Abstract
AIM: To assess whether metformin, which has a chemopreventive effect in chronic liver disease, has any chemotherapeutic effect in hepatocellular carcinoma.
METHODS: This was a retrospective study of 701 patients with newly diagnosed hepatocellular carcinoma (HCC) seen between January 2005 and June 2011 at Mayo Clinic, Rochester, Minnesota. This patient cohort was a part of the global HCC BRIDGE study, which is a large longitudinal study of HCC determining the real-world experience of HCC characteristics, management and patient outcomes. We defined significant metformin exposure as continuation of this agent at least 90 d beyond diagnosis of HCC, and compared survival of diabetic patients on metformin to diabetic patients not on metformin and non-diabetics.
RESULTS: Our cohort was 72.9% male, with a mean ± SD age of 62.6 ± 12.3 years. The most common etiologies of liver disease were hepatitis C (34%), alcoholic liver disease (29%), fatty liver disease (15%) and hepatitis B (9%). By univariate analysis, using diabetics not on metformin as the reference group, diabetic patients with HCC on metformin had no survival advantage, with a HR (95%CI) of 1.0 (0.8-1.3). Non-diabetic HCC patients also did not appear to have a survival advantage as compared to diabetic HCC patients not on metformin, as demonstrated by a HR (95%CI) of 1.1 (0.7-1.7). Diabetics on metformin beyond 90 d after HCC diagnosis had a longer median survival at 34.2 mo, as compared to 25.5 mo among diabetic patients who were not on metformin or had discontinued metformin within 90 d after HCC diagnosis. This finding was likely due to potential survival bias among those who lived long enough to receive metformin.
CONCLUSION: Although the literature suggests a chemotherapeutic effect in other malignancies, our study demonstrates no survival benefit to the use of metformin in diabetic patients with HCC.
Keywords: Hepatocellular carcinoma, Metformin, Diabetes, Survival, Liver disease, Lactic acidosis
Core tip: Metformin has been shown to prevent the development of hepatocellular carcinoma (HCC) among patients with diabetes and chronic liver disease in retrospective studies. This agent results in inhibition of the mTOR pathway, integral to many malignancies. We investigated the role of metformin as a chemotherapeutic agent in HCC, by assessing whether its use in patients newly diagnosed with this cancer had improved survival as compared to diabetics on other hypoglycemic agents and those without diabetes. Our analysis clearly reveals that there is no overall survival benefit in using metformin for those patients newly diagnosed with HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) arises in the context of chronic liver disease and has been rising in incidence across North America, as the number of people with hepatitis C cirrhosis and non-alcoholic steatohepatitis-induced cirrhosis increases[1]. It is often diagnosed at an advanced stage, when curative treatments such as resection or liver transplant are no longer feasible, resulting in a poor overall 5-year survival rate of less than 15%[2]. In fact, HCC is the most rapidly rising cause of cancer-related deaths among men in the United States[3]. The only chemotherapeutic option that has been shown to modestly increase survival in advanced HCC is sorafenib, a Ras-Raf kinase and vascular endothelial growth factor (VEGF) inhibitor[4]. Given that HCC develops through the upregulation of various pathways including the mammalian target of rapamycin (mTOR) pathway, mTOR inhibitors are currently being investigated as chemotherapy for HCC[5]. Growth factors such as insulin-like growth factor (IGF-1) stimulate the mTOR pathway, a survival pathway whose upregulation has been detected in up to 50% of HCC tumors[6]. This stimulation of the mTOR pathway leads to selectively increased translation of mRNAs key to fuelling tumor development and progression. The mTOR pathway as a pro-survival pathway is particularly affected by cellular energetics. AMP-activated protein kinase (AMPK), an intracellular sensor serving to maintain energy balance, is stimulated by increased energy consumption as reflected by an elevated AMP/ATP ratio. AMPK activation in turn inhibits the mTOR pathway and anabolic processes, including the energy-consuming process of protein synthesis.
