Abstract
AIM: To investigate the effect of perioperative restricted fluid therapy on circulating CD4+/CD8+ T lymphocyte ratio, percentage of regulatory T cells (Treg) and postoperative complications in patients with colorectal cancer.
METHODS: A total of 185 patients met the inclusion criteria and were included in the randomized clinical trial. These patients were divided into two groups according to receipt of either perioperative standard (S, n = 89) or restricted (R, n = 96) fluid therapy. Clinical data of these patients were collected in this prospective study. Perioperative complications and cellular immunity changes (CD4+/CD8+ and Treg) were analyzed comparatively between the two groups.
RESULTS: Both during surgery and on postoperative days, the total volumes of fluids administered in the R group were significantly lower than those in the S group (1620 ± 430 mL vs 3110 ± 840 mL; 2090 ± 360 mL vs 2750 ± 570 mL; 1750 ± 260 mL vs 2740 ± 490 mL; 1620 ± 310 mL vs 2520 ± 300 mL; P < 0.05). Decreased ratios of circulating CD4+/CD8+ T lymphocytes (1.47 ± 0.28 vs 2.13 ± 0.26; 1.39 ± 0.32 vs 2.21 ± 0.24; P < 0.05) and Treg percentage values (2.79 ± 1.24 vs 4.26 ± 1.04; 2.46 ± 0.98 vs 4.30 ± 1.12; P < 0.05) were observed after surgery in both groups. However, in the R group, these values restored more quickly starting from postoperative day 2 (1.44 ± 0.24 vs 1.34 ± 0.27; 2.93 ± 1.08 vs 2.52 ± 0.96; P < 0.05). The proportion of patients with complications was significantly lower in the restricted group (36 of 89 vs 59 of 96, P < 0.01).
CONCLUSION: Perioperative restricted intravenous fluid regimen leads to a low postoperative complication rate and better cellular immunity preservation in patients with colorectal cancer.
Keywords: Colorectal cancer, Restricted fluid therapy, Standard fluid therapy, Postoperative complications, Immunological function
Core tip: This prospective study revealed that perioperative restricted intravenous fluid regimen results in a low postoperative complication rate and better preservation of cellular immunity, which at least in part, explains the improved postoperative clinical outcomes associated with the restricted fluid regimen in patients with colorectal cancer.
INTRODUCTION
In the hospital setting, intravenous fluid administration has became one of the most usual therapies[1,2]. The exact quantity of intravenous fluid is not known or is roughly calculated. The correlation between extracellular water volume and fluid administration in surgery is poorly understood, and is a matter for debate. In current practice, the volume of fluids administered in major abdominal procedures can reach 4-6 L during the operation and approximately 10-20 L postoperatively[3]. As a result, an increase in the patient’s weight caused by an overload of intravenous fluids is often observed in the postoperative period. Traditional fluid therapy in major surgery causes a body weight increase of 3-6 kg[4]. Interstitial oedema, disturbed coagulation, impaired wound healing and cardiopulmonary complications are associated with fluid overload, which has been shown to decrease muscular oxygen tension and delay recovery of gastrointestinal function[5,6]. Furthermore, postoperative weight gain and intraoperative fluid overload have been associated with poor survival and complications[7-9].
Several studies have demonstrated that the volume and type of administered liquids differentially influenced the immunity and inflammation, which was in line with another study showing that volume replacement using colloid could significantly reduce inflammatory response in patients with major abdominal surgery compared with crystalloid-based treatment[1,9-11].
Therefore, we supposed that a perioperative fluid restriction regimen could decrease postoperative complications by regulating inflammatory responses. In addition, it was previously reported that CD4/CD8 ratio and regulatory T cells (Treg) play an important role in the outcomes of gastrointestinal cancer surgery[12,13]. However, the detailed mechanisms of the perioperative fluid restriction therapy to reduce postoperative complications and regulate inflammatory function remain unknown to date[9,14,15]. The aim of this study was to compare the effect of a restricted perioperative intravenous fluid regimen to a standard regimen on complications and immunological function in patients with colorectal resection.
