, and the final yield from the original dried mixture was 10.1% (w/w). Lyophilised CGX extract (100 mg) was dissolved in 50% and 90% methanol (20 mL for qualification analysis and quantitative analysis) and mixed. The solution was filtered through an Acrodisc® LC 13-mm syringe filter (0.45-μm pore size; Ann Arbor, MI, United States).
Quantitative analysis of CGX using high-performance liquid chromatography
For reproducibility of CGX, quantitative analysis was performed with nine of reference compounds solutions; scopoletin, liquiritin, naringin, esculetin, rosmarinic acid, salvianolic acid B, poncirin, glycyrrhizin, and tanshinone IIA (200 μg/mL) were prepared in 90% methanol and stored at < 4 °C. The standard solutions were prepared by six concentrations of diluted solutions (methanol).
All calibration curves of each chemical compound were attained by assessing the peak areas at six concentrations in the range of 3.13-100 μg/mL for all reference compounds. The linearity of the peak area (y) vs concentration (x, μg/mL) curve for each component was used to calculate the contents of the main CGX components.
Quantitative analysis was performed under identical conditions using an 1100 series high-performance liquid chromatography (HPLC) device (Agilent Technologies, Santa Clara, CA, United States) equipped with an autosampler (G11313A), column oven (GA1316A), binary pump (G1312), diode-array detector, and degasser (GA1379A). The analytical column with a Gemini C18 (4.6 mm × 250 mm; particle size, 5 μm; Phenomenex, Torrance, CA, United States) was kept at 30 °C during the analysis. Data were acquired and processed using ChemStation software (Agilent Technologies). The mobile phase conditions contained 10% acetonitrile in water with 0.05% formic acid (A) and 90% acetonitrile in water (B). The gradient flow was as follows; 0-30 min, 0%-20% B; 30-50 min, 20%-75% B; and 50-60 min, 75%-100%. The analysis was operated at a flow rate of 1.0 mL/min and detected at 280 nm. The injection volume was 10 μL.
Reagents and chemicals
1,1,3,3-tetraethoxypropane (TEP), acetaldehyde, chloramine T, collagen type I, and trichloroacetic acid (TCA) were purchased from Sigma (St. Louis, MO, United States); perchloric acid was obtained from GFS Chemical Co. (Columbus, OH, United States); and thiobarbituric acid (TBA) was purchased from Lancaster Co. (Lancashire, United Kingdom). Histofine was from Nichirei Biosciences (Tokyo, Japan); hydrochloric acid and phosphoric acid were from Kanto Chemical Co., Inc. (Tokyo, Japan); n-butanol was purchased from J.T. Baker (Center Valley, PA, United States); Mayer’s haematoxylin and isopropanol were obtained from Wako Pure Chemical Industries (Osaka, Japan); TRI reagent was obtained from Invitrogen (Carlsbad, CA, United States); and goat anti-human CTGF antibody, CTFG standard solution, rabbit anti-human CTGF antibody, and anti-rabbit immunoglobulin G horseradish peroxidase conjugate were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States).
Animals and experimental design
Thirty-nine specific pathogen-free male Sprague-Dawley rats (6-wk-old, 190-210 g) were purchased from Koatech (Gyeonggi-do, Rep. of Korea). After 7 d of acclimation to an environmentally controlled room at 22 °C ± 2 °C under a 12/12-h light/dark cycle with pellet food (Koatech) and tap water provided ad libitum, the rats were divided randomly into five groups of six to nine animals each: normal (n = 6, distilled water), control (n = 9, alcohol with distilled water), alcohol with 100 mg/kg CGX (n = 9), alcohol with 200 mg/kg CGX (n = 9), and CGX alone (n = 6, 200 mg/kg CGX) groups. Chronic hepatic injury and hepatofibrosis were induced by oral administration of 30% ethanol solution (10 mL/kg) for 4 wk (six times weekly), with the exception of the normal and CGX-only groups. All animals given 30% alcohol were administrated distilled water or CGX (100 or 200 mg/kg) with gastric gavages 6 h before alcohol consumption.
Body weight was recorded twice weekly, and whole blood was collected from the abdominal common artery under ether anaesthesia at the end of the experiment. The liver and spleen of each rat were removed and weighed, and liver tissues were then fixed in 10% formalin solution or stored in RNAlater (Ambion, Austin, TX, United States) or at -70 °C for the examination of histomorphology, RNA expression, or biochemical parameters, respectively. Experiments were designed and performed in strict accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 1985) and approved by the Institutional Animal Care and Use Committee of Daejeon University (animal ethical clearance No. DJUARB 2011-035).
Serum biochemical analysis
Whole blood was collected from the abdominal aorta under the ether anaesthesia condition on the final day of the experiment after a 12-h fast. Serum was separated by centrifugation (3000 g, 15 min) following blood clotting. The serum levels of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were determined with an auto chemistry analyser (Chiron, Emeryville, CA, United States).
Histopathological analysis
For histopathological evaluation, a portion of liver tissue in 10% formalin solution was re-fixed in Bouin’s solution. The paraffin-embedded liver tissue was sectioned (5-μm thickness) for haematoxylin and eosin (HE) and Masson’s trichrome staining. Characteristic histopathological features, such as hepatocyte destructions and fibrosis, were examined under microscopy (IX71; Olympus, Tokyo, Japan).
Determination of hydroxyproline in liver tissues
Hydroxyproline determination was performed using a slight modification of the previous method[16]. Briefly, liver tissues (200 mg) which were stored at -70 °C were homogenized in 2 mL 6 N of hydrogen chloride, and incubated overnight at 110 °C. After filtering the acid hydrolysates using filter paper (Toyo Roshi Kaisha, Tokyo, Japan), the 50 μL of hydrolysis samples or hydroxyproline standards were incubated at 50 °C until perfectly dried. The dried samples were dissolved with same volumes of absolute methanol, and then added 1.2 mL of 50% isopropanol and 200 μL of chloramine-T solution to each sample, followed by incubation at room temperature for 10 min. After 10 min of incubation, the Ehrlich’s solution (1.3 mL) was added to each samples mixture and incubated at 50 °C for 90 min. The optical density of the reaction product was read at 558 nm using a spectrophotometer (Cary 50; Varian, Palo Alto, CA, United States). A standard curve was constructed using serial dilutions of 1.0 mg/mL solutions of hydroxyproline.
