Abstract
AIM: To investigate whether dairy product consumption is a risk factor for gastric cancer.
METHODS: We searched the PubMed and Web of Science databases for English-language studies on dairy product consumption and gastric cancer risk that were published between October 1980 and September 2013. One author independently extracted data and assessed study quality. Based on the heterogeneity results, we used either the fixed effects model or the random effects model to compute the summary relative risks and 95% confidence intervals (CIs). We also analyzed subgroups according to the study design, geographic region, sex, and whether there were adjustments for confounders (smoking and drinking) with respect to the sources of heterogeneity.
RESULTS: We found 39 studies that were potentially eligible for inclusion in this meta-analysis, including 10 cohort studies and 29 case-control studies. The summary relative risk for gastric cancer, comparing the highest and lowest dairy product consumption categories, was 1.06 (95%CI: 0.95-1.18). Specific analyses for milk, butter, and margarine yielded similar results, but the results for cheese and yogurt were different. There was significant heterogeneity for all studies (Q = 112.61; P = 0.000; I2 = 67.1%). No publication bias was observed (Egger’s test: P = 0.135; Begg’s test: P = 0.365). There was a nonsignificant association between dairy product consumption and gastric cancer risk in the subgroup analysis for the study design, sex, geographic region, and whether there were adjustments for confounders (smoking and drinking).
CONCLUSION: In our meta-analysis, dairy product consumption was associated with a nonsignificantly increased risk of gastric cancer. However, this result should be verified using large, well-designed prospective studies.
Keywords: Milk, Dairy product, Gastric cancer, Meta-analysis
Core tip: Previously published epidemiologic studies have presented inconclusive results on the association between dairy product consumption and gastric cancer risk. Therefore, we performed a meta-analysis to further explore the possibility of an association. To the best of our knowledge, this is the first meta-analysis that explores the association between dairy product consumption and gastric cancer risk. We analyzed the effects of consuming individual dairy product and the total effects of all dairy product on gastric cancer risk, and we conducted subgroup analyses for the study design, sex, region, and adjustment factors. Our study offers new insight into gastric cancer prevention.
INTRODUCTION
Gastric cancer remains the fourth most common cancer and the second leading cause of cancer mortality worldwide, even though the incidence and mortality rates have steadily decreased over the last half century[1-3]. Epidemiological investigations have associated the risk of gastric cancer with living habits[4-6]. Because dietary intake may be an important factor in the etiology of gastric cancer[7], it has recently become popular to analyze the dietary factors that may be associated with gastric cancer.
Although milk is thought to contain all of the substances that are essential for human nutrition[8], some dairy products, such as cheese and whole milk, have a high fat content. One study associated a higher intake of high-fat dairy product with an increased risk of gastric cardia adenocarcinoma[9].
Dairy product consumption may affect various carcinogenesis pathways. Dairy intake is thought to modify cancer risk through the following biological effects: a higher circulation of insulin-like growth factor 1[10,11], modification of the vitamin D status[12,13], a higher intake of conjugated linolenic acid[14,15], and exposure to contaminants such as polychlorinated biphenyls[16-18]. Some meta-analyses have reported that dairy product consumption could increase the risk of ovarian[19] and prostate[20] cancers while reducing the risk of colorectal cancer[21,22].
It is unclear whether dairy product consumption is a risk factor for gastric cancer because the previously published epidemiologic studies have presented inconclusive results on this topic[23,24]. Therefore, we performed a meta-analysis of cohort and case-control studies to analyze the possibility of an association between dairy consumption and gastric cancer risk.
MATERIALS AND METHODS
Search strategy
We searched the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and Web of Science (http://isiknowledge.com) databases for English-language studies on dairy product consumption and gastric cancer risk that were published between October 1980 and September 2013. We performed our search using the following terms: (dairy product or dairy or milk or food or diet) and (stomach or gastric) and (cancer or neoplasm or carcinoma or tumor).
Study selection
Studies were included if they met the following criteria: (1) had been published as an original article; (2) had a case-control or prospective cohort design; (3) had clearly defined outcomes such as gastric or stomach cancer; (4) presented relative risk (RR) estimates, odds ratios (ORs), or hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for the association between gastric cancer and dairy product consumption; and (5) were published in English between October 1980 and September 2013. If data were duplicated in more than one study, the most recent or informative study was used. In this meta-analysis, we considered “dairy”, “milk product”, and “milk and dairy product” as equivalent to “dairy product.”
One cohort study[25] and 21 case-control studies[9,26-45] were excluded for the following reasons: CIs were not provided[26-35], anatomic subsites or Lauren gastric cancer classifications were presented[9,25,36-38], more than one RR that involved single or multiple gastric cancer was found[39], gastric cancer was divided into two types according to the microsatellite instability status or promoter hypermethylation of the hMLH1 gene[40,41], or the exposure was nonspecific (e.g., mixed with coffee or tea)[42-45].
Data extraction
One investigator (Y.S.) extracted the following data from each publication: basic study information (first author and year of publication), the country where the study was conducted, the study design, the type of control subjects used in the case-control study (population- or hospital-based), the number of cases, the sample size, the follow-up duration, the type of dairy product and consumption categories, the RRs with 95%CIs for the association between gastric cancer and dairy product consumption, and covariate adjustments. We selected the most fully adjusted RRs for inclusion in the multivariate model.
Quality assessment
To assess the study quality, we adopted an evaluation system that was based on the Newcastle-Ottawa Scale. The quality of the included studies was evaluated based on the following three aspects: the selection of the study populations, the comparability of the populations, and the ascertainment of exposure. The highest possible score was 9 stars, and a high quality study was defined as a study with ≥ 6 stars.
Statistical analysis
The RR and its corresponding 95%CI were used to measure the effect of interest. Because gastric cancer risk is relatively low in the general population, the ORs from case-control studies were assumed to be the same as the RRs and HRs. For simplicity, we report all results as the RR[46]. One of the included case-control studies used two control groups (population- and hospital-based); we used the RR in relation to the population control subjects. We assessed the statistical heterogeneity among the studies with both the Q and I2 statistics. The null hypothesis that the studies are homogeneous was refused if the P-value for heterogeneity was < 0.10 or the I2 was > 50%. Based on the heterogeneity results, we used either the fixed effects model or the random effects model to compute the summary relative risks (SRRs) and 95%CIs. The causes of heterogeneity were investigated by subgroup analyses. We conducted a sensitivity analysis by omitting one study in turn and examining whether the results were influenced by any single study. Funnel plots, the Begg’s adjusted rank correlation test[47], and the Egger’s regression asymmetry test[48] were used to assess the publication bias (P < 0.10 was taken to indicate significant publication bias). We performed all analyses with STATA11.0 software (STATA, College Station, TX, United States). All statistical tests were two-sided.
RESULTS
Study characteristics and quality assessment
We found 39 studies[4,24,39,49-84] that were potentially eligible for inclusion in this meta-analysis, including 10 cohort studies[49-58] and 29 case-control studies[4,24,39,59-84]. The process of selecting studies is shown in Figure 1. Of the 10 cohort studies, 3 were carried out in the United States, 4 in Japan, 2 in Europe, and 1 in South Korea (Table 1). Of the 29 case-control studies, 5 were carried out in Japan; 4 in the United States; 3 in China; 2 each in Iran, Poland, Turkey, and Italy; and 1 each in Germany, Sweden, Portugal, France, Mexico, Venezuela, South Korea, Serbia, and Uruguay. One case-control study[39] had two control groups (population- and hospital-based) (Table 2).
Figure 1.