Metformin, a biguanide medication used commonly in diabetics, is known to inhibit the mTOR pathway through AMPK activation. Metformin has been shown to inhibit tumor growth in vitro and in vivo by inducing apoptosis in various malignancies including breast[7], lung[8] and melanoma[9]. A greater effect on HCC could be anticipated, given that the organic cation transporter 1 (OCT1) is most highly expressed in hepatocytes, enabling increased uptake of metformin in the liver[10]. Retrospective studies have suggested that metformin prevents development of HCC among patients with diabetes[11] and diabetic patients with chronic liver disease[12]. The former large population-based study by Chen et al[11] demonstrated a dose-dependent decrease in the risk of HCC among diabetic patients. A recent meta-analysis further confirmed a 50% decreased HCC incidence among diabetics on metformin[13]. This hypoglycemic agent has been shown to have a potent tumor suppressive effect in various malignancies, through AMPK activation and subsequent inhibition of the mTOR pathway. The observed activation of the mTOR pathway in about 50% of HCCs makes mTOR inhibition relevant to their treatment[3]. Based on the above convincing literature for a chemopreventive effect in HCC and in vivo animal data on a chemotherapeutic effect of metformin on various malignancies including HCC, the goal of our study was to assess whether metformin might have a chemotherapeutic effect in patients newly-diagnosed with HCC.
We performed a retrospective study at Mayo Clinic to investigate two clinical questions: firstly, whether metformin had a significant effect on survival of patients newly-diagnosed with HCC, and secondly, whether it was safe for patients with HCC developing in the context of cirrhosis to continue on metformin.
MATERIALS AND METHODS
This study was comprised of 701 patients aged ≥ 18 newly diagnosed with HCC between January 2005 and June 2011. This patient cohort was a part of the global HCC BRIDGE study, which is a large longitudinal cohort study of HCC determining the real-world experience of HCC characteristics, management and patient outcomes. The diagnosis of HCC was made by histopathology or noninvasive criteria according to the American Association for the Study of Liver Disease (AASLD) or European Association for the Study of the Liver (EASL) guidelines. Data were collected retrospectively and prospectively as recorded in the medical record into the BRIDGE database. Information on metformin was additionally abstracted from the medical record. Significant metformin exposure was defined as intake of this medication at the time of HCC diagnosis, and continuation beyond 90 d following diagnosis. The study was approved by the Mayo Clinic Rochester Institutional Review Board.
Statistical analysis
We separated the HCC patients into the following categories: non-diabetics, diabetics not on metformin or who discontinued metformin within 90 d of HCC diagnosis, and diabetics who continued metformin beyond 90 d after HCC diagnosis. Follow-up was censored on January 1, 2013. Death was the primary endpoint. Median survival, defined as the time from day 91 after the first diagnosis date to the date of last follow-up or death, was estimated using the Kaplan-Meier method and compared using the log-rank test. Patients who were lost to follow-up or died within 90 d after HCC diagnosis were excluded from the analysis. The association between age, gender, etiology of chronic liver disease, the Barcelona-Clınic Liver Cancer (BCLC) stage, diabetes or metformin use and risk of death was determined by HR and 95%CI calculated by Cox-proportional hazards regression. Variables with a P value of < 0.05 were included in a multivariate model. Gender was also included in the multivariate model as a potential confounder. A P value of < 0.05 was considered statistically significant.
RESULTS
Our cohort was 72.9% male, with a mean ± SD age of 62.6 ± 12.3 years. The most common etiologies of liver disease were hepatitis C (34%), alcoholic liver disease (29%), fatty liver disease (15%) and hepatitis B (9%). The BCLC stage distribution at the time of diagnosis is shown in Table 1.
Table 1.