Determination of lipid peroxidation in liver tissue and serum
Lipid peroxidation levels in liver tissue and serum were determined by measuring malondialdehyde (MDA), an end product of lipid peroxidation, using the thiobarbituric acid-reactive substances (TBARS) method as described previously[17,18]. The concentration of TBARS was expressed as μmol/g tissue or μmol in serum, using TEP as a standard. MDA levels in liver tissue were determined as follows. Briefly, 0.15 g liver tissue was homogenised in 1.5 mL ice-cold 1.15% potassium chloride buffer, and 130 μL homogenate was mixed 80 μL 1% phosphoric acid. The mixture was incubated for 45 min at 100 °C after added to 260 μL 0.67% TBA solution. The serum levels of MDA were determined with another method contrary to tissue MDA as follows. Fifty microlitres of serum samples or standard solutions were mixed with 500 μL 20% TCA and 200 μL 0.67% TBA, and incubated for 30 min at 100 °C. After incubation, the mixture was cooled on ice with vigorous vortexing with 1.03 mL n-butanol. After centrifugation of each mixture at 3000 g for 15 min, the absorbance of the upper organic layer was measured at 520 and 535 nm with a spectrophotometer (Cary 50; Varian) and compared with the value from freshly prepared TEP as a standard.
Determination of cytokines in liver tissue and HSC-T6 cells
Levels of TGF-β1, PDGF-BB, and interferon-gamma (IFN-γ) in liver tissue were measured using commercial enzyme-linked immunosorbent assay kits (BioSource, San Jose, CA, United States; RD Systems, Minneapolis, MN, United States). CTGF levels were measured manually, as we described previously with simply modification[14]. The units were picomoles or nanomoles per mg protein, and protein concentrations were determined using a bicinchoninic acid protein assay kit (Sigma). In addition, the levels of the above-mentioned cytokines were measured in culture media of HSC-T6 cells. Briefly, HSC-T6 cells (2 × 106 cells) were seeded in six-well plates with 2 mL Dulbecco’s modified eagle medium (DMEM) with 10% foetal bovine serum (FBS) and incubated overnight. After 24 h of incubation at 37 °C and 5% CO2, the cell culture media were changed to serum-free DMEM and the cells were pre-treated with CGX (100 μg/mL) for 2 h, followed by treatment with 100 μmol/L acetaldehyde for 24 h. The culture media were used for measurement of TGF-β1, PDGF-BB, and CTGF according to the above mentioned.
Real-time polymerase chain reactions in tissues and HSC-T6 cells
Total 17 kinds of genes were analysis for the mRNA expression levels in liver tissue samples or HSC-T6 cells. Total RNA was isolated from liver tissue samples using TRIzol reagent (Molecular Research Center, Cincinnati, OH, United States). cDNA was then synthesized from total RNA (2 μg) in a 20-μL reaction using a high-capacity cDNA reverse transcription kit (Ambion). The primers for alpha-smooth muscle actin (α-SMA), collagen type 1 alpha 1 (ColT1a1), collagen type 1 alpha 2 (ColT1a2), TGF-β1, PDGF-β, CTGF, tissue inhibitor of metalloproteinases (TIMP)-1, TIMP-2, matrix metalloproteinase (MMP)-2, MMP-9, IFN-γ, toll-like receptor-4 (TLR-4), chemokine (C-C motif) ligand 5 (CCL 5), monocyte chemotactic protein-1 (MCP-1), acetaldehyde dehydrogenase (ALDH), CYP2E1, and β-actin were as follows (forward and reverse, respectively): TGF-β, AGG AGA CGG AAT ACA GGG CTT T and AGC AGG AAG GGT CGG TTC AT; PDGF-β, ACC ACT CCA TCC GCT CCT TT and TGT GCT CGG GTC ATG TTCA A; CTGF, CAG TTG GCT CGC ATC ATA GTT G and GTG TGT GAT GAG CCC AAG GA; IFN-γ, TGC TCA TGA ATG CAT CCT TTT T and GAA AGA CAA CCA GGC CAT CAG; α-SMA, AAC ACG GCA TCA TCA CCA ACT and TTT CTC CCG GTT GGC CTT A; ColT1a1, CCC AGC GGT GGT TAT GAC TT and GCT GCG GAT GTT CTC AAT CTG; ColT1a2, CCC AGA GTG GAA GAG CGA TTA and GCT GCG GAT GTT CTC AAT CTG; TIMP-1, CTC CTC GCT GCG GTT CTG and CGA CGC TGT GGG AAA TGC; TIMP-2, GTC CAT CCA GAG GCA CTC ATC and CCC AGA AGA AGA GCC TAA ACC A; MMP-2, TGT GGC AGC CCA TGA GTT C and TCG GAA GTT CTT GGT GTA GGT GTA; MMP-9, TCG AGG GAC GCT CCT ATT TGT and CCA TAT TTT CTG TCT GTG TCG TAG TCA; ALDH; GAG TCT TCT ACC ATC AAG GCC AAT and GCT CGC TCA ACA CTC TTT CTC A; CYP2E1, AGA AGG AAA AAC ACA GCC AAG AA and GTT GTG CTG GTG TCA GTT C; CCL 5, AGG AGT ATT TTT ACA CCA GCA GCA A and CTT CTC TGG GTT GGC ACA CA; MCP-1, CCA CTC ACC TGC TGC TAC TCA T and CTG CTG CTG GTG ATT CTC TTG T; TLR-4, GAG GCA GCA GGT CGA ATT GTA and AAC AGG GCT TTT TTG AGT CTT CTC; and β-actin, CTA AGG CCA ACC GTG AAA AGA T and GAC CAG AGG CAT ACA GGG ACA A. Reactions were performed with 8 μL iQ SYBR Green Supermix, 1 μL 10 pM primer pair, 8.5 μL distilled water, and 2.5 μL cDNA. Each polymerase chain reaction was performed under the following conditions: 95 °C for 5 min followed by 40 cycles of 95 °C for 1 min, 58 °C for 40 s and 72 °C for 40 s, followed by a single fluorescence measurement.