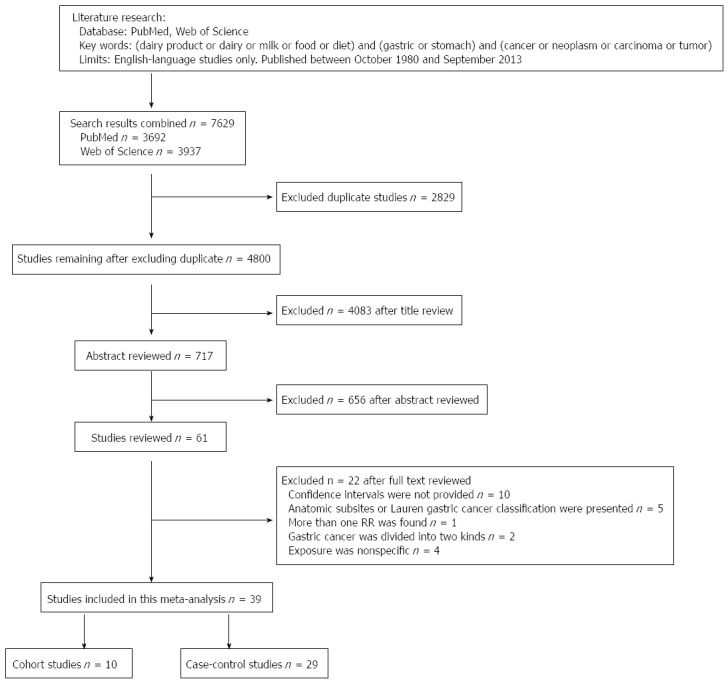
Flow chart of the selection of publications included in this meta-analysis.
Table 1.
Characteristics of published cohort studies on dairy product consumption and gastric cancer risk
| Ref. | Region and design | No. of cases (outcome) | Sample size | Follow-up (yr) | Type of meat and consumption categories | Adjusted RR (95%CI) | Covariate adjustments |
| Nomura et al[49], 1990 | United States; cohort | 150 (incidence) | 7990 men | 19 | Milk (times/wk) ≤ 1 2-4 ≥ 5 Ice cream (times/wk) ≤ 1 2-4 ≥ 5 Butter, margarine, cheese (times/wk) ≤ 1 2-4 ≥ 5 | 1.0 1.5 (0.8-2.5) 1.2 (0.8-1.6) 1.0 0.9 (0.6-1.3) 1.1 (0.7-1.8) 1.0 1.1 (0.5-2.1) 1.4 (0.9-2.2) | Age |
| Kneller et al[50], 1991 | United States; cohort | 75 (mortality) | 17633 men | 20 | Dairy Quartiles Milk (glasses/d) < 1 1 2-3 ≥ 4 Used before but not currently | 1.00 1.2 (0.67-2.26) 1.1 (0.61-2.10) 1.2 (0.61-2.44) 1.00 1.4 (0.76-2.74) 0.9 (0.45-1.70) 2.4 (1.10-5.04) 1.2 (0.50-2.68) | Year of birth and current cigarette smoking |
| Galanis et al[51], 1998 | United States; cohort | 108 (44 women, 64 men) (incidence) | 11907 (6297 women, 5610 men) | 14.8 | Milk (cups/d) 0 ≥ 1 Milk (cups/d) 0 ≥ 1 Milk (cups/d) 0 ≥ 1 | Women 1.0 1.0 (0.5-1.8) Men 1.0 1.0 (0.6-1.7) Total (women and men) 1.0 1.0 (0.7-1.5) | Age, years of education, Japanese place of birth, gender. Among man also adjusted for cigarette smoking and alcohol intake status |
| Ngoan et al[52], 2002 | Japan; cohort | 116 (39 women, 77 men) (mortality) | 13250 (7333 women, 5917 men) | 10.5 | Fresh milk ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Fresh milk ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Fresh milk ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Milk products ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Milk products ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Milk products ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Margarine ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d Margarine ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d | Women 0.9 (0.3-2.3) 1.3 (0.6-2.6) Men 1.3 (0.7-2.4) 0.9 (0.5-1.6) Total (women and men) 1.5 (0.8-3.0) 0.8 (0.4-1.6) Women 1.2 (0.3-5.6) 3.1 (0.8-11.6) Man 1.7 (0.8-3.6) 1.5 (0.5-4.2) Total (women and men) 1.3 (0.6-2.8) 1.4 (0.5-3.6) Women 0.6 (0.2-1.9) 0.9 (0.3-2.9) Man 1.1 (0.5-2.1) 1.1 (0.5-2.6) | Women or men: adjust for age Total: adjust for age, sex, smoking, processed meat, liver, cooking or salad oil, suimono and pickled food |
| Margarine ≤ 2-4 times/mo 2-4 times/wk ≥ 1 time/d | Total (women and men) 0.8 (0.4-1.8) 0.7 (0.3-1.8) | ||||||
| Khan et al[53], 2004 | Japan; cohort | 51 (15 women and 36 men) (mortality) | 3158 (1634 women, 1524 men ) | 14.8 | Milk A B Milk A B Butter/margarine A B Butter/margarine A B Cheese A B Cheese A B | Women 1.0 (0.4-3.0) Men 1.1 (0.5-2.2) Women 0.6 (0.2-2.3) Men 1.7 (0.9-3.2) Women 1.2 (0.3-5.4) Men 1.2 (0.5-3.0) | Women: adjust for age, health status, health education, health screening and smoking Men: adjust for age, smoking |
| Tokui et al[54], 2005 | Japan; cohort | 859 (285 women, 574 men) (mortality) | 110792 women and men | 11 | Milk None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Milk None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Yogurt None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Yogurt None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Cheese None 1-2 times/mo 1-2 times/wk 3-4 times/wk ≥ 1 times/d Cheese None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Butter None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Butter None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d | Women 1.00 (Referent) 0.62 (0.33-1.18) 0.90 (0.58-1.38) 0.78 (0.50-1.21) 0.83 (0.60-1.13) Men 1.00 (Referent) 1.13 (0.79-1.63) 1.17 (0.87-1.58) 1.08 (0.79-1.48) 1.06 (0.84-1.35) Women 1.00 (Referent) 0.95 (0.63-1.43) 1.38 (0.93-2.05) 0.85 (0.44-1.63) 0.88 (0.47-1.64) Men 1.00 (Referent) 0.69 (0.49-0.98) 0.86 (0.58-1.28) 1.22(0.76-1.97) 0.82 (0.50-1.37) Women 1.00 (Referent) 1.31(0.92-1.85) 0.93 (0.57-1.52) 0.60 (0.24-1.46) 1.18 (0.52-2.69) Men 1.00 (Referent) 0.93 (0.72-1.20) 1.08 (0.80-1.48) 1.32 (0.86-2.04) 0.79 (0.39-1.61) Women 1.00 (Referent) 0.76 (0.50-1.17) 1.27 (0.85-1.90) 0.37 (0.14-1.01) 1.22 (0.65-2.26) Men 1.00 (Referent) 0.97 (0.75-1.27) 0.96 (0.70-1.33) 0.92 (0.57-1.47) 0.70 (0.38-1.29) | Age |
| Margarine None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d Margarine None 1-2/mo 1-2/wk 3-4/wk ≥ 1/d | Women 1.00 (Referent) 0.98 (0.64-1.50) 1.09 (0.74-1.59) 0.69 (0.39-1.22) 0.82 (0.50-1.33) Men 1.00 (Referent) 0.83 (0.61-1.12) 1.14 (0.87-1.49) 0.92 (0.62-1.36) 0.72 (0.48-1.10) | ||||||
| Van der Pols et al[55], 2007 | United Kingdom, Scotland; cohort | 770 (mortality) | 4383 children | 65 | Total dairy Group 1 (low) Group 2 Group 3 Group 4 (high) Milk (cups/d) < 0.5 0.5-0.8 > 0.8 - < 1.2 ≥ 1.2 | 1.00 1.17 (0.39-3.47) 1.46 (0.44-4.89) 0.81 (0.09-7.34) 1.00 1.17 (0.41-3.34) 1.40(0.46-4.26) 0.79 (0.11-5.73) | Age, sex, energy, fruit, and calcium intakes |
| Pham et al[56], 2010 | Japan; cohort | 477 (157 women, 320 men) (mortality) | 63403 (women 37 673, men 25 730) | - | Dairy products pattern (factor score) Quartiles Dairy products pattern (factor score) Quartiles | Women 1.00 0.96 (0.64-1.45) 0.74 (0.47-1.18) 0.77 (0.48-1.23) Men 1.00 0.82 (0.61-1.10) 0.74 (0.54-1.01) 0.72 (0.52-0.99) | Age, tobacco smoking status, history of gastric ulcer, stomach cancer screening, body mass index, educational level, and total energy intake |