Baseline characteristics of patients in the study
| Variables | Non-diabetic (n = 438) | Diabetics not on metformin (n = 207) | Diabetics on metformin (n = 56) | P value |
| Age, yr | ||||
| Mean ± SD | 61.4 ± 13.3 | 64.7 ± 10.3 | 64.4 ± 9.7 | 0.003 |
| Median | 61.1 | 65.5 | 62 | |
| Q1, Q3 | 53.9, 71.4 | 56.7, 72.0 | 57.7, 71.4 | |
| Range | 19.0-91.5 | 19.8-89.4 | 43.3-88.0 | |
| Age group | 0.001 | |||
| < 20 yr | 2 (0.5) | 1 (0.5) | 0 (0.0) | |
| 21-40 yr | 22 (5.0) | 2 (1.0) | 0 (0.0) | |
| 41-60 yr | 189 (43.2) | 64 (30.9) | 19 (33.9) | |
| ≥ 61 yr | 225 (51.4) | 140 (67.6) | 37 (66.1) | |
| Gender | 0.060 | |||
| Female | 132 (30.1) | 47 (22.7) | 11 (19.6) | |
| Male | 306 (69.9) | 160 (77.3) | 45 (80.4) | |
| Caucasian | 326 (74.4) | 168 (81.2) | 43 (76.8) | 0.170 |
| Etiology of liver disease | ||||
| Hepatitis C | 161 (37.2) | 61 (29.9) | 12 (21.8) | 0.030 |
| Hepatitis B | 49 (11.3) | 6 (2.9) | 5 (9.1) | 0.002 |
| Alcoholic liver disease | 129 (30.0) | 59 (28.9) | 10 (18.5) | 0.210 |
| Nonalcoholic fatty liver disease | 25 (5.8) | 63 (30.9) | 14 (25.9) | < 0.0001 |
| Other | 74 (16.9) | 18 (8.7) | 15 (26.8) | 0.012 |
| BCLC stage | ||||
| Missing | 125 | 45 | 17 | 0.080 |
| 0/A | 72 (23.0) | 26 (16.0) | 11 (28.2) | |
| B | 43 (13.7) | 15 (9.3) | 4 (10.3) | |
| C | 165 (52.7) | 96 (59.3) | 23 (59.0) | |
| D | 33 (10.5) | 25 (15.4) | 1 (2.6) |
All data except for age are shown in n (%). BCLC: Barcelona-Clınic Liver Cancer.
Table 2 shows univariate and multivariate Cox Proportional Hazards analysis of survival predictors of HCC patients in this study. Given that BCLC stage is a known predictor of survival in HCC patients, only the 514 patients for whom data on the BCLC stage was available were included in the multivariate Cox Proportional Hazards analysis. By univariate analysis, using diabetics not on metformin as the reference group, diabetic patients with HCC on metformin had no survival advantage, with a HR (95%CI) of 1.0 (0.8-1.3). Non-diabetic HCC patients also did not appear to have a survival advantage as compared to diabetic HCC patients not on metformin, as demonstrated by a HR (95%CI) of 1.1 (0.7-1.7).
Table 2.
Survival of patients newly diagnosed with hepatocellular carcinoma, categorized according to demographics, etiologies of liver disease, stage, diabetes status and metformin use
| Number of deaths/total | Median survival (mo) | 1-yr Kaplan-meier estimate (95%CI) | Unadjusted HR (95%CI) | P value | Adjusted HR (95%CI) | P value | |
| Male | 314/511 | 25.2 | 61.8% (57.7-66.3) | 1.1 (0.9-1.3) | 0.54 | 1.0 (0.8-1.3) | 0.73 |
| Age, per 10 yr | - | - | - | 1.2 (1.1-1.3) | < 0.001 | 1.2 (1.1-1.3) | < 0.001 |
| Caucasian | 329/537 | 24.6 | 64.6% (60.6-68.9) | 0.9 (0.8-1.1) | 0.36 | ||
| Etiology of liver disease | |||||||
| Hepatitis C | 127/234 | 29 | 65.6% (59.7-72.1) | 0.8 (0.6-0.9) | 0.0095 | ||
| Hepatitis B | 30/60 | 30.2 | 58.1% (46.3-72.8) | 0.9 (0.6-1.3) | 0.52 | ||
| Alcoholic liver disease | 126/198 | 19.8 | 60.8% (54.3-68.1) | 1.0 (0.8-1.3) | 0.75 | ||
| NAFLD | 64/102 | 29.4 | 67.6% (58.8-77.7) | 1.0 (reference) | 0.98 | ||