To determine mRNA expressions in HSC-T6 cells, the cells (2 × 106 cells) were seeded in six-well plates with 2 mL DMEM with 10% FBS and incubated overnight at 37 °C and 5% CO2, after which the cell culture media were changed to serum-free DMEM. Next, various concentrations of CGX (25, 50, or 100 μg/mL) were added to the wells. After 2 h of incubation with CGX, the 100 μmol/L acetaldehyde was treated for 24 h, and total RNA was extracted using TRIzol reagent. The cDNA synthesis and gene expression experiment in cells were according to the same method of liver tissues. For analysis of data, the gene expression levels were compared with those of β-actin as a reference gene.
Quantification of intracellular collagen levels in HSC-T6 cells
To evaluate the effect of CGX on the production of collagen in HSC-T6 cells, the collagen content was determined according to a previous method, with slight modification[19]. Briefly, HSC-T6 cells (5 × 106 cells) were seeded onto 60-mm dishes with 3 mL DMEM with 10% FBS and incubated overnight at 37 °C and 5% CO2, and the cell culture media were then changed to serum-free DMEM. After a 2-h pre-treatment with various concentrations of CGX (25, 50, or 100 μg/mmol/L), the cells were added to 100 mmol/L acetaldehyde. After 48 h of incubation, the cells were harvested using 500 μL protease inhibitor cocktail (10 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L n-ethylmaleimide, and 1 mmol/L phenylmethylsulfonyl fluoride in 10 mmol/L phosphate-buffered saline) with an ice scraper. Total protein was obtained by vigorously vortexing (three 30-s iterations separated by 15-s intervals on ice). The tubes were submitted to collagen precipitation reaction with 25% saturated ammonium sulphate for 24 h at 4 °C. The collagen was isolated by centrifugation (24000 g, 1 h, 4 °C), the supernatants were discarded from the tubes, and the pellet was dissolved in 2 mL 0.5 mol/L acetic acid. One hundred microlitres of collagen aliquots were transferred to the 1.8-mL tube and added to 1 mL Sirius Red dye solution (50 μmol/L dye solution in 0.5 mol/L acetic acid). This was followed by vortexing, placing the tube at room temperature for 30 min, and then centrifuging at 24000 g for 40 min. The pellet was eluted with 1 mL 0.1 N potassium hydroxide. Next, absorbance of the sample with type 1 collagen as a standard was determined at 540 nm using a spectrophotometer (Soft Max 5.1; Molecular Devices, Sunnyvale, CA, United States).
Statistical analysis
The results are expressed as means ± SD. The statistical significance of differences among groups was determined by one-way analysis of variance, followed by Student’s unpaired t-test. In all analyses, P < 0.05, P < 0.01, or P < 0.001 was taken to indicate statistical significance.
RESULTS
Fingerprint analysis of CGX
The major nine of reference compounds were matched with components of CGX. In the quantitative analysis, the standard curves for the nine compounds which were containing scopoletin, liquiritin, naringin, esculetin, rosmarinic acid, salvianolic acid B, poncirin, glycyrrhizin, and tanshinone IIA were y = 11.887x - 8.247 (R2 = 0.999), y = 16.447x - 11.451 (R2 = 0.999), y = 15.211x - 11.069 (R2 = 0.999), y = 11.713x - 9.079 (R2 = 0.999), y = 17.497x - 16.149 (R2 = 0.999), y = 7.937x - 16.028 (R2 = 0.998), y = 17.155x - 8.843 (R2 = 0.999), y = 0.728x - 0.245 (R2 = 0.991), and y = 29.448x - 21.802 (R2 = 0.999), respectively. CGX and its standard mixtures analysis by HPLC were performed with detection at 280 nm (Figure 1A and B). The retention times of each components was as follows; scopoletin for 22.13 min, liquiritin for 23.16 min, naringin for 28.30 min, esculetin for 31.21 min, rosmarinic acid for 32.05 min, salvianolic acid B for 37.63 min, poncirin for 38.22 min, glycyrrhizin for 46.38 min, and tanshinone IIA for 59.12 min. The contents of components were in the range of 1.823-228.79 μg/g (Figure 1A-C).
Figure 1.
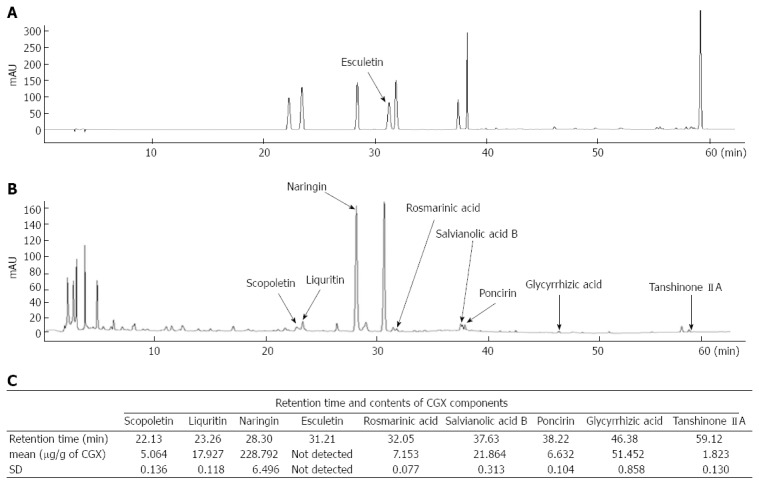
Fingerprint analysis of chunggan extract. Chunggan extract (CGX) and its main compounds were subjected to high-performance liquid chromatography (HPLC). Histograms of the reference compounds mixture (A) and the CGX sample using HPLC (B) was constructed, and quantitative analysis was performed for each reference compounds in CGX (C).
Effects on body weight and relative organ weight
Four weeks of alcohol consumption notably inhibited body weight gain compared with the normal group (P < 0.05), and CGX treatment had no significant effect on body weights (Table 2). No remarkable difference among groups was observed in the absolute or relative change in spleen or liver weight.
Table 2.