| Buckland et al[57], 2010 | European countries; cohort | 449 (190 women, men 259) (incidence) | 485044 (women 340467, men 144577) | 8.9 | Dairy products Tertiles Dairy products Tertiles Dairy products Tertiles | Women 1 (reference) 1.22 (0.84-1.77) 0.97 (0.65-1.45) Men 1 (reference) 0.86 (0.63-1.17) 0.98 (0.70-1.37) Total (women and men) 1 (reference) 1.01 (0.80-1.27) 0.97 (0.75-1.25) | Sex, body mass index, educational level, smoking status, cigarette smoking intensity, and total energy intake. |
| Ko et al[58], 2013 | South Korea; cohort | 166 (incidence) | 9724 women and men | 8.5 | Dairy products Almost never 1-4 times/mo 1-4 times/wk ≥ 1 time/d Dairy products Low intake High intake Dairy products Low intake High intake | Total (women and men) 1.15 (0.78-1.70) 1.25 (0.82-1.92) 1.30 (0.83-2.06) Women 1.10 (0.91-1.33) Men 1.05 (0.92-1.19) | Total: adjust for age, sex, cigarette smoking, body mass index, alcohol drinking, and area of residence Women or men: adjust for age, cigarette smoking, body mass index, alcohol drinking, and area of residence |
A: Reference group = took never + took several times per year + took several times per month; B: Comparison group = took several times per week + took every day. RR: Relative risk (rate ratio or hazard ratio); CI: Confidence interval.
Table 2.
Characteristics of published case-control studies on dairy product consumption and gastric cancer risk
| Ref. | Region and design | Cases/controls | Type of item and consumption categories | Adjusted RR (95%CI) | Covariate adjustments |
| Correa et al[59], 1985 | United States; case control (hospital based) | 391/391 | Dairy products Dairy products | Whites 1.06 (0.68-1.63) Blacks 0.85 (0.53-1.34) | Age, sex, respondent status, education, income, tobacco, and alcohol use |
| Wu-Williams et al[60], 1990 | United States; case control (population based) | 137/137 | Milk ≤ 1/wk ≥ 2-4/wk ≥ 5/wk | 1.0 0.8 (0.4-1.7) 1.0 (0.6-1.7) | |
| Mettlin et al[24], 1990 | United States; case control (hospital based) | 115/1300 | Whole Milk None < Daily Daily 2%Milk None < Daily Daily Skim Milk None < Daily Daily | 1.0 2.9 (1.7-4.9) 3.9 (2.3-6.6) 1.0 0.5 (0.3-0.9) 0.4 (0.2-0.6) 1.0 1.0 (0.5-2.3) 0.5 (0.2-1.3) | Age, sex, smoking history, education, and country of residence |
| Boeing et al[61], 1991 | Germany; case control (hospital based) | 143/579 | Milk Tertiles Dairy products Tertiles Cheese Tertiles | 1.0 1.23 (0.77-1.97) 1.31 (0.82-2.10) 1.0 0.71 (0.45-1.12) 0.63 (0.39-1.03) 1.0 1.09 (0.67-1.75) 0.55 (0.30-0.98) | Milk and dairy products: adjust for age, sex, hospital Cheese: adjust for age, sex, hospital, raw vegetables, citrus fruit, processed meat, whole-meal bread |
| Boeing et al[62], 1991 | Poland; case control (hospital based) | 741/741 | Cheese score Low Moderate High | 1.0 0.92 (0.73-1.17) 0.92 (0.67-1.26) | Age, sex, education, occupation, residence |
| Yu et al[63], 1991 | China, case control (population based) | 84/2676 | Milk Nonusers Users | 1.0 0.9 (0.5-1.7) | Age, sex, family income, family history of stomach cancer, family history of other cancer, history of tuberculosis, blood type, cigarette smoking, alcohol, strong tea, fruit, and milk consumption |
| Hoshiyama et al[39], 1992 | Japan; case control population based hospital based | 294/294 294/202 | Dairy products ≤ 1/wk 2-4/wk ≥ 5/wk Dairy products ≤ 1/wk 2-4/wk ≥ 5/wk | 1.0 0.6 (0.4-1.0) 0.8 (0.6-1.2) 1.0 0.9 (0.5-1.6) 1.0 (0.7-1.6) | Age, sex, administrative division, and smoking status Age, sex, area, smoking status |
| Memik et al[64], 1992 | Turkey; case control (population based) | 252/609 | Milk 0-200 mL/wk 200-600 mL/wk 600 mL/wk | 1.0 0.91 (0.43-1.94) 5.33 (3.09-9.26) | |
| Hansson et al[65], 1993 | Sweden; case control (population based) | 338/669 | Whole milk (mL/wk) ≤ 199 > 199-2700 > 2700-4100 > 4100-6900 > 6900 Skimmed milk (mL/wk) 0 > 0 | 1.19 (0.68-2.05) 1.58 (0.97-2.59) 1.35 (0.83-2.20) 1.73 (1.02-2.94) 0.77 (0.53-1.12) | Age, gender, SES |
| Soured milk (times/mo) ≤ 0.9 > 0.9-7 | 0.82 (0.54-1.24) | ||||
| > 7-11 > 11-19 > 19 Cheese (times/mo) ≤ 7 > 7-29 > 29-59 > 59 | 0.84 (0.55-1.26) 0.81 (0.51-1.30) 0.90 (0.58-1.42) 1.03 (0.64-1.65) 0.84 (0.53-1.31) 0.79 (0.48-1.32) | ||||
| Inoue et al[66], 1994 | Japan; case control (hospital based) | 668/668 | Milk < 3-4 times/wk ≥ 3-4 times/wk | 1.0 (0.80-1.25) | Sex |
| Falcao et al[67], 1994 | Portugal; case control (hospital based) | 74/193 | Milk ≤ 0.51/d | 0.33 (0.11-0.99) | |
| Cornée et al[68], 1995 | France; case control (hospital based) | 92/128 | Total dairy products Tertiles Milk (all types) Tertiles Hard cheese Tertiles Soft cheese Tertiles Yoghurt Tertiles Butter and cream Tertiles | 1.0 1.10 (0.53-2.30) 1.80 (0.89-3.66) 1.0 1.53 (0.73-3.19) 1.57(0.75-3.29) 1.0 1.09 (0.52-2.26) 1.48 (0.74-2.96) 1.0 0.64 (0.31-1.30) 0.92 (0.47-1.80) 1.0 0.86 (0.40-1.88) 0.75 (0.37-1.54) 1.0 1.31 (0.64-2.71) 1.44 (0.71-2.93) | Age, sex, occupation and total energy intake |
| Muñoz et al[4], 1997 | Italy; case-control (hospital based) | 88/103 | Butter (score) Low Intermediate/high Margarine (score) Low Intermediate/high | 1.0 1.88 (1.03-3.44) 1.0 2.42 (1.06-5.51) | Age, sex, area of residence, and education |
| Watabe et al[69], 1998 | Japan; case control (population based) | 242/484 | Milk Daily Cheese ≥ 3/wk Butter ≥ 3/wk Yogurt Daily | 0.6 (0.43-0.83) 0.83 (0.51-1.33) 1.57 (0.96-2.53) 0.66 (0.38-1.09) | |
| Ward et al[70], 1999 | Mexico; case control (population based) | 220/752 | Dairy products (times/wk) < 5 5-10 11-16 ≥ 17 | 1.0 2.1 (1.2-3.7) 2.3 (1.2-4.2) 2.7 (1.4-5.0) | Age, gender, total calories, chili pepper consumption, added salt, history of peptic ulcer, cigarette smoking, SES |