MATERIALS AND METHODS
Patients
We conducted a prospective, randomized study from March 2009 to December 2012 in the Department of General Surgery of Taizhou People’s Hospital of the Medical College of Nantong University (China). Adult patients admitted for elective colorectal resection were considered eligible if they had no life-threatening systemic diseases [American Society of Anesthesiologist (ASA) I-III]and did not meet the following exclusion criteria: current lactation, pregnancy, language problems, smoking within 2 wk, diabetes mellitus, renal insufficiency, disseminated or secondary cancer, inflammatory bowel disease, alcohol overconsumption, mental disorder and contraindications to epidural analgesia. Randomization was achieved by using computer-generated random numbers, and was stratified for colonic or rectal surgery. A list of randomized numbers for grouping according to receipt of either perioperative standard (S) or restricted (R) fluid therapy was generated by computer and sealed in opaque envelopes by a research assistant blinded to the study objective and design. Sequential distribution of these envelopes determined the perioperative approach used on each study subject. Both oral and written consents were collected from patients or the next-of-kin relatives. This study was conducted in accordance with the principle of good clinical practice, the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board in Taizhou People’s Hospital.
Restricted and standard perioperative fluid therapy
Perioperative fluid regimens in both R and S groups are demonstrated in Table 1. Operative blood losses in both S and R groups were estimated by weighing blood-soaked sponges, and by measuring the volume collected in drains and suction bottles. 6% hydroxyethyl starch (HAES) in saline was used to replace the loss of blood, with an allowance for 500 mL of extra normal saline as decided by the anesthesiologist according to clinical findings. The blood component treatment was started when the estimation of blood loss reached 1500 mL, with the goal of obtaining 25%-35% hematocrit (patients with cardiovascular diseases were given a higher percentage of hematocrit) in both groups. A maximum of 25 mL/kg of lactated Ringer’s (RL) solution was used. In order to correct intraoperative blood loss, HAES (6%) was also used to obtain further requirements of fluid. 5% albumin solution was administered if the maximum allowable dose of 6% HAES reached (33 mL/kg per day). After surgery, 30 mL/h of oral fluid was allowed in all patients. The total oral fluids were increased to 60 mL/h after 12 h, and to unrestricted oral fluids after 24 h unless nausea was observed. If 200 mL of drinking water was tolerated in patients in 0.5 h, intravenous fluid treatment was discontinued. In addition, whole blood lactate was measured to guide the fluid treatment as previously described[8]. In order to adjust the above-mentioned fluid regimens, blood lactate was monitored during the operation (every hour) and after the operation (2, 6, 12, 24, 36, 48, and 72 h). A maximum of allowed HAES (6%) was 1500 mL, only when active bleeding was observed. If the maximum allowable dose of HAES had been reached (33 mL/kg per day), 5% albumin solution was administered.
Table 1.
Perioperative fluid regimens
| Perioperative period | Restricted fluid | Standard fluid |
| Preloading of epidural analgesia | No preloading | 500 mL 6% HAES |
| During the operation | 7 mL/kg RL in first hour; 5 mL/kg per hour RL in following hours | 12 mL/kg per hour RL |
| Remainder of the operation day | 1000 mL 5% glucose (with potassium if needed) | 12 mL/kg per hour RL |
| Days following operation | 1000-1500 mL crystalloid | 2000-2500 mL crystalloid |
HAES: Hydroxyethyl starch; RL: Lactated Ringer’s solution.
Presurgery and postsurgery management
Patients received intravenous cephoxitin (1-2 g/8 h) as antibiotic prophylaxis initiated on induction of anesthesia 0.5-1 h before incision unless contraindicated, repeated every 4 h if the operation had not been terminated, and continued for 24 h after operation. Re-feeding was programmed to begin on the day after operation, via either the oral or the enteral route, unless contraindicated, and nasogastric tubes were not routinely used. Prophylaxis of deep vein thrombosis was administered to all moderate- and high-risk patients with 20-40 mg of enoxaparin 1-2 h before surgery and repeated daily after surgery on an individual basis. All patients were encouraged to perform early mobilization after operation. General anesthesia or combined (general and thoracic epidural) was employed at the anesthesiologist’s discretion.