Body weight, organ weights, serum biochemistry parameters and oxidative stress parameters
| Groups | Normal | Alcohol |
Alcohol with CGX treatment |
CGX 200 | |
| 100 | 200 | ||||
| Body mass (g) | 347.3 ± 15.6 | 325.4 ± 20.4a | 327.8 ± 24.6 | 329.5 ± 16.0 | 332.3 ± 16.5 |
| Liver mass (g) | 10.2 ± 0.7 | 10.1 ± 1.5 | 9.3 ± 1.2 | 9.6 ± 0.7 | 9.2 ± 0.6 |
| Spleen mass (g) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Liver mass (%) | 2.9 ± 0.1 | 3.1 ± 0.5 | 2.8 ± 0.3 | 2.9 ± 0.1 | 2.8 ± 0.1 |
| Spleen mass (%) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| AST (IU/L) | 119.7 ± 18.1 | 226.3 ± 120.2a | 125.1 ± 18.1c | 105.3 ± 9.8c | 106.7 ± 5.2 |
| ALT (IU/L) | 45.8 ± 2.5 | 91.0 ± 39.5a | 70.3 ± 10.9c | 54.2 ± 6.5c | 45.7 ± 7.7 |
| ALP (IU/L) | 203.0 ± 29.7 | 269.9 ± 111.2 | 190.7 ± 33.4 | 174.9 ± 17.3 | 162.8 ± 20.0a |
| LDH (IU/L) | 1406.4 ± 68.3 | 1646.1 ± 224.3a | 1338.0 ± 444.6 | 1009.6 ± 164.1d | 925.2 ± 115.2a |
Rats were orally administered with alcohol (10 mL of 30% ethanol/kg) with/without CGX (100 or 200 mg/kg) for 4 wk. Body weight were recorded twice weekly during experiment. At the end of the experiment, whole blood was collected and liver and spleen were removed. Data were expressed as mean ± SD (n = 6-9);
P < 0.05 vs normal group;
P < 0.05 vs alcohol group;
P < 0.01 vs alcohol group. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; LDH: Lactic dehydrogenase.
Effects on changes in serum biochemical parameters
Four weeks of alcohol consumption considerably elevated serum AST, ALT, ALP, and LDH levels by 1.7-, 2.0-, 1.3- and 1.2-fold, respectively. These elevations were significantly ameliorated by CGX treatment (AST, P < 0.05 for 100 and 200 mg/kg; ALT, P < 0.05 for 100 and 200 mg/kg; LDH, P < 0.001 for 200 mg/kg, respectively). Serum levels of ALP and LDH were significantly lower in the CGX-only group than in the normal group (P < 0.05; Table 2).
Histopathological analysis
Four weeks of alcohol consumption induced minor hepatocyte destructions. CGX administration ameliorated these alterations, as demonstrated by HE staining (Figure 2A). In addition, slight fibrotic changes around the hepatic central vein were observed in the control group treated with alcohol, and CGX administration (100 and 200 mg/kg) attenuated this histological change, as demonstrated by Masson’s trichrome staining (Figure 2B).
Figure 2.
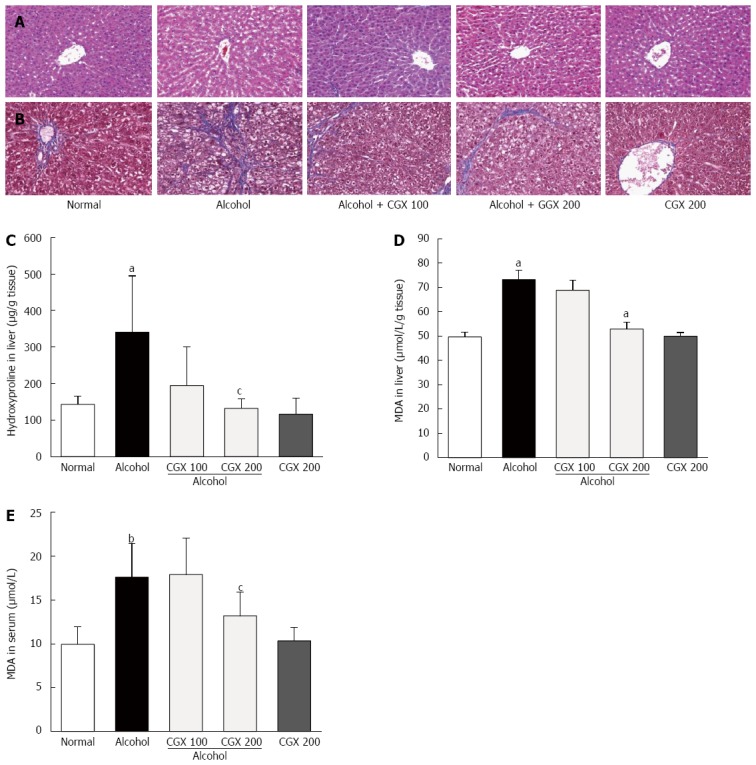
Histopathological examinations and contents of hydroxyproline and malondialdehyde. Rats were orally administered 30% alcohol (10 mL/kg) with or without Chunggan extract (CGX; 100 or 200 mg/kg) for 4 wk. The removed liver tissues were examined using haematoxylin and eosin (A) and Masson’s trichrome (B) staining under an optical microscope (× 200 magnifications). Hydroxyproline (C) and malondialdehyde (MDA) (D) contents in the liver tissue and serum MDA concentrations (E) were measured. Data are expressed as means ± SD (n = 6-9). aP < 0.05 vs normal group, bP < 0.01 vs normal group, cP < 0.05 vs control group.
Effects on hydroxyproline and MDA contents in liver tissue
Four weeks of alcohol consumption increased hepatic hydroxyproline content 2.4-fold compared with the normal group, and CGX treatment significantly ameliorated this increase compared with the control group (P < 0.05 for 200 mg/kg; Figure 2C). Hepatic and serum MDA levels were notably elevated after four weeks of alcohol consumption by 1.5- and 1.8-fold, respectively, compared with those in the normal group. CGX treatment significantly reduced these increased MDA levels compared with the control group (P < 0.05 for 200 mg/kg; Figure 2D and E).
Effects on pro-fibrogenic cytokines and IFN-γ in liver tissue
Alcohol treatment notably increased TGF-β1 and PDGF-BB levels in hepatic tissue compared with the normal group. CGX treatment significantly attenuated the elevation of TGF-β (P < 0.05 for 200 mg/kg) and PDGF-BB (P < 0.05 for 100 and 200 mg/kg) levels. The CTGF level was increased slightly by alcohol administration and normalised by CGX treatment, but this effect was not significant. Four weeks of alcohol consumption remarkably decreased the IFN-γ level in hepatic tissue, whereas CGX treatment significantly recovered this level compared with the control group (P < 0.05 for 200 mg/kg; Figure 3A).