| Muñoz et al[71], 2001 | Venezuela, case control (population based) | 292/485 | Dairy products Quartiles | 1.0 1.58 (0.98-2.55) 2.08 (1.30-3.32) 2.43 (1.46-4.04) | Age, sex, tobacco, alcohol, total calories and SES |
| Kim et al[72], 2002 | Korea; case control (hospital based) | 136/136 | Milk and milk products Low Medium High | 1.00 0.75 (0.42-1.35) 0.68 (0.34-1.36) | Age, sex, SES, family history and refrigerator use |
| Chen et al[73], 2002 | United States; case control (population based) | 124/449 | Dairy products (times/wk) Quartiles | 0.79 (0.35-1.7) 1.40 (0.68-2.8) 0.76 (0.34-1.7) | Age, sex, energy, respondent type, body mass index, alcohol use, tobacco use, education, family history, vitamin supplement |
| Milk (times/wk) Quartiles | 0.72 (0.33-1.6) 1.7 (0.85-3.5) 0.86 (0.39-1.9) | ||||
| Ito et al[74], 2002 | Japan; case control (hospital based) | 508/36490 | Milk Almost never Occasionally 3-4 times/wk Everyday | 1.0 0.98 (0.75-1.27) 1.09 (0.85-1.39) 0.85 (0.62-1.18) | Age, year, season of fist hospital visit, smoking habit, family history of gastric cancer |
| Lissowska et al[75], 2004 | Poland; case control (population based) | 274/463 | Diary product (times/wk) < 18.9 18.9-25.8 25.9-32.9 > 32.9 | 1.0 0.96 (0.63-1.46) 0.87 (0.54-1.40) 0.94 (0.57-1.54) | Age, sex, education, smoking, and calories from food |
| De Stefani et al[76], 2004 | Uruguay; case control (hospital based) | 240/960 | Dairy foods Tertiles Dairy foods Highest tertile vs Lowest tertile Dairy foods Highest tertile vs Lowest tertile | Total (women and men) 1.0 1.24 (0.85-1.80) 0.89 (0.59-1.33) Women 1.45 (0.64-3.29) Men 0.75 (0.46-1.20) | Total: adjust for age, sex, residence, urban/rural status, education, body mass index, and total energy intake Women or men: adjust for age, residence, urban/rural status, education, body mass index, tobacco smoking, alcohol drinking, and total energy intake |
| Huang et al[77], 2004 | Japan; case control (hospital based) | GCFH(+) 464/6 310 GCFH(-) 1524/44 396 | Milk < 1/d ≥ 1/d Milk < 1/d ≥ 1/d | 0.86 (0.70-1.05) 0.98 (0.88-1.09) | Age, sex |
| Fei et al[78], 2006 | China; case control (hospital based) | 189/567 | Milk products High vs low | 0.690 (0.524-0.907) | |
| Lucenteforte et al[79], 2008 | Italy; case control (hospital based) | 230/547 | Milk and yogurt (servings/wk) ≤ 0.5 > 0.5-4.5 > 4.5-7 > 7-9 > 9-24 Cheese (servings/wk) ≤ 1.8 > 1.8-3.0 > 3.0-3.8 > 3.8-5.1 > 5.1-10.7 | 1.0 0.77 (0.45-1.33) 0.81 (0.50-1.30) 0.88 (0.50-1.54) 1.06 (0.64-1.78) 1.0 1.38 (0.79-2.41) 1.43 (0.82-2.49) 1.22 (0.70-2.15) 1.63 (0.92-2.90) | Age, sex, education, year of interview, body mass index, tobacco smoking, family history of stomach cancer, and total energy intake |
| Chen et al[80], 2009 | China; case control (hospital based) | 41/205 | Dairy products (times/wk) < 3 ≥ 3 Milk (/d) No Yes | 1.00 0.72 (0.13-4.15) 1.00 1.02 (0.16-7.08) | Age and years of schooling |
| Pourfarzi et al[81], 2009 | Iran; case control (population based) | 217/394 | Dairy products ≤ 2 times/wk 3-4/wk > 1/d Cheese ≤ 2 times/wk 3-4/wk > 1/d | 1.00 3.77 (1.92-7.42) 2.28 (1.23-4.22) 1.00 1.00 (0.39-2.56) 1.16 (0.54-2.51) | Age, sex, education, family history of gastric cancer, citrus fruits, garlic, onion, red meat, fish, dairy products, strength and warmth of tea, preference for salt intake and H. pylori |
| Lazarevic et al[82], 2010 | Serbia; case control (hospital based) | 102/204 | Milk Tertiles Dairy Tertiles | 1.00 2.60 (0.86-7.87) 5.08 (1.59-10.16) 1.00 0.42 (0.20-1.23) 0.63 (0.33-1.72) | Age, sex, residence, education, meals regularity, tobacco smoking, and history of cancer in the first degree relatives |
| Icli et al[83], 2011 | Turkey; case control (hospital based) | 253/253 | Milk Low Moderate High | 1.0 1.4 (0.7-2.6) | |
| Yogurt Low Moderate High | 1.0 0.8 (0.4-1.5) 1.0 (0.2-4.9) | ||||
| Pakseresht et al[84], 2011 | Iran; case control (population based) | 286/304 | Dairy Per 100 g | 1.01 (0.90-1.13) | Age, sex, education, living area, smoking, gastric symptoms, income, owning refrigerator, duration of using refrigerator, seeds preparing method, frying H. pylori infection. |
RR: Relative risk (rate ratio or hazard ratio); CI: Confidence interval; SES: Socio-economic status; PCC: Population-based case-control; HCC: Hospital-based case-control; H. pylori: Helicobacter pylori.
The quality scores of the included studied are presented in Tables 3 and 4. The quality scores ranged from 5 to 8 for the case-control studies and 7 to 9 for the cohort studies.
Table 3.
Methodological quality of cohort studies included in this meta-analysis
| Ref. | Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Control for important factors1 | Assessment of outcome | Follow-up long enough for outcomes to occur2 | Adequacy of follow-up of cohorts3 | Total quality scores |
| Nomura et al[49], 1990 | * | * | * | * | * | * | * | * | 8 |
| Kneller et al[50], 1991 | * | * | * | * | * | * | * | * | 8 |
| Galanis et al[51], 1998 | * | * | * | * | ** | * | * | * | 9 |
| Ngoan et al[52], 2002 | * | * | * | * | ** | * | * | * | 9 |
| Khan et al[53], 2004 | * | * | * | * | ** | * | * | * | 9 |
| Tokui et al[54], 2005 | * | * | * | * | * | * | * | * | 8 |
| Van der Pols et al[55], 2007 | * | * | - | * | * | * | * | * | 7 |
| Pham et al[56], 2010 | * | * | * | * | ** | * | - | * | 8 |
| Buckland et al[57], 2010 | * | * | * | * | * | * | * | * | 8 |
| Ko et al[58], 2013 | * | * | * | * | ** | * | * | * | 9 |
A maximum of two stars could be awarded for this item. Studies that controlled for age received one start, whereas studies that controlled for smoking or drinking received an additional start;
A cohort study with a follow-up time > 8 years was awarded one start;
A cohort study with a follow-up rate > 70% was awarded one start.