| BCLC stage | |||||||
| 0/A | 39/109 | > 97.3 | 88.4% (82.5-94.8) | 1.0 (reference) | < 0.001 | 1.0 (reference) | < 0.001 |
| B | 32/62 | 41.0 | 70.0% (59.3-82.7) | 1.9 (1.2-3.1) | 0.005 | 2.0 (1.2-3.1) | 0.005 |
| C | 205/284 | 15.2 | 50.1% (44.5-56.4) | 3.8 (2.7-5.4) | < 0.001 | 3.7 (2.6-5.2) | < 0.001 |
| D | 32/59 | 30.6 | 58.9% (47.2-73.5) | 2.4 (1.5-3.8) | < 0.001 | 2.5 (1.6-4.0) | < 0.001 |
| Diabetes | |||||||
| metformin status | |||||||
| Non-diabetic | 257/438 | 27.3 | 61.4% (56.9-66.3) | 1.0 (0.7-1.4) | 0.98 | 1.1 (0.7-1.7) | 0.59 |
| Continue metformin | 37/56 | 34.2 | 60.6% (48.8-75.4) | 0.9 (0.7-1.1) | 0.30 | 1.0 (0.8-1.3) | 0.77 |
| No metformin or | 133/207 | 25.5 | 65.5% (59.2-72.4) | 1.0 (reference) | 0.52 | 1.0 (reference) | 0.86 |
| Discontinue within 90 d | |||||||
| In patients with NAFLD | |||||||
| Non-diabetic | 11/25 | 46.7 | 79.5% (64.9-97.3) | 0.4 (0.2-0.7) | 0.005 | ||
| Continue metformin | 7/14 | 36.5 | 65.7% (43.1-100.0) | 0.6 (0.3-1.4) | 0.24 | ||
| No metformin or discontinue within 90 d | 46/63 | 16.3 | 63.0% (51.8-76.7) | 1.0 (reference) | 0.01 | ||
BCLC: Barcelona-Clınic Liver Cancer; NAFLD: Nonalcoholic fatty liver disease.
By the time the data was censored, 427 of the 701 (60.9%) patients had died. The median survival of the entire cohort was 26.9 mo with a 1-year survival rate of 26.9% (95%CI: 59.0-66.3). Figure 1 illustrates the Kaplan-Meier curves of these groups, with the diabetics on metformin beyond 90 d of HCC diagnosis having the longest median survival of 34.2 mo as compared to 25.5 mo among diabetic patients who were not on metformin or had discontinued metformin within 90 d after HCC diagnosis (Table 2).
Figure 1.
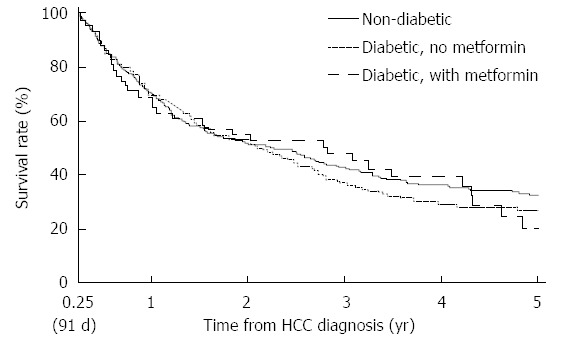
Kaplan-Meier survival curves of patients with hepatocellular carcinoma according to the following categories: (1) non-diabetics; (2) diabetics not on metformin; and (3) diabetics on metformin.
Assessment of survival in patients with HCC in the context of fatty liver disease also showed no benefit to the use of metformin. Although nonalcoholic fatty liver disease (NAFLD)-related HCC patients with diabetes who were on metformin had longer median survival than those not on metformin (36.5 mo vs 16.3 mo), the survival difference was not statistically significant (P = 0.24), likely due to the small number of NAFLD-related HCC patients who were on metformin (n = 14).
DISCUSSION
Type 2 diabetes is a significant risk factor for the development of malignancies, including HCC[14]. Diabetes remains a risk factor even after having adjusted for traditional liver disease risk factors such as alcoholic and viral liver disease[3,15]. This is most likely due to the higher prevalence of non-alcoholic steatohepatitis among diabetics, which is in itself a risk factor for HCC. An insulin-resistant state leads to activation of the IGF-1/mTOR signalling cascade and consequent hepatic steatosis. Additionally, patients with cirrhosis have defective insulin release and sensitivity, which can further contribute to hyperglycemia[16].