Laboratory tests
Samples of venous EDTA blood were collected serially before 8 a.m. on the day before surgery as well as on days 1, 2, 3, and 5 after surgery. CD4, CD8 and Treg (CD4+CD25+Foxp3+) were detected as described previously[13]. Briefly, 50 μL of heparinized blood were incubated at 4 °C with 10 μL of each monoclonal antibody specific for a surface antigen (i.e., CD4, CD8, CD25) or an irrelevant isotype-matched monoclonal antibody, conjugated to a different fluorochrome for 30 min. Peripheral blood samples were depleted of red blood cells by incubation with 1 mL of FACS lysing solution (BD Biosciences) at room temperature for 10 min at the end of this step. And the intracellular staining procedure was started when all samples were washed twice with PBS. To this end, cells were first resuspended in 0.25 mL of Cytofix/Cytoperm solution (BD Biosciences) and allowed to sit for further 20 min. In order to favor penetration of anti-Foxp3 in the subsequent incubation, samples were then washed twice with BD Perm/Wash (BD Biosciences) to keep cells permeabilized. Samples were maintained in a minute amount of BD Perm/Wash solution (50 μL) containing FITC-conjugated anti-Foxp3 or FITC-conjugated isotype-matched irrelevant antibody (10 μL) at 4 °C for 30 min, then washed twice with BD Perm/Wash solution, and finally resuspended in 250 μL of PBS. Flow cytometry was immediately performed on a FACS Calibur instrument (BD Biosciences) and a minimum of 105 events were acquired for each analysis.
Clinical data collection and assessment
Fluid administration and loss were both recorded from the initiation of abrosia to postoperative day (POD)5. Intraoperative and postoperative physiologic parameters were monitored, venous blood was sampled daily until POD5 or until hospital discharge, and arterial blood was collected as needed. Any complication recorded within 30 d as well as death and other adverse effects, including ischemia and impairment of renal function after surgery were defined as the primary outcomes and the secondary outcomes, respectively. Baseline and surgical characteristics, and postoperative complications were recorded.
Statistical analysis
Before the trial was started, it was estimated that a sample size of 70 patients in each group had a power of 80% to detect an effect size of 1.00 standard deviation (SD) using a two-group t-test with a two-sided significant level of 0.05.
Statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc.). Data are expressed as means with their SD. The Mann-Whitney U test was used to analyze non-parametric ordinal data and the Student’s t-test was used to analyze normally distributed continuous data. The χ2 test or Fisher’s exact test were used to compare complications between groups, and results were considered statistically significant if P values were < 0.05.
RESULTS
In all, 364 patients underwent colorectal resection at the Department of Surgery, Taizhou People’s Hospital, during the study period. Of these, 185 patients completed the trial at last, including 96 in the S group and 89 in the R group (Figure 1).
Figure 1.
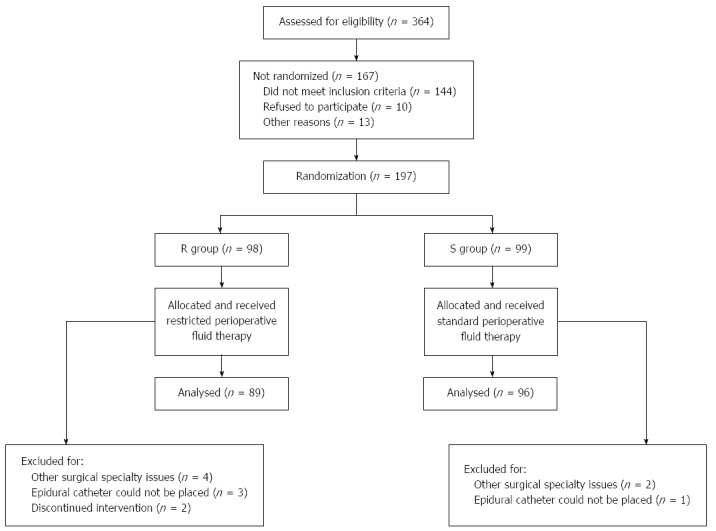
CONSORT diagram for the trial. R group: Restricted fluid therapy group; S group: Standard fluid therapy group.
Baseline characteristics are shown in Table 2. The clinical characteristics were similar in two groups (P > 0.05).
Table 2.