Figure 3.
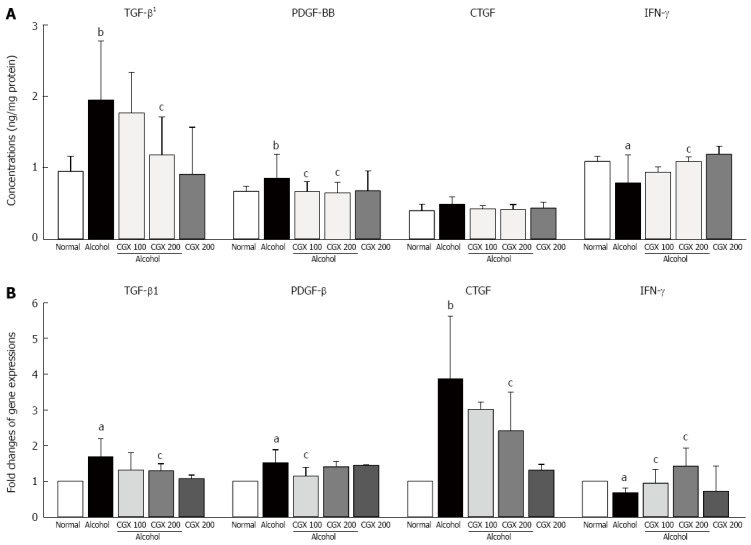
Changes in hepatofibrosis-associated cytokines in protein and gene expression. Rats were orally administered 30% alcohol (10 mL/kg) with or without Chunggan extract (CGX; 100 or 200 mg/kg) for 4 wk. The levels of transforming growth factor-beta (TGF-β), platelet-derived growth factor-BB (PDGF-BB), connective tissue growth factor (CTGF), and interferon-gamma (IFN-γ) were determined in liver homogenates by enzyme-linked immunosorbent assays (A), and their gene expressions were determined by real-time polymerase chain reactions (B). Data are expressed as means ± SD (n = 6-9). aP < 0.05 vs normal group, bP < 0.01 vs normal group, cP < 0.05 vs control group, dP < 0.01 vs control group. 1Only TGF-β was expressed as ng/100 μg protein. Others were according to the mg/protein, but TGF-β1 was only according to the 100 μg/mg protein.
Effects on mRNA expressions in liver tissue
Four weeks of alcohol consumption caused remarkable up-regulation of TGF-β1, PDGF-BB and CTGF gene expressions, but down-regulated IFN-γ in gene expression. These alterations in gene expressions were significantly ameliorated by CGX treatment (P < 0.05 for 100 or 200 mg/kg; Figure 3B). Alcohol consumption significantly up-regulated the gene expressions of α-SMA, ColT1a1, ColT1a2, TIMP-1, TIMP-2, CYP2E1, TLR-4, CCL 5, and MCP-1, and CGX administration significantly attenuated these changes, with the exception of CCL 5. On the other hand, the gene expression of ALDH was notably down-regulated by alcohol consumption, and CGX administration normalised this alteration (Table 3).
Table 3.
Relative gene expressions in hepatic tissues
| Groups | Normal | Alcohol only |
Alcohol with CGX treatment |
CGX 200 only | |
| 100 | 200 | ||||
| α-SMA | 1.00 | 1.26 ± 0.51a | 1.26 ± 0.42 | 0.97 ± 0.18c | 1.19 ± 0.18 |
| ColT1a1 | 1.00 | 1.72 ± 0.08b | 1.18 ± 0.14 | 1.01 ± 0.01d | 0.94 ± 0.53 |
| ColT1a2 | 1.00 | 1.63 ± 0.80 | 0.81 ± 0.10d | 0.74 ± 0.25d | 1.01 ± 0.41 |
| TIMP-1 | 1.00 | 1.64 ± 0.11b | 0.78 ± 0.04d | 0.79 ± 0.02d | 1.16 ± 0.06 |
| TIMP-2 | 1.00 | 1.72 ± 0.18b | 0.92 ± 0.03d | 0.92 ± 0.12d | 1.43 ± 0.14b |
| MMP-2 | 1.00 | 0.84 ± 0.63 | 1.23 ± 0.30c | 1.27 ± 0.17c | 1.27 ± 0.48 |
| MMP-9 | 1.00 | 0.72 ± 0.34 | 1.00 ± 0.81 | 1.10 ± 0.16c | 1.04 ± 0.78 |
| CYP2E1 | 1.00 | 1.33 ± 0.04b | 0.87 ± 0.03d | 0.84 ± 0.07d | 0.90 ± 0.14 |
| ALDH | 1.00 | 0.73 ± 0.18a | 0.81 ± 0.27 | 0.83 ± 0.13 | 1.13 ± 0.38 |
| TLR-4 | 1.00 | 1.44 ± 0.27a | 1.22 ± 0.08d | 0.95 ± 0.24c | 1.06 ± 0.04 |
| CCL 5 | 1.00 | 1.17 ± 0.07b | 1.06 ± 0.35 | 0.74 ± 0.31 | 1.08 ± 0.06 |
| MCP-1 | 1.00 | 1.48 ± 0.21b | 1.26 ± 0.10d | 1.50 ± 0.13 | 1.63 ± 0.09 |
Rats were orally administered with alcohol (10 mL of 30% ethanol/kg) with/without CGX (100 or 200 mg/kg) for 4 wk. At the end of the experiment, liver tissues were collected for mRNA expressions by real-time PCR. Data were expressed as mean of fold changes ± SD (n = 4)
P < 0.05,
P < 0.01 vs normal group,
P < 0.05,
P < 0.01 vs alcohol group. ALDH: Acetaldehydedehydrogenas; CCL 5: Chemokine (C-C motif) ligand-5; ColT1a1: Collagen type 1a1; ColT1a2: Collagen type 1a2; CYP2E1: cytochrome p450 2E1; MCP-1: Monocyte chemotactic protein-1; MMP-2: Matrix metalloproteinase-2; MMP-9: Matrix metalloproteinase-9; TIMP-1: Tissue inhibitor of metalloproteinases-1; TIMP-2: Tissue inhibitor of metalloproteinases-2; α-SMA: alpha-smooth muscle actin.