Table 4.
Methodological quality of case-control studies included in this meta-analysis
| Ref. | Adequate definition of cases | Representativeness of cases | Selectionof control | Definition of control | Control for important factor or additional factor1 | Exposure assessment | Same method of ascertainment for cases and cohorts | Nonresponse rate2 | Total quality scores |
| Correa et al[59], 1985 | * | * | - | * | ** | * | * | - | 7 |
| Wu-Williams et al[60], 1990 | * | * | * | - | - | * | * | - | 5 |
| Mettlin et al[24], 1990 | - | * | - | * | ** | * | * | - | 6 |
| Boeing et al[61], 1991 | * | * | - | * | * | * | * | - | 6 |
| Boeing et al[62], 1991 | * | * | - | * | * | * | * | - | 6 |
| Yu et al[63], 1991 | * | * | * | * | ** | * | * | - | 8 |
| Hoshiyama et al[39], 1992 | * | * | * | - | ** | * | * | - | 7 |
| Memik et al[64], 1992 | * | * | * | * | - | - | * | - | 5 |
| Hansson et al[65], 1993 | * | * | * | - | * | * | * | - | 6 |
| Inoue et al[66], 1994 | * | * | - | * | - | * | * | * | 6 |
| Falcao et al[67], 1994 | * | * | - | * | - | * | * | - | 5 |
| Cornée et al[68], 1995 | * | * | - | * | * | * | * | - | 6 |
| Muñoz et al[4], 1997 | * | * | - | * | * | * | * | - | 6 |
| Watabe et al[69], 1998 | * | * | * | - | - | * | * | - | 5 |
| Ward et al[70], 1999 | * | * | * | * | ** | * | * | - | 8 |
| Muñoz et al[71], 2001 | * | * | * | - | ** | * | * | - | 7 |
| Kim et al[72], 2002 | * | * | - | * | * | * | * | - | 6 |
| Chen et al[73], 2002 | * | * | * | - | ** | * | * | - | 7 |
| Ito et al[74], 2002 | * | * | - | * | ** | * | * | - | 7 |
| Lissowska et al[75], 200 | * | * | * | - | ** | * | * | - | 7 |
| De Stefani et al[76], 2004 | * | * | - | * | ** | * | * | - | 7 |
| Huang et al[77], 2004 | - | * | - | * | * | * | * | - | 5 |
| Fei et al[78], 2006 | * | * | - | * | - | * | * | - | 5 |
| Lucenteforte et al[79], 2008 | * | * | - | * | ** | * | * | - | 7 |
| Chen et al[80], 2009 | * | * | - | * | * | * | * | - | 6 |
| Pourfarzi et al[81], 2009 | * | * | * | * | * | * | * | - | 7 |
| Lazarevic et al[82], 2010 | * | * | - | * | ** | * | * | - | 7 |
| Icli et al[83], 2011 | * | * | - | * | - | * | * | - | 5 |
| Pakseresht et al[84], 2011 | * | * | * | * | ** | * | * | - | 8 |
A maximum of two stars could be awarded for this item. Studies that controlled for age received one start, whereas studies that controlled for smoking or drinking received an additional start;
One star was assigned if there was no significant difference in the response rate between case and control subjects by using the χ2 test (P = NS). NS: Not significant.
Dairy products
Highest vs lowest intake categories: Thirty-eight studies[4,24,39,49-83] presented results on the comparison between the highest vs lowest dairy consumption categories and gastric cancer risk. We eliminated one study[83] because dairy product consumption was analyzed as a continuous variable. The SRR for gastric cancer, comparing the highest and lowest dairy product consumption categories, was 1.06 (95%CI: 0.95-1.18). Significant heterogeneity was seen among these studies (Q = 112.61; P = 0.000; I2 =67.1%). Both Egger’s test (P = 0.135) and Begg’s test (P = 0.365) had symmetric funnel plots and lacked any indication of publication bias (Figure 2).
Figure 2.
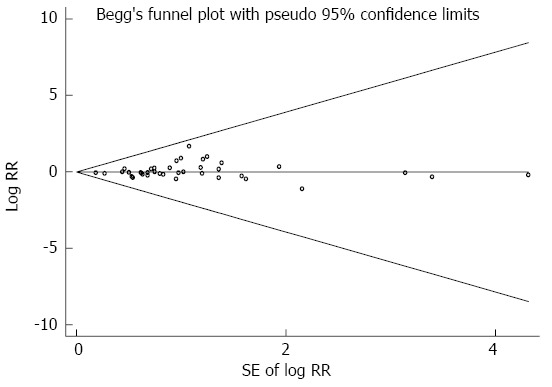
Funnel plot of studies evaluating the association between dairy product consumption and gastric cancer risk.
Sensitivity analysis: We conducted a sensitivity analysis by omitting one study at a time and observing its influence on the overall estimate. The SRR for dairy product consumption and gastric cancer risk was 1.06 (95%CI: 0.94-1.18) after excluding a study by Khan et al[53], which had 9 stars in the quality assessment. The SRR was 1.06 (95%CI: 0.94-1.19) after excluding another study by Correa et al[59] that had divided participants into two ethnic groups. The SRR changed from 1.06 to 1.07 (95%CI: 0.94-1.23) after excluding the study by Huang et al[77] in which participants had a family history of gastric cancer.
Subgroup analysis: In a subgroup analysis performed according to the study design, the SRR for dairy product consumption in hospital-based case-control studies[4,24,59,61,62,66-68,72,74,76-80,82,83] was 0.94 (95%CI: 0.83-1.08). The SRR in population-based case-control studies[39,60,63-65,69-71,73,75,81] was 1.36 (95%CI: 0.94-1.96); for cohort studies[49-58], it was 1.00 (95%CI: 0.89-1.14) (Figure 3A). The population-based case-control studies had significant heterogeneity (Q = 59.14; P = 0.000; I2 = 83.1%). The cohort studies and hospital-based case-control studies did not have significant heterogeneity (Q = 12.90; P = 0.167; I2 = 30.2% and Q = 30.50; P = 0.016; I2 = 47.5%, respectively).
Figure 3.