Baseline and surgical characteristics of the patients in the two study groups
| Restricted fluid (n = 89) | Standard fluid (n = 96) | P value | |
| Age (yr) | 64.7 ± 16.8 | 65.4 ± 17.6 | 0.78 |
| Male/Female | 50/39 | 56/40 | 0.77 |
| Body mass index | 21.9 ± 4.2 | 22.3 ± 3.8 | 0.50 |
| ASA grade | 0.98 | ||
| I | 29 | 30 | |
| II | 37 | 41 | |
| III | 23 | 25 | |
| Dukes stage | 0.61 | ||
| A | 9 | 8 | |
| B | 54 | 65 | |
| C | 26 | 23 | |
| Comorbidity | 0.78 | ||
| Cardiovascular diseases | 46 | 43 | |
| Pulmonary diseases | 11 | 14 | |
| Other diseases | 16 | 15 | |
| Smokers | 21 | 19 | 0.53 |
| Site of surgery | 0.81 | ||
| Colon | 59 | 62 | |
| Rectum | 30 | 34 | |
| Type of surgery | 0.38 | ||
| Laparoscopic | 27 | 35 | |
| Open | 62 | 61 | |
| Stoma | 12 | 15 | 0.68 |
| Blood loss (mL) | 120 ± 46 | 133 ± 62 | 0.11 |
| Length of operation (min) | 146 ± 52 | 154 ± 48 | 0.28 |
ASA: American Society of Anesthesiologists.
Patients in the R group received significantly lower volumes of fluids than those in the S group both during operation and on POD1-3 (Figure 2A-C). The numbers of patients receiving higher volumes of fluids than planned to resolve hyperlactatemia were similar in each group [R group: 27 (30.3%) vs S group: 23 (24.0%), P = 0.33]. In addition, the administration of dopamine and epinephrine was also similar between the R group and S group [R group: 22 (24.7%) vs S group: 19 (19.8%), P = 0.42]. There was no significant difference in time to return of bowel function (passage of flatus) between the restricted and standard groups (2.8 ± 0.8 d vs 3.0 ± 0.9 d, P = 0.11). In the first 3 d after surgery the median weight gain was smaller in the restricted group (Figure 3).
Figure 2.
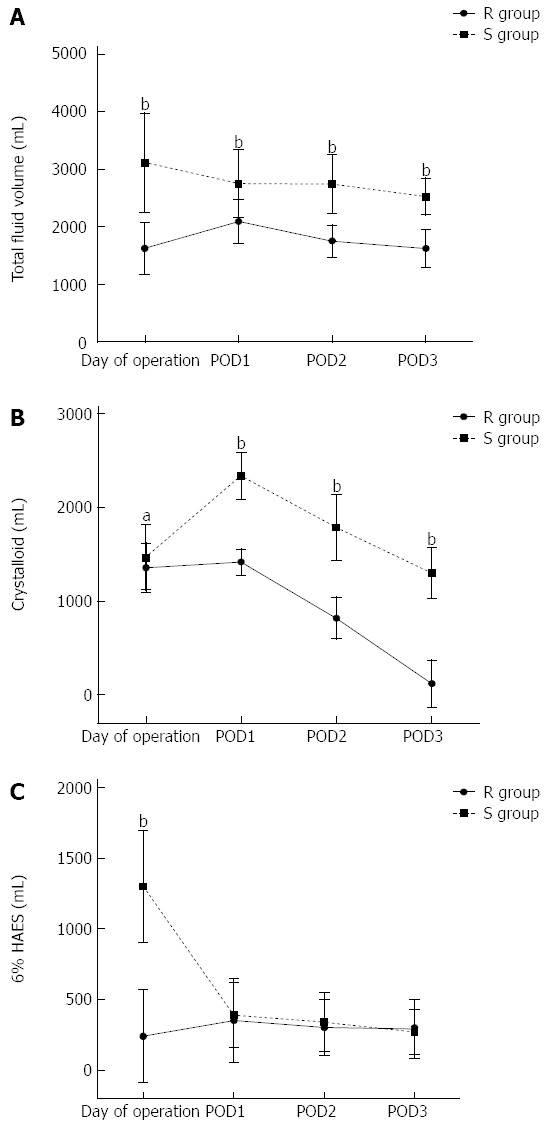
Evolution of intravenous fluids received by the patients in two groups. Data are mean ± SD. POD: Postoperative day; HAES: Hydroxyethyl starch; R group: Restricted fluid therapy group; S group: Standard fluid therapy group. aP < 0.05, bP < 0.01 R group vs S group.
Figure 3.
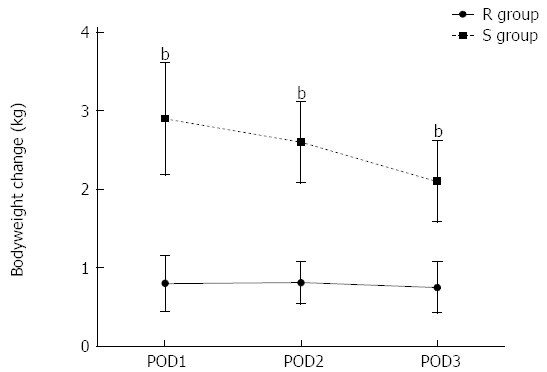
Body weight changes in the first 3 d after surgery in two groups. Data are mean ± SD. POD: Postoperative day; R group: Restricted fluid therapy group; S group: Standard fluid therapy group. bP < 0.01 R group vs S group.