Effects on collagen production and cytokines in HSC-T6 cell
Intracellular collagen contents were approximately 1.4-fold higher in T6 cells compared with those not treated with acetaldehyde, and pre-treatment with CGX (100 μg/mL) significantly inhibited the production of collagen at the intracellular level (P < 0.05, Figure 4A). Acetaldehyde treatment remarkably elevated pro-fibrogenic cytokines by 3.1-, 1.1-, and 2.1-fold for TGF-β, PDGF-BB, and CTGF, respectively, compared with non-treatment with acetaldehyde in T6-cell culture medium (P < 0.05 for 100 μg/mL in TGF-β and CTGF; P < 0.01 for 100 μg/mL in PDGF-BB, respectively; Figure 4A). The mRNA expression levels of ColT1a1, TGF-β1, PDGF-β, and CTGF were remarkably up-regulated by 1.2- 1.3-, 1.4-, and 1.7-fold, respectively, due to acetaldehyde stimulation in T6 cells, and CGX efficiently down-regulated these abnormal changes (P < 0.05 for 50 μg/mL in PDGF-β and 100 μg/mL in ColT1a1; P < 0.01 for 50 μg/mL in TGF-β1 and 100 μg/mL in TGF-β1, PDGF-β, and CTGF, respectively; Figure 4B).
Figure 4.
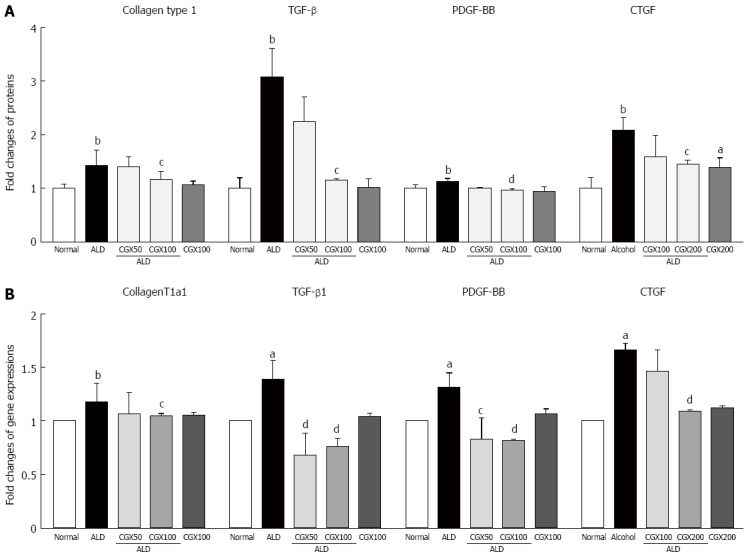
Effects of Chunggan extract on collagen production and pro-fibrogenic cytokines in T6 cells. HSC-T6 cells (2 × 106 cells) were pre-treated with CGX (50 or 100 μg/mL) 2 h before acetaldehyde (ALD) treatment (100 μmol/L) for 24 h. The intracellular collagen type 1 level was measured using Sidney’s method, and levels of transforming growth factor-beta (TGF-β), platelet-derived growth factor-BB (PDGF-BB), and connective tissue growth factor (CTGF) in media were determined using enzyme-linked immunosorbent assays (A). The gene expressions for collagen type 1a1, TGF-β, PDGF-β and CTGF were measured using real-time polymerase chain reactions (B). The results are expressed as the fold-change relative to the normal group. Data are expressed as means ± SD (n = 4). aP < 0.05 vs normal group; bP < 0.01 vs normal group; cP < 0.05 vs ALD-only group, dP < 0.01 vs ALD-only group.
DISCUSSION
Chronic alcohol consumption is one of the greatest concerns in hepatic injuries which can lead to steatohepatitis, hepatofibrosis, cirrhosis, and hepatocellular carcinoma. The liver is very vulnerable organ to alcohol abuse because it is the main organ to metabolize and detoxify it. Hepatic injury caused by alcohol abuse is detected in approximately 15% of all patients with hepatofibrosis[4].
Many of studies have focused on the treatment of alcohol-associated chronic liver injury, especially using herbal plants[20,21]. In current study, we purposed to investigate hepatoprotective and anti-fibrotic effects of CGX in a rat model of chronic alcohol consumption.
In our experiment, a 4-wk administration of 30% ethanol (10 mL/kg) induced typical characters of alcoholic liver injury, as evidenced by approximately 2-fold increases in biochemical parameters, including AST, ALT, ALP, and LDH (Table 2). These alterations were significantly ameliorated by CGX treatment. CGX treatment also resulted in improved histopathological findings with Masson’s trichrome staining, although 4 wk of 30% ethanol administration did not fully induce a fibrotic change (Figure 2A and B). This limitation in the fibrotic change achieved in oral ethanol administration models is well recognized[22-25]. However, hepatic hydroxyproline content was significantly increased by 2.4-fold in the control group compared with the normal group. Hydroxyproline is a major component of the protein collagen and a critical biomarker of fibrotic change[26]. As we expected, CGX treatment significantly normalised this change (Figure 2C). These results indicate the anti-hepatic injury action of CGX, especially focused on the amelioration of fibrotic changes in the liver tissue.
The liver fibrosis is a consequence of HSC activation that leads to the over-production of collagen and accumulation of ECM molecules. Four weeks of alcohol consumption activated HSCs, as evidenced by up-regulation of the gene expressions of α-SMA, ColT1a1, and ColT1a2 (Table 3). Oxidative stress is known to play a key role in the pathogenesis of alcohol-induced hepatic injuries, including fibrosis[27,28].
Continuous oxidative stress readily damages hepatocytes and accelerates the stimulation of HSC activation, leading to transformation into collagen-producing myofibroblasts[29]. The 4-wk alcohol treatment drastically increased MDA levels in serum and hepatic tissue, and CGX significantly reduced these alterations (Figure 2D and E). In our experiment, antioxidant components, including superoxide dismutase, catalases, and glutathione-redox enzymes, were depleted in the control group, and these distortions were notably attenuated by CGX treatment (data not shown).