The increased risk of HCC among patients with an insulin-resistant state is the reason metformin has garnered such interest as a chemopreventive and chemotherapeutic agent. Recently, research into therapeutics for malignancy has turned towards agents that modify aspects of metabolism. The pro-survival mTOR pathway is particularly affected by cellular energetics. AMP-activated protein kinase (AMPK), an intracellular sensor that serves to maintain energy balance, is stimulated by increased energy consumption as reflected by an elevated AMP/ATP ratio. AMPK activation in turn inhibits the mTOR pathway and anabolic processes, including the energy-consuming process of protein synthesis. Metformin is thought to affect tumor growth by two mechanisms: (1) through inhibition of mitochondrial oxidative phosphorylation which activates AMPK thereby resulting in mTOR pathway inhibition; and (2) through decreased serum glucose, which inhibits IGF-R, thereby preventing downstream mTOR pathway activation in insulin-responsive cancers[17]. Over the last few years, metformin has demonstrated promising results in various malignancies, including breast cancer[7], lung cancer[8] and melanoma[9]. Retrospective studies have suggested that metformin prevents development of HCC among patients with diabetes[11] and those with chronic liver disease[12]. A greater effect on HCC could be anticipated, given that the OCT1 transporter is most highly expressed in hepatocytes, enabling increased uptake of metformin in the liver[10]. Therefore, the reality of in vivo pharmacokinetics favours accumulation of metformin in the liver. Metformin is absorbed from the gut into the portal vein circulation, which drains directly into the liver. In vitro, by inhibiting the mTOR pathway, rather than activating it as insulin would, metformin arrests the cell cycle and induces cancer cell apoptosis[18]. In vivo, metformin has been shown to inhibit DEN-induced liver tumorigenesis by affecting lipogenesis[19] and inhibit tumor growth in mouse xenograft models of HCC[20]. Induction of cancer cell apoptosis following cell cycle arrest appears to be the mechanism underlying tumor growth inhibition in these xenograft models[21].
We therefore set out to evaluate whether metformin had a therapeutic effect on HCC once diagnosed. Based on our findings, one can conclude that in patients with a new diagnosis of HCC, continuation of metformin beyond 90 d after this diagnosis does not appear to have a beneficial chemotherapeutic effect as measured by survival. This result was independent of BCLC stage and patient age. Additionally, patients with HCC caused by fatty liver disease had no greater survival advantage on metformin as compared to those patients not on metformin. It may be that HCCs that develop in patients on metformin break through this agent, and are either resistant to mTOR inhibition or dependent on other pro-carcinogenic pathways. Two recent retrospective studies of metformin use among breast cancer patients revealed no evidence of improved survival, even when adjusting for cumulative metformin duration[22,23]. However, we did also find that metformin appears to be well tolerated without major side effects, in particular lactic acidosis in this patient population with advanced liver disease. This finding supported our recent report showing that metformin can be used safely in patients with cirrhosis, regardless of severity of liver impairment, if there is no specific contraindication[24].
An important point to note is our definition of significant exposure, wherein only those patients who had continued metformin beyond 90 d after the diagnosis of HCC were considered as having been significantly exposed. The censoring of patients who passed away within 90 d of diagnosis accounts for the paradoxically increased median survival of the remaining stage D patients as compared to that of stage C patients. This is because a significant proportion of the stage D patients died within 90 d after HCC diagnosis. Nonetheless, the confidence intervals for the two median survivals have substantial overlap, indicating that the median survivals were in fact similar.
This study is the first to evaluate whether metformin has any positive impact on HCC once diagnosed. The main limitations are the retrospective study design and the relatively small population of 701 patients. Due to the retrospective study design, we were not able to obtain information on dose and duration of metformin use. Additionally, data on BCLC stage was missing for some patients. Because information on the first treatment for HCC was not always recorded in the BRIDGE database, the analysis was not adjusted for HCC treatment modalities. Nonetheless, given that BCLC Stage itself dictates the type of treatments patients undergo, adjusting for BCLC Stage accounts for the type of treatment the patients have undergone. Whether metformin had a different effect on patients who received different treatments was not investigated. Despite the suggestive finding of benefit of metformin in the subgroup of HCC patients with NAFLD, the analysis may not have adequate power to show statistical significance due to the small number patients of those who were on metformin. This finding needs to be further examined in a larger cohort of HCC patients with NAFLD. Finally, this study was conducted at a tertiary care referral centre, and therefore may be weighted more heavily towards a patient population with more advanced liver disease/cancer.