The circulating CD4+/CD8+ T cells ratios were decreased in both groups after surgery (P < 0.05; Figure 4A). However, the ratios in the R group were restored much more quickly starting from POD2 compared with the S group (P < 0.05; Figure 4A). Treg play a nonredundant role in the maintenance of immune homeostasis. Interestingly, the Treg percentage values after surgery were also decreased in both S and R groups. After the operation, suppressed Treg percentage values were found on POD1, which were gradually restored on POD2-5 in both groups. However, expression of Treg in the S group was consistently lower than that in the R group on POD1-5 (P < 0.05; Figure 4B).
Figure 4.
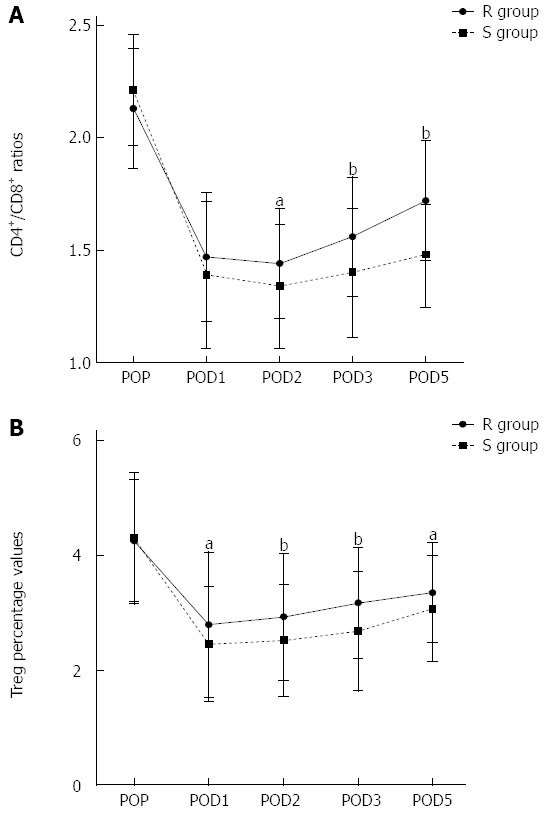
Evolution of peripheral blood CD4+/CD8+ ratios and Treg percentage values in two groups. Data are mean ± SD. POP: Pre-operation; POD: Postoperative day; R group: Restricted fluid therapy group; S group: Standard fluid therapy group. aP < 0.05, bP < 0.01 R group vs S group.
The number of patients with complications, as well as the total number of complications, was significantly lower in the restricted group (Table 3). The incidence of anastomotic leakage was similar in the two groups (2 in the R group vs 8 in the S group, P = 0.10). However, more heart insufficiency complications were observed in the restricted group (8 vs 1, P = 0.02). In the postoperative period 3 patients in the restricted group had reversible acute renal failure (P = 0.11).
Table 3.
Postoperative complications of the patients in the two study groups n (%)
| Restricted fluid (n = 89) | Standard fluid (n = 96) | P value | |
| Patients with major surgical complications | 5 (5.6) | 13 (14.6) | 0.069 |
| Total major surgical complications | 7 | 19 | |
| Sepsis/shock | 2 | 5 | 0.29 |
| Anastomotic bleeding | 1 | 3 | 0.62 |
| Anastomotic leakage | 2 | 8 | 0.10 |
| Peritonitis without leakage | 1 | 1 | 1.00 |
| Patients with minor surgical complication | 22 (24.7) | 38 (39.6) | 0.03 |
| Total minor surgical complications | 25 | 44 | |
| Wound dehiscence, infection, haematoma | 13 | 15 | 0.85 |
| Blood transfusion | 7 | 18 | 0.03 |
| Patients with organ-specific complications | 21 (23.6) | 30 (31.3) | 0.24 |
| Total organ-specific complications | 28 | 34 | |
| Urinary tract infection | 5 | 10 | 0.23 |
| Heart insufficiency | 8 | 1 | 0.02 |
| Respiratory insufficiency | 1 | 1 | 1.00 |
| Renal insufficiency | 3 | 0 | 0.11 |
| Cognitive disorder | 3 | 7 | 0.34 |
| Total postoperative complications | 60 | 97 | |
| Total patients with complications | 36 (40.4) | 59 (61.5) | < 0.01 |
DISCUSSION
This study assessed the effect of perioperative restricted intravenous fluid therapy on immunological function and postoperative complications. Our results showed that patients treated with a perioperative restricted intravenous fluid regimen had significantly fewer postoperative complications after elective colorectal surgery.