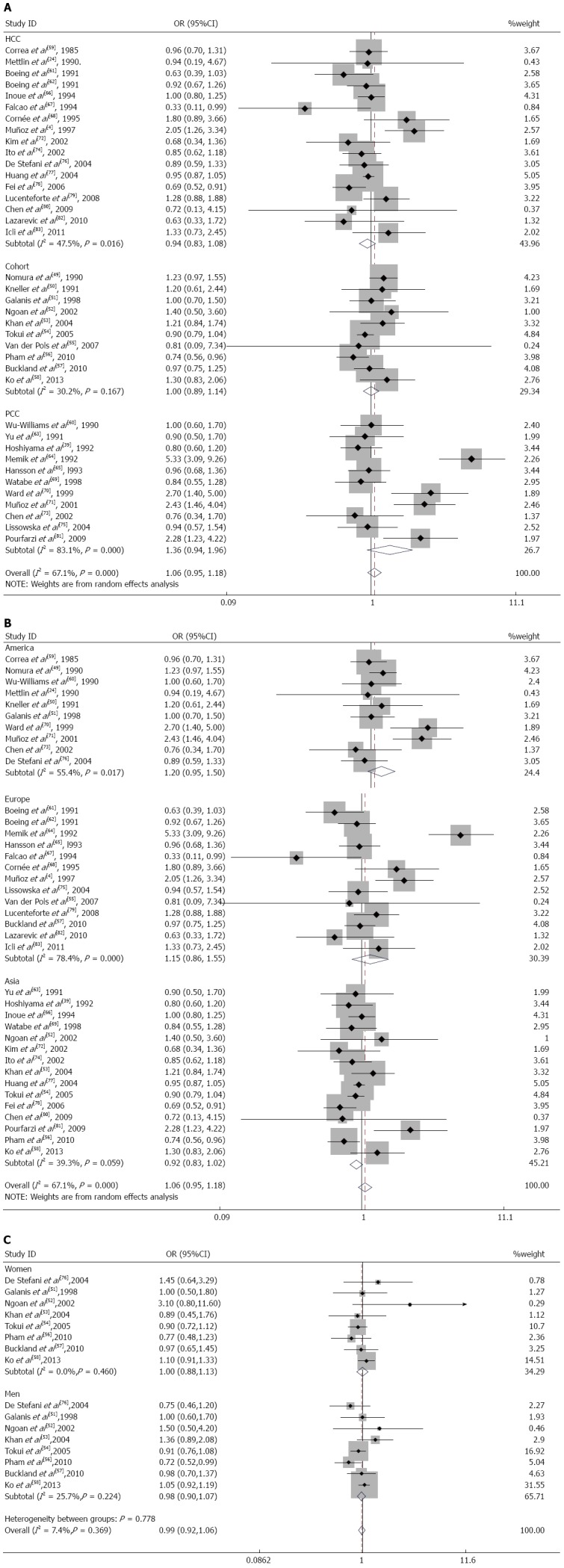
Forest plots of gastric cancer risk associated with dairy product consumption, stratified by study design (A), geographic region (B) and sex (C).
When we analyzed the subgroups according to the geographic region, there was a modest, nonsignificant effect of dairy product consumption on gastric cancer for studies performed in United States[24,49-51,59,60,70,71,73,76] (SRR = 1.20; 95%CI: 0.95-1.50; Q = 20.18; P = 0.017; I2 = 55.4%) and Europe[4,55,57,61,62,64,65,67,68,75,79,82,83] (SRR = 1.15; 95%CI: 0.86-1.55; Q = 55.50; P = 0.000; I2 = 78.4%), but this relationship did not hold for studies in Asia[39,52-54,56,58,63,66,69,72,74,77,78,80,81] (SRR = 0.92; 95%CI: 0.83-1.02; Q = 23.08; P = 0.059; I2 = 39.3%) (Figure 3B).
In the subgroup analysis according to sex, the SRR was 0.98 (95%CI: 0.90-1.07) in men[51-54,56-58,76] and 1.00 (95%CI: 0.88-1.13) in women[51-54,56-58,76]. These studies lacked heterogeneity (Q = 9.42; P = 0.224; I2 =25.7% for men and Q = 6.70; P = 0.460; I2 = 0.0% for women) (Figure 3C).
We also stratified these studies, adjusting for smoking and drinking. The SRR for gastric cancer in 17 studies that were adjusted for smoking[24,39,50,52,53,56-59,63,70,71,73-75,79,82] was 1.06 (95%CI: 0.90-1.25); the SRR in 5 studies that were adjusted for drinking[58,59,63,71,73] was 1.19 (95%CI: 0.81-1.74). There was significant heterogeneity between these smoking-adjusted studies (Q = 35.00; P = 0.004; I2 = 54.3%) and drinking-adjusted studies (Q = 11.47; P = 0.022; I2 = 65.1%) (Table 5).
Table 5.
Subgroup analysis of relative risks for the association between dairy product consumption and gastric cancer risk
| No. of studies | RR (95%CI) | Q-value | pH | I2 | |
| Study design | |||||
| All studies | 38 | 1.06 (0.95-1.18) | 112.61 | 0.000 | 67.1% |
| Cohort studies | 10 | 1.00 (0.89-1.14) | 12.90 | 0.167 | 30.2% |
| HCC studies | 17 | 0.94 (0.83-1.08) | 30.50 | 0.016 | 47.5% |
| PCC studies | 11 | 1.36 (0.94-1.96) | 59.14 | 0.000 | 83.1% |
| Sex | |||||
| Women | 8 | 1.00 (0.88-1.13) | 6.70 | 0.460 | 0.0% |
| Men | 8 | 0.98 (0.90-1.07) | 9.42 | 0.224 | 25.7% |
| Region | |||||
| Asia | 15 | 0.92 (0.83-1.02) | 23.08 | 0.059 | 39.3% |
| Europe | 13 | 1.15 (0.86-1.55) | 55.50 | 0.000 | 78.4% |
| America | 10 | 1.20 (0.95-1.50) | 20.18 | 0.017 | 55.4% |
| Adjustments | |||||
| Smoking No smoking | 1721 | 1.06 (0.90-1.25)1.06 (0.91-1.23) | 35.00 77.31 | 0.0040.000 | 54.3%74.1% |
| DrinkingNo drinking | 533 | 1.19 (0.81-1.74)1.04 (0.93-1.17) | 11.47 98.41 | 0.0220.000 | 65.1%67.5% |
RR: Relative risk (rate ratio or hazard ratio); CI: Confidence interval.
Individual dairy product items
Seven cohort studies[49-55], 6 population-based case-control studies[60,63-65,69,73], and 10 hospital-based case-control studies[24,61,66-68,74,77,80,82,83] of milk consumption were included in our meta-analysis. When we analyzed the effects of the study design, the SRR was 1.05 (95%CI: 0.89-1.23) in cohort studies, 1.16 (95%CI: 0.62-2.17) in population-based case-control studies, and 1.09 (95%CI: 0.87-1.35) in hospital-based case-control studies (Figure 4A). There was significant heterogeneity for population-based case-control studies (Q = 45.23; P = 0.000; I2 = 88.9%) and hospital-based case-control studies (Q = 21.23; P = 0.012; I2 = 57.6%) but not for cohort studies (Q = 6.48; P = 0.372; I2 = 7.4%). We analyzed the geographic region, including studies performed in America[24,49-51,60,73] (SRR = 1.13; 95%CI: 0.92-1.39; Q = 4.99; P = 0.417; I2 = 0.0%), Europe[55,61,64,65,67,68,82,83] (SRR = 1.58; 95%CI: 0.89-2.80; Q = 37.37; P = 0.000; I2 = 81.3%), and Asia[52-54,63,66,69,74,77,80] (SRR = 0.93; 95%CI: 0.86-1.00; Q = 8.30; P = 0.404; I2 = 3.7%). For subgroup analysis according to sex, the SRR of gastric cancer associated with milk consumption was 1.03 (95%CI: 0.85-1.26) in men[51-54] and 0.91 (95%CI: 0.71-1.18) in women[51-54]. These studies lacked heterogeneity (Q = 0.30; P = 0.959; I2 = 0.0% for men and Q = 1.35; P = 0.717; I2 = 0.0% for women).
Figure 4.