In summary, our study demonstrates that there is no survival benefit to metformin use in diabetic patients with a new diagnosis of HCC. This is in keeping with similar outcomes in the recent literature of metformin use among patients already diagnosed with cancer. Our study at least provides a preliminary indication that metformin does not help prevent progression of HCC in real life.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a malignancy that has been increasing in incidence worldwide, concordant with the rising prevalence of chronic liver disease secondary to hepatitis C, and fatty liver disease. Sorafenib is currently the only targeted chemotherapeutic agent proven to minimally improve overall survival in patients with advanced HCC, and there has been an active search for further chemotherapeutic agents. Metformin has been found in retrospective studies to have a chemopreventive effect against HCC among patients with chronic liver disease and diabetes.
Research frontiers
The mammalian target of rapamycin (mTOR) pathway upregulated in up to 50% of HCC tumors, and metformin as an inhibitor of the mTOR pathway has been discovered to have tumor suppressive properties in in vivo models of HCC. In this retrospective study performed at the Mayo Clinic, Rochester, Minnesota, the authors have found that metformin, an antidiabetic medication that affects the mTOR pathway, does not improve survival in diabetic patients newly diagnosed with HCC.
Innovations and breakthroughs
There has been much study into the use of metformin as a chemopreventive and chemotherapeutic agent in the recent oncology literature. Metformin has been of particular interest in the treatment of malignancies because it is easily tolerated and less toxic than standard of care chemotherapy agents. The authors demonstrate for the first time that metformin use does not benefit survival among patients with HCC, as compared to survival of diabetic patients on other hypoglycemic agents and patients without diabetes who have HCC.
Applications
Despite the promising recent literature on metformin, this study reveals that metformin use does not positively impact survival. Therefore, there is no indication to continue metformin with the intent of using metformin as a chemotherapeutic agent in patients newly diagnosed with HCC. On the other hand, the authors did not find any evidence that the use of metformin was unsafe in this patient population. This finding supports previous reports of the safety of metformin use in cirrhotic patients. These questions merit additional prospective study.
Peer review
The article is a retrospective study of metformin effect on HCC patients between Jan 2005 and June 2011. The paper is well designed. The antitumor effect of metformin in several types of cancers, including hepatocellular carcinoma, has recently been a matter of investigations. The presented study gives some information about the topic.
Footnotes
Supported by National Institutes of Health, No. NCI CA165076; Mayo Clinic Center for Cell Signaling in Gastroenterology, No. NIDDK P30DK084567; Mayo Clinic Cancer Center, No. NCI CA15083; and Mayo Clinic Center for Clinical and Translational Science, No. NCATS UL1 TR000135
P- Reviewer: Fan JG, Garcovich M, Hsieh CC, Ozen H, Paik SW S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006;25:3771–3777. doi: 10.1038/sj.onc.1209560. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, Muzikansky A, Clark JW, Kwak EL, Schrag D, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–183, 1972-183. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 8.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder JM, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi: 10.1038/cddis.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonker JW, Schinkel AH. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3) J Pharmacol Exp Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 11.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 12.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–91; quiz 892. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baig NA, Herrine SK, Rubin R. Liver disease and diabetes mellitus. Clin Lab Med. 2001;21:193–207. [PubMed] [Google Scholar]
- 17.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 18.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 19.Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, Saxena NK, Biswal S, Girnun GD. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544–552. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, et al. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2013:Dec 30; Epub ahead of print. doi: 10.3892/ijo.2013.2233. [DOI] [PubMed] [Google Scholar]
- 21.Qu Z, Zhang Y, Liao M, Chen Y, Zhao J, Pan Y. In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatol Res. 2012;42:922–933. doi: 10.1111/j.1872-034X.2012.01007.x. [DOI] [PubMed] [Google Scholar]
- 22.Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 2013;36:3018–3026. doi: 10.2337/dc12-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aksoy S, Sendur MA, Altundag K. Demographic and clinico-pathological characteristics in patients with invasive breast cancer receiving metformin. Med Oncol. 2013;30:590. doi: 10.1007/s12032-013-0590-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014:Epub ahead of print. doi: 10.1002/hep.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]