Interstitial oedema due to fluid overload may predispose to complications[16]. Further effects of fluid overload may be poorer wound healing and a delayed return of bowel function[16,17]. Many studies have demonstrated that postoperative immunity dysfunction is associated with high complication rates[18-20]. Additionally, it was reported that sustained suppression of lymphocyte function and numbers may be caused during the postsurgical period of colorectal surgery, which may promote the rates of postoperative complications[18,21-25]. It was also reported that increased lymphocyte cell death by Fas-mediated circulating lymphocyte subset apoptosis can be found in patients with major surgery[26,27]. These findings were in line with those from other studies showing that the surgical procedures decreased the T-lymphocyte population[15,20,28,29]. A previous study demonstrated an increased CD4/CD8 ratio during the postoperative period in patients with colorectal cancer receiving anti-inflammatory therapy using fish oil[30]. Furthermore, Zhu et al[31] demonstrated that CD4/CD8 ratio to return to normal might indicate recovery of the anti-infection mechanism and may have reduced septic events. Th1, Th17 and Treg cells represent three CD4+ T-cell subsets that share important developmental elements, but ultimately bifurcate into distinct phenotypes with remarkably opposite activities, with Th1 and Th17 cells being pro-inflammatory, and Treg being anti-inflammatory[32,33]. Treg are positively correlated with effector T-cells, and the activity of all these effector T cells is attenuated by the anti-inflammatory action of Treg[33]. These cells are critical for the prevention of excessive immune activation and autoimmune responses perpetrated by self-reactive T cells[13,32]. In the present study, patients received the perioperative restricted intravenous fluid regimen underwent an attenuated reduction and faster recovery of CD4+/CD8+ ratios and Treg percentage values compared with those received the standard perioperative intravenous fluid treatment.
These results indicated that better preserved immune function may contribute to a better regulated inflammatory response in these patients. Although the detailed mechanism underlying this effect is unclear to date, cellular swelling caused by the perioperative restricted intravenous fluid regimen may have led to disruption of intracellular signaling mechanisms, which ultimately resulted in poor clinical outcomes[34-36].
The greater number of perioperative blood transfusions in the standard group was most likely explained by the excess amount of fluid with low haemoglobin concentration and haemodilution, triggering transfusion of erythrocytes. Interestingly, the loss of estimated blood between the two groups was similar. In the present study, there were more cardiac complications in the restricted group. Furthermore, more patients had mild, reversible renal failure in the postoperative period in the restricted group. However, there was no statistically significant difference. The renal failure may have been triggered by the restricted amount of fluid in these patients. In addition, increased creatinine levels were also noted in previous studies, but renal failure was not more common when fluid was restricted[37]. These findings indicate that the use of a restricted fluid regimen needs to be accompanied by increased observation of cardiac and renal status.
There were no deaths in the present trial, in contrast to some previous studies of restricted fluid regimens in colorectal surgery[9,16]. Holte et al[38] demonstrated that patients in the restricted group had improved pulmonary function, and a trend towards fewer postoperative complications including major complications such as small bowel obstruction and anastomotic leakage. However, major surgical complications were comparable between two groups in this study.
In conclusion, perioperative restricted intravenous fluid therapy leads to a low postoperative complication rate and better cellular immunity preservation after surgery in patients with colorectal cancer, which could at least in part, explains the improvement of clinical outcomes associated with the restricted fluid therapy regimen. However, this is a single clinical center study and a multi-center study with a larger number of patients is necessary to strengthen our findings.
COMMENTS
Background
Several studied have demonstrated that the type and volume of liquid have been suggested to differentially influence inflammation and immunity. However, the detailed mechanisms of perioperative fluid restriction therapy to reduce postoperative complications and regulate inflammatory function remain unknown to date.
Research frontiers
CD4/CD8 ratio and regulatory T cells (Treg) play an important role in the outcomes of gastrointestinal cancer surgery. The effect of a restricted perioperative intravenous fluid regimen and a standard regimen on complications and immunological function in patients with colorectal resection warrants further investigation.