In HSC activation and ECM production, pro-fibrogenic cytokines such as TGF-β, PDGF-β, and CTGF play pivotal roles. In the case of alcoholic liver injury, these three cytokines repeatedly inhibit the regeneration of hepatocytes[30]. TGF-β not only activates HSCs, but also positively affects the receptor expressions of PDGF-β and CTGF[31]. PDGF-β plays a critical role in the proliferation and activation of HSCs[32,33]. The hepatic tissue levels of TGF-β and PDGF-BB were considerably up-regulated in terms of protein and gene expressions by the 4-wk alcohol treatment, and CGX significantly normalized these alterations (Figure 3). CTGF is synthesized from hepatocytes or HSCs, and is up-regulated by TGF-β1 in hepatic injury due to alcohol consumption[34-36]. The up-regulation of CTGF gene expression by alcohol was more pronounced than were those of TGF-β1 and PDGF-β. CGX treatment significantly regulated this change in gene expression, but not in protein level (Figure 3).
IFN-γ is well known for the anti-fibrogenic cytokine that can inhibit HSC proliferation[30]. In our study, IFN-γ was notably down-regulated in terms of protein and gene expression levels, and these abnormalities were normalized by CGX treatment (Figure 3). Liver fibrosis is a very dynamic phenomenon resulting from an imbalance in ECM production and its degradation[37]. MMPs selectively degrade ECMs, whereas TIMPs act to inhibit MMP functions in hepatic fibrogenesis[38]. Alcohol consumption notably induced the up-regulation of TIMP-1 and TIMP-2, but the down-regulation of MMP-2 and MMP-9. These alterations in gene expression were significantly ameliorated by CGX treatment (Table 3).
Furthermore, we partially investigated the effects of CGX on alcohol metabolic enzymes, such as CYP2E1 and ALDH. As a detoxification enzyme, CYP2E1 contribute to generate ROS or CYP2E1 adducts, which acts as free radicals in case of excessive alcohol ingestion in liver tissue[5,39,40]. ALDH is a main enzyme that rapidly converts acetaldehyde, a typical free radical in the alcohol metabolism process, to non-toxic acetate[41]. Additionally, TLR-4, CCL 5, and MCP-1 are linked directly or indirectly to the pathogenesis of alcohol-induced hepatic injury and hepatic fibrosis.
TLR-4 plays a major role in chronic alcohol consumption-induced hepatic injury[42]. CCL 5 and MCP-1 are chemokines that act in the inflammatory response during hepatic injury[43]. Our results exhibited the effects of CGX on the regulation of the above-mentioned gene expressions, which were altered by alcohol consumption (Table 3).
To verify the pharmacological actions of CGX in this study, we adapted an in vitro model using rat-derived HSCs, a T6 cell line, under acetaldehyde-treated conditions. Acetaldehyde is known as the direct stimulation of HSCs activation[44]. Upon Stimulation with acetaldehyde to the T6 cells produced collagen type 1, TGF-β1, PDGF-β, and CTGF gene expression as well as protein levels. However, these overall alterations were significantly stabilized by CGX (Figure 4). These results demonstrate that the protective effect of CGX and its regulation of pro-fibrogenic cytokines both in in vivo and in vitro experiments.
In conclusion, our results strongly suggest that CGX efficiently affects alcohol consumption-induced hepatic injury through regulation of pro-fibrogenic cytokines as well as alcohol metabolism.
COMMENTS
Background
Alcohol abuse can cause severe liver injuries including alcoholic steatohepatitis as well as hepatofibrosis, even hepato cellular carcinoma via oxidative liver damage and fibrotic change. Until recent days, there is no therapeutic drug to care alcohol abuse-induce liver injury. Chunggan extract (CGX) has been developed to cure various liver diseases in clinical fields. Previous studies reported that CGX was potentially applied to prevent or treat various liver disease including chemical-induced acute and chronic liver fibrosis and NASH using animal models.
Research frontiers
CGX, which was developed from Traditional Chinese Medicine and it is composed of 13 different herbal materials and it has been used to treat patients with various liver diseases such as viral hepatitis, fatty liver or alcoholic liver disorder since 2001. Moreover, CGX was studied on the safety and toxicology studies using Rats and beagle dogs. In alcoholic liver injury, the research hotspot is how to modulate the pathological changes such as oxidative damages or fibrotic changes by CGX and to improve its effectiveness on preventing those alterations.
Innovations and breakthroughs
The study revealed the pharmacological properties of CGX mainly focused on the pro-fibrogenic cytokines as well as alcohol metabolism related molecules. Moreover, the corresponded mechanisms were observed in rat derived hepatic stellate cells (HSC) cell line using HSC-T6 cells.
Applications
There is no therapeutic way to treat or cure alcoholic liver injury. Therefore, it is important to develop a new drug to treat the alcoholic liver injury. Results in present study suggest that the CGX is a potential therapeutic material that could be used in preventing alcohol-induced hepatofibrotic change, oxidative damage and alcohol metabolism.
Terminology
CGX, which means “cleaning the liver” and has been used to treat various liver disorders, was invented according to the Traditional Chinese Medicine based theory, and it is composed of different 13 kinds of herbal materials. Pro-fibrogenic cytokines: The major composition of Pro-fibrogenic cytokines are growth factors including transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF)-BB and connective tissue growth factor (CTGF). During hepatofibrosis these cytokines were released from activated hepatostellate cells and leads to collagen accumulation in liver tissues; HSC: HSC is a kind of liver cells, which is responsible for formation of hepatofibrosis through the activation of HSC. When it is activated the myofibroblasts were formed via the release of pro-fibrogenic cytokines such as TGF-β, PDGF-BB and CTGF.
Peer review
The present manuscript is a good descriptive study in which authors analyze the anti-hepatofibrotic properties of CGX on 4 wk alcohol consumption induced hepatic injury in rat model. In this study, CGX significantly reduced liver enzymes in the serum level and also considerably ameliorated the abnormal values of pro-fibrogenic cytokines as well as oxidative stress damages in the liver tissues. Moreover, its pharmacological mechanisms of CGX were also observed in acetaldehyde-stimulated HSC-T6 cells.