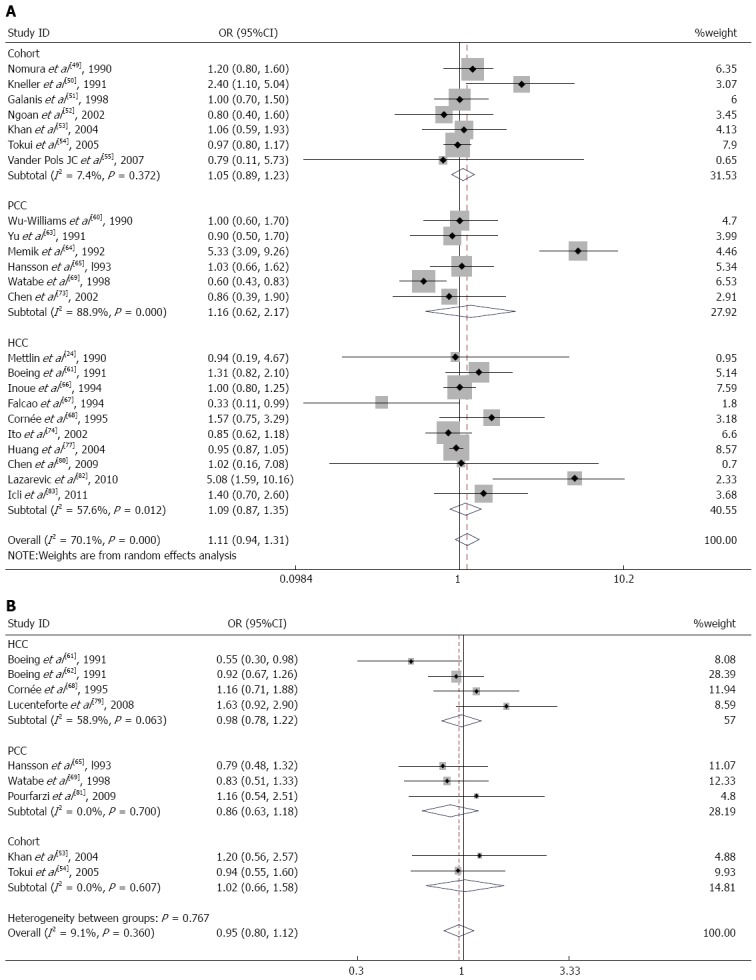
Forest plots of gastric cancer risk associated with milk (A) or cheese (B) consumption, stratified by study design.
Nine studies on cheese consumption were included in our meta-analysis. When we examined these 9 studies together, the SRR for gastric cancer was 0.95 (95%CI: 0.80-1.12) for individuals in the highest compared with the lowest category of cheese consumption. These studies did not have significant heterogeneity (Q = 8.80; P = 0.360; I2 = 9.1%). There was no observed publication bias (Egger’s test: P = 0.621; Begg’s test: P = 0.754). Additionally, we calculated the SRR for gastric cancer separately for three study designs: hospital-based case-control studies[61,62,68,79] (SRR = 0.98; 95%CI: 0.78-1.22), population-based case-control studies[65,69,81] (SRR = 0.86; 95%CI: 0.63-1.18), and cohort studies[53,54] (SRR = 1.02; 95%CI: 0.66-1.58) (Figure 4B). In the subgroup analysis according to the geographic region, studies in Europe[61,62,65,68,79] had an SRR of 0.95 (95%CI: 0.77-1.16; Q = 7.87; P = 0.097; I2 = 49.2%), whereas studies in Asia[53,54,69,81] had an SRR of 0.96 (95%CI: 0.71-1.29; Q = 0.92; P = 0.820; I2 = 0.0%).
We included four studies specifically reporting on yogurt consumption[54,68,69,83] in our meta-analysis. The SRR for gastric cancer for the highest vs lowest yogurt consumption category was 0.79 (95%CI: 0.41-1.51) in hospital-based case-control studies[68,83], 0.66 (95%CI: 0.39-1.12) in population-based case-control studies[69], and 0.84 (95%CI: 0.57-1.24) in cohort studies[54]. When all four studies were examined together, the SRR was 0.77 (95%CI: 0.58-1.03). There was no evidence of heterogeneity (Q = 0.62; P = 0.891; I2 = 0.0%) or publication bias (Egger’s test: P = 0.923; Begg’s test: P = 1.000) when comparing these 4 studies. In the subgroup analysis by geographic region, the SRR was 0.79 (95%CI: 0.41-1.51; Q = 0.10; P = 0.747; I2 = 0.0%) for studies in Europe[68,83] and 0.77 (95%CI: 0.56-1.06; Q = 0.52; P = 0.172; I2 =0.0%) for studies in Asia[54,69].
Only 3 studies[4,54,69] on butter consumption and gastric cancer risk were analyzed in our meta-analysis. An analysis of all 3 studies showed an SRR of 1.35 (95%CI: 0.88-2.08) for high vs low butter consumption. Significant heterogeneity was seen among these 3 studies (Q = 4.40; P = 0.111; I2 = 54.6%), but there was no publication bias (Egger’s test: P = 0.349; Begg’s test: P = 0.296).
A limited number of studies (3 in total)[4,52,54] on margarine were included in our meta-analysis. The SRR for gastric cancer was 1.04 (95%CI: 0.51-2.12) in the pooled analysis, which was obtained from comparing the highest and lowest margarine consumption categories. There was significant heterogeneity in these 3 studies (Q = 6.83; P = 0.033; I2 = 70.7%), but there was no publication bias (Egger’s test: P = 0.601: Begg’s test: P = 1.000).
DISCUSSION
To the best of our knowledge, this is the first meta-analysis to report on an association between dairy product consumption and gastric cancer risk. Twenty-eight case-control studies and 10 cohort studies were combined to expand the sample size and obtain a more creditable result. The SRR for gastric cancer according to the highest vs lowest dairy product consumption category was 1.06 (95%CI: 0.95-1.18). Ten publications[26-35] were excluded from this meta-analysis because they did not provide 95%CIs. The exclusion of those studies reduced the study population, which may have affected our result.
Among the 38 included publications, 1[64] reported an extremely positive association between dairy product consumption and gastric cancer incidence (RR = 5.33). When we excluded this study and recalculated the SRR with the remaining studies, we found that the SRR decreased from 1.06 to 1.01. The modest, nonsignificant risk of gastric cancer from dairy product consumption in this meta-analysis may be attributable to this single study.
Significant heterogeneity was seen among the 38 studies. To investigate the reasons for heterogeneity, we conducted subgroup analyses for the study design, geographic region, sex, and whether there were adjustments for confounders (smoking and drinking). However, we did not find a possible source of heterogeneity, despite the subgroup analyses.
After performing the subgroup analysis for the study design, discrepancies were observed between case-control studies and the cohort studies. Because exposure information is collected after diagnosing gastric cancer, case-control studies can result in selection bias. Individuals in case-control studies, especially in hospital-based case-control studies, may change their earlier long-term dietary habits to avoid disease-related digestive symptoms. For example, patients with gastric cancer may stop drinking milk because of the onset of symptoms (stomachache, vomiting, and nausea). Patients in hospital-based case-control studies were likely to be health conscious, which could strongly influence their dietary habits and confound the observed association. Moreover, a possible reason for the difference in the risk estimates is that the participants in the prospective cohort and case-control studies had different exposure levels for the highest consumption categories.
We found a nonsignificant, protective effect for dairy product consumption on gastric cancer in Asian populations but did not observe this effect for European and American populations. Ethnic differences, different eating habits, and the gap in economic development may explain this discrepancy. For example, milk is a major animal source of dietary protein in an Asian diet, especially in developing countries; however, milk is essentially a breakfast food in the West. Cheese and yogurt are also popular foods in the West. Approximately 60% of the studies used in our meta-analysis were conducted in Europe and America. Differences in dairy production and aseptic technology may also have affected these results.