Innovations and breakthroughs
The ratios of circulating CD4+/CD8+ T lymphocytes and Treg percentage values were restored much more quickly in the restricted group starting from postoperative day 2. The proportion of patients with complications was significantly lower in the restricted group.
Applications
This study revealed that the perioperative restricted intravenous fluid regimen is effective in reducing postoperative complication rates and preservation of cellular immunity after surgery in patients with colorectal cancer.
Peer review
It is an interesting theme and an interesting article and this paper provides some useful information on the perioperative intravenous fluid regimen for patients with colorectal cancer.
Footnotes
P- Reviewer: Nedrebo BS, Tang CL S- Editor: Nan J L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Bamboat ZM, Bordeianou L. Perioperative fluid management. Clin Colon Rectal Surg. 2009;22:28–33. doi: 10.1055/s-0029-1202883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–1692. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Brandstrup B. Fluid therapy for the surgical patient. Best Pract Res Clin Anaesthesiol. 2006;20:265–283. doi: 10.1016/j.bpa.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Wise WE, Padmanabhan A, Meesig DM, Arnold MW, Aguilar PS, Stewart WR. Abdominal colon and rectal operations in the elderly. Dis Colon Rectum. 1991;34:959–963. doi: 10.1007/BF02049957. [DOI] [PubMed] [Google Scholar]
- 5.Kalyan JP, Rosbergen M, Pal N, Sargen K, Fletcher SJ, Nunn DL, Clark A, Williams MR, Lewis MP. Randomized clinical trial of fluid and salt restriction compared with a controlled liberal regimen in elective gastrointestinal surgery. Br J Surg. 2013;100:1739–1746. doi: 10.1002/bjs.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 7.Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173:1058–1064. doi: 10.1001/jamainternmed.2013.552. [DOI] [PubMed] [Google Scholar]
- 8.Wenkui Y, Ning L, Jianfeng G, Weiqin L, Shaoqiu T, Zhihui T, Tao G, Juanjuan Z, Fengchan X, Hui S, et al. Restricted peri-operative fluid administration adjusted by serum lactate level improved outcome after major elective surgery for gastrointestinal malignancy. Surgery. 2010;147:542–552. doi: 10.1016/j.surg.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang K, Suttner S, Boldt J, Kumle B, Nagel D. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anaesth. 2003;50:1009–1016. doi: 10.1007/BF03018364. [DOI] [PubMed] [Google Scholar]
- 11.Gombocz K, Beledi A, Alotti N, Kecskés G, Gábor V, Bogár L, Koszegi T, Garai J. Influence of dextran-70 on systemic inflammatory response and myocardial ischaemia-reperfusion following cardiac operations. Crit Care. 2007;11:R87. doi: 10.1186/cc6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang ZM, Wilmore DW, Wang XR, Wei JM, Zhang ZT, Gu ZY, Wang S, Han SM, Jiang H, Yu K. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97:804–809. doi: 10.1002/bjs.6999. [DOI] [PubMed] [Google Scholar]
- 13.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22:327–334. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrillow SJ, Weinberg L, Parker F, Calzavacca P, Licari E, Aly A, Bagshaw S, Christophi C, Bellomo R. Perioperative fluid prescription, complications and outcomes in major elective open gastrointestinal surgery. Anaesth Intensive Care. 2010;38:259–265. doi: 10.1177/0310057X1003800206. [DOI] [PubMed] [Google Scholar]
- 15.Tartter PI. Preoperative lymphocyte subsets and infectious complications after colorectal cancer surgery. Surgery. 1988;103:226–230. [PubMed] [Google Scholar]
- 16.MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O’Dwyer PJ. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93:1469–1474. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 17.Abraham-Nordling M, Hjern F, Pollack J, Prytz M, Borg T, Kressner U. Randomized clinical trial of fluid restriction in colorectal surgery. Br J Surg. 2012;99:186–191. doi: 10.1002/bjs.7702. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki T, Ogata M, Kawasaki C, Okamoto K, Sata T. Effects of epidural anaesthesia on surgical stress-induced immunosuppression during upper abdominal surgery. Br J Anaesth. 2007;98:196–203. doi: 10.1093/bja/ael334. [DOI] [PubMed] [Google Scholar]