Footnotes
Supported by Oriental Medicine Research and Development Project, Ministry of Health and Welfare, South Korea No. HI12C-1920-01001
P- Reviewer: Caboclo JLF, Koch TR, Wu SL S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Johnson D, Morrison DS. Opportunities for prevention of alcohol-related death in primary care: results from a population-based cross-sectional study. Alcohol. 2012;46:703–707. doi: 10.1016/j.alcohol.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 4.Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24:1151–1161. doi: 10.1111/j.1365-2036.2006.03110.x. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 7.Fallowfield JA. Therapeutic targets in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G709–G715. doi: 10.1152/ajpgi.00451.2010. [DOI] [PubMed] [Google Scholar]
- 8.Kawelke N, Vasel M, Sens C, Au Av, Dooley S, Nakchbandi IA. Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-β. PLoS One. 2011;6:e28181. doi: 10.1371/journal.pone.0028181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 10.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang JH, Batey RG, George J. Role of ethanol in the regulation of hepatic stellate cell function. World J Gastroenterol. 2006;12:6926–6932. doi: 10.3748/wjg.v12.i43.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi WJ, Shin JW, Son JY, Seo DS, Park HS, Han SH, Sung HJ, Cho JH, Cho CK, Yoo HS, et al. Toxicological study of the hepatotherapeutic herbal formula, chunggan extract, in beagle dogs. World J Gastroenterol. 2006;12:7497–7502. doi: 10.3748/wjg.v12.i46.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu XP, Shin JW, Wang JH, Cho JH, Son JY, Cho CK, Son CG. Antioxidative and hepatoprotective effect of CGX, an herbal medicine, against toxic acute injury in mice. J Ethnopharmacol. 2008;120:51–55. doi: 10.1016/j.jep.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Kwak KG, Wang JH, Shin JW, Lee DS, Son CG. A traditional formula, Chunggan extract, attenuates thioacetamide-induced hepatofibrosis via GSH system in rats. Hum Exp Toxicol. 2011;30:1322–1332. doi: 10.1177/0960327110389502. [DOI] [PubMed] [Google Scholar]
- 15.Shin JW, Son JY, Oh SM, Han SH, Wang JH, Cho JH, Cho CK, Yoo HS, Lee YW, Lee MM, et al. An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats. World J Gastroenterol. 2006;12:6142–6148. doi: 10.3748/wjg.v12.i38.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol. 2003;29:669–676. doi: 10.1165/rcmb.2002-0046OC. [DOI] [PubMed] [Google Scholar]
- 17.Kamal AA, Gomaa A, el Khafif M, Hammad AS. Plasma lipid peroxides among workers exposed to silica or asbestos dusts. Environ Res. 1989;49:173–180. doi: 10.1016/s0013-9351(89)80062-3. [DOI] [PubMed] [Google Scholar]
- 18.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 19.Keira SM, Ferreira LM, Gragnani A, Duarte IS, Barbosa J. Experimental model for collagen estimation in cell culture. Acta Circulica Brasileira. 2004;19:17–22. [Google Scholar]
- 20.Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh N, Ghosh R, Mandal V, Mandal SC. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol. 2011;49:970–988. doi: 10.3109/13880209.2011.558515. [DOI] [PubMed] [Google Scholar]
- 22.de la M Hall P, Lieber CS, DeCarli LM, French SW, Lindros KO, Järveläinen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res. 2001;25:254S–261S. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
- 23.Nanji AA, French SW. Animal models of alcoholic liver disease--focus on the intragastric feeding model. Alcohol Res Health. 2003;27:325–330. [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto H, French SW, Benson N, Delgado G, Rao GA, Larkin EC, Largman C. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985;5:224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–187. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolarin DM, Azinge EC. Biochemical markers, extracellular components in liver fibrosis and cirrhosis. Nig Q J Hosp Med. 2007;17:42–52. doi: 10.4314/nqjhm.v17i1.12541. [DOI] [PubMed] [Google Scholar]
- 27.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez-Valle V, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr Med Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 30.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 31.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–140. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 32.Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:d1720–d1726. doi: 10.2741/A875. [DOI] [PubMed] [Google Scholar]
- 33.Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, et al. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148:785–800. [PMC free article] [PubMed] [Google Scholar]
- 34.Ehnert S, Knobeloch D, Blankenstein A, Müller A, Böcker U, Gillen S, Friess H, Thasler WE, Dooley S, Nussler AK. Neohepatocytes from alcoholics and controls express hepatocyte markers and display reduced fibrogenic TGF-ß/Smad3 signaling: advantage for cell transplantation? Alcohol Clin Exp Res. 2010;34:708–718. doi: 10.1111/j.1530-0277.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 35.Hora C, Negro F, Leandro G, Oneta CM, Rubbia-Brandt L, Muellhaupt B, Helbling B, Malinverni R, Gonvers JJ, Dufour JF. Connective tissue growth factor, steatosis and fibrosis in patients with chronic hepatitis C. Liver Int. 2008;28:370–376. doi: 10.1111/j.1478-3231.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 36.Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 37.Minne JF, Bonneterre J. Prophylactic cranial irradiation for lung cancer patients. Int J Radiat Oncol Biol Phys. 1992;24:187. doi: 10.1016/0360-3016(92)91044-n. [DOI] [PubMed] [Google Scholar]
- 38.Roderfeld M, Hemmann S, Roeb E. Mechanisms of fibrinolysis in chronic liver injury (with special emphasis on MMPs and TIMPs) Z Gastroenterol. 2007;45:25–33. doi: 10.1055/s-2006-927388. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Ingelman-Sundberg M, Johansson I. Mechanisms of hydroxyl radical formation and ethanol oxidation by ethanol-inducible and other forms of rabbit liver microsomal cytochromes P-450. J Biol Chem. 1984;259:6447–6458. [PubMed] [Google Scholar]
- 41.Meier P, Seitz HK. Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care. 2008;11:21–26. doi: 10.1097/MCO.0b013e3282f30564. [DOI] [PubMed] [Google Scholar]
- 42.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H, Schmitz P, Streetz KL, Berg T, Gassler N, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–4140. doi: 10.1172/JCI41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegmund SV, Dooley S, Brenner DA. Molecular mechanisms of alcohol-induced hepatic fibrosis. Dig Dis. 2005;23:264–274. doi: 10.1159/000090174. [DOI] [PubMed] [Google Scholar]