- 19.Utoh J, Yamamoto T, Utsunomiya T, Kambara T, Goto H, Miyauchi Y. Effect of surgery on neutrophil functions, superoxide and leukotriene production. Br J Surg. 1988;75:682–685. doi: 10.1002/bjs.1800750719. [DOI] [PubMed] [Google Scholar]
- 20.Nichols PH, Ramsden CW, Ward U, Sedman PC, Primrose JN. Perioperative immunotherapy with recombinant interleukin 2 in patients undergoing surgery for colorectal cancer. Cancer Res. 1992;52:5765–5769. [PubMed] [Google Scholar]
- 21.Jensen LS, Hokland M, Nielsen HJ. A randomized controlled study of the effect of bedside leucocyte depletion on the immunosuppressive effect of whole blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1996;83:973–977. doi: 10.1002/bjs.1800830727. [DOI] [PubMed] [Google Scholar]
- 22.Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236:759–766; disscussion 767. doi: 10.1097/01.SLA.0000036269.60340.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wichmann MW, Hüttl TP, Winter H, Spelsberg F, Angele MK, Heiss MM, Jauch KW. Immunological effects of laparoscopic vs open colorectal surgery: a prospective clinical study. Arch Surg. 2005;140:692–697. doi: 10.1001/archsurg.140.7.692. [DOI] [PubMed] [Google Scholar]
- 24.Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT, Chiang JM, Lin JR. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27:1347–1357. doi: 10.1007/s00384-012-1459-x. [DOI] [PubMed] [Google Scholar]
- 25.Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A Preoperative Neutrophil to Lymphocyte Ratio of 3 Predicts Disease-Free Survival After Curative Elective Colorectal Cancer Surgery. Ann Surg. 2014;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 26.Evans C, Galustian C, Kumar D, Hagger R, Melville DM, Bodman-Smith M, Jourdan I, Gudgeon AM, Dalgleish AG. Impact of surgery on immunologic function: comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am J Surg. 2009;197:238–245. doi: 10.1016/j.amjsurg.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Finco C, Magnanini P, Sarzo G, Vecchiato M, Luongo B, Savastano S, Bortoliero M, Barison P, Merigliano S. Prospective randomized study on perioperative enteral immunonutrition in laparoscopic colorectal surgery. Surg Endosc. 2007;21:1175–1179. doi: 10.1007/s00464-007-9238-4. [DOI] [PubMed] [Google Scholar]
- 28.Moug SJ, Smith D, Leen E, Angerson WJ, Horgan PG. Selective continuous vascular occlusion and perioperative fluid restriction in partial hepatectomy. Outcomes in 101 consecutive patients. Eur J Surg Oncol. 2007;33:1036–1041. doi: 10.1016/j.ejso.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Vretzakis G, Kleitsaki A, Stamoulis K, Bareka M, Georgopoulou S, Karanikolas M, Giannoukas A. Intra-operative intravenous fluid restriction reduces perioperative red blood cell transfusion in elective cardiac surgery, especially in transfusion-prone patients: a prospective, randomized controlled trial. J Cardiothorac Surg. 2010;5:7. doi: 10.1186/1749-8090-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, Xie QW, Yin MJ. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14:2434–2439. doi: 10.3748/wjg.14.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev. 2013;252:52–77. doi: 10.1111/imr.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Tax AW, Orsi AJ, Lafferty M, Barsevick A, Luborsky L, Prystowsky M, Nahass D, Lowery B, McCorkle R. A descriptive study of immune status in patients undergoing colorectal surgery: lymphocyte phenotypes. Oncol Nurs Forum. 1994;21:1539–1544. [PubMed] [Google Scholar]
- 35.Liu C, Liu J, Zhang S. Laparoscopic versus conventional open surgery for immune function in patients with colorectal cancer. Int J Colorectal Dis. 2011;26:1375–1385. doi: 10.1007/s00384-011-1281-x. [DOI] [PubMed] [Google Scholar]
- 36.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 37.Cook JA, Fraser IA, Sandhu D, Everson NW, Rossard DP. A randomised comparison of two postoperative fluid regimens. Ann R Coll Surg Engl. 1989;71:67–69. [PMC free article] [PubMed] [Google Scholar]
- 38.Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, Kehlet H. Liberal or restrictive fluid administration in fast-track colonic surgery: a randomized, double-blind study. Br J Anaesth. 2007;99:500–508. doi: 10.1093/bja/aem211. [DOI] [PubMed] [Google Scholar]