Smoking and drinking may increase the risk of gastric cancer. We found a nonsignificant risk of developing gastric cancer from dairy product consumption after adjusting for the two factors independently. In our meta-analysis, nearly 50% of the studies were adjusted for smoking, and < 20% of the studies were adjusted for drinking. Because not enough studies were adjusted for the subjects’ smoking and drinking habits, we could not obtain a convincing result.
Different types of individual dairy product produce different effects on the gastric cancer risk. Frequent, long-term dairy product consumption (especially whole milk and butter, which have the highest fat content) can lead to obesity. An animal experiment study[85] has suggested that obesity increases pro-inflammatory immune responses and accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and Th17 response. We found an inverse association between gastric cancer risk and yogurt consumption, but this association was not significant. Yogurt is a type of fermented milk; one study[86] found that Propionibacterium freudenreichii (P. freudenreichii) - the sole bacterium contained in some fermented milk - could inhibit the adhesion of the causative agent for gastric cancer, Helicobacter pylori (H. pylori), to digestive epithelial cells and inhibit H. pylori-induced damage. The study also reported that the aqueous phase of this fermented milk kills human gastric cancer cells via metabolites, including propionate and acetate that are released by the bacterium P. freudenreichii. In an animal experiment, P. freudenreichii-fermented dairy had an anti-inflammatory effect[87]. Together, these findings imply that P. freudenreichii-fermented milk can act as a prophylaxis for gastric cancer. Dairy products, especially milk, contain many essential nutrients, such as conjugated linoleic acid, vitamins, and minerals, which may promote positive health effects[88]. Studies[89] performed in animals and in vitro have shown the protective effects of conjugated linoleic acid against carcinogenesis in the forestomach, potentially by inhibiting the cyclooxygenase-2 or lipoxygenase pathway or by inducing the expression of apoptotic genes.
Our meta-analysis has several strengths. First, to the best of our knowledge, this is the first meta-analysis to explore an association between dairy product consumption and gastric cancer risk. We included 10 prospective cohort studies and 29 case-control studies. Second, we conducted a comprehensive search of the literature on the association between dairy product consumption and gastric cancer risk. Third, our research included a large sample size (837072 subjects and 11791 cases). Fourth, by using a large number of studies from 1980 to the present, we enhanced our statistical power for examining the association between dairy product consumption and gastric cancer risk. Fifth, our study was not subject to publication bias such that the probability of publishing a study did not rely on the strength and direction of the association.
Despite these strengths, our meta-analysis has some limitations. First, we calculated the gastric cancer RR according to the highest vs lowest dairy product consumption; as a result, we could not evaluate associations between different dairy product consumption levels and gastric cancer risk. Second, because we could only collect data from published investigations, we did not include any relevant unpublished data, which may affect our results. Moreover, we limited our study to assessing studies that were published in English. Third, the majority of studies reported in this meta-analysis utilized a case-control design, which is more susceptible to recall and selection biases than a cohort design. Fourth, most of studies in our meta-analysis provided RR estimates that were adjusted for a common set of variables (age, sex, and body mass index), but none of the studies could fully adjust for all confounders. For example, infection with H. pylori is a known risk factor for gastric noncardia cancer[90]. In our meta-analysis, only two case-control studies[81,84] were adjusted for H. pylori. Fifth, heterogeneity may be introduced through the methodological differences among the studies, including different intake measurements. Additionally, because most of the studies presented here used food-frequency questionnaires, the results were likely to be affected by misclassification of dairy product consumption. Sixth, few studies were designed to investigate the risk of gastric cancer from dairy product consumption. Seventh, the studies included in our meta-analysis were only conducted in United States, Europe, and Asia, which limits the findings to the studied populations. Thus, studies of other populations are warranted to generalize our findings.
In conclusion, dairy product consumption was associated with a nonsignificantly increased risk of gastric cancer. However, this result should be verified using large, well-designed prospective cohort and case-control studies, especially in Africa. Future studies should control for more potential confounders, especially for confounders with known gastric cancer risks. In addition, further investigation is warranted to determine whether the effect of dairy product consumption varies by gastric cancer type.
COMMENTS
Background
Gastric cancer remains the fourth most common cancer and the second leading cause of cancer-related mortality. It has recently become popular to analyze the risk factors that may be associated with gastric cancer. Some meta-analyses have found that the risk of gastric cancer is associated with processed meat and coffee, and some meta-analyses have reported that dairy product consumption could increase the risk of ovarian and prostate cancers. It remains unclear whether dairy product consumption is a risk factor for gastric cancer.
Research frontiers
Many case-control and cohort studies have researched the association between gastric cancer and dairy product consumption, but the conclusions have been inconsistent. Therefore, the authors performed a meta-analysis to analyze this association.
Innovations and breakthroughs
In this study, the authors found that dairy product consumption was associated with a nonsignificantly increased risk of gastric cancer. To the best of our knowledge, this is the first meta-analysis to report an association between dairy product consumption and gastric cancer risk.
Applications
This study may offer new insight into gastric cancer prevention. The authors observed that the gastric cancer risk is not significantly related to dairy product consumption. However, this result should be verified using large and well-designed studies, especially in Africa. An exploration of the mechanism for this association will be conducted with a future animal experiment.
Terminology
A meta-analysis is a method for explaining the association between two factors. We collected as much case-control and cohort study data as possible to expand our sample size and obtain a more creditable result. Subgroup analyses were used to investigate the reasons for heterogeneity.
Peer review
This paper is potentially interesting since it deals with a very interesting issue in gastroenterology, as well as in public health medicine. This is a well written article in spite of heterogeneity of data of relevant articles. This issue is important and the work is well conducted.
Footnotes
P- Reviewer: Greco L, Kim JH, Nagarajan P, Torre GL S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz SE, Ferraroni M, La Vecchia C, Decarli A. Gastric cancer risk factors in subjects with family history. Cancer Epidemiol Biomarkers Prev. 1997;6:137–140. [PubMed] [Google Scholar]
- 5.Nagase H, Ogino K, Yoshida I, Matsuda H, Yoshida M, Nakamura H, Dan S, Ishimaru M. Family history-related risk of gastric cancer in Japan: a hospital-based case-control study. Jpn J Cancer Res. 1996;87:1025–1028. doi: 10.1111/j.1349-7006.1996.tb03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70:50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 8.Laakkonen A, Pukkala E. Cancer incidence among Finnish farmers, 1995-2005. Scand J Work Environ Health. 2008;34:73–79. doi: 10.5271/sjweh.1167. [DOI] [PubMed] [Google Scholar]
- 9.Navarro Silvera SA, Mayne ST, Risch H, Gammon MD, Vaughan TL, Chow WH, Dubrow R, Schoenberg JB, Stanford JL, West AB, et al. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer. 2008;123:852–860. doi: 10.1002/ijc.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voskuil DW, Vrieling A, van’t Veer LJ, Kampman E, Rookus MA. The insulin-like growth factor system in cancer prevention: potential of dietary intervention strategies. Cancer Epidemiol Biomarkers Prev. 2005;14:195–203. [PubMed] [Google Scholar]
- 11.Hoppe C, Udam TR, Lauritzen L, Mølgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am J Clin Nutr. 2004;80:447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 12.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr. 2005;81:1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E. Dietary influences of 1,25(OH)2 vitamin D in relation to prostate cancer: a hypothesis. Cancer Causes Control. 1998;9:567–582. doi: 10.1023/a:1008835903714. [DOI] [PubMed] [Google Scholar]
- 14.Larsson SC, Bergkvist L, Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort. Am J Clin Nutr. 2005;82:894–900. doi: 10.1093/ajcn/82.4.894. [DOI] [PubMed] [Google Scholar]
- 15.Belury MA. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–2998. doi: 10.1093/jn/131.10.2995. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Millikan RC, Bell DA, Cui L, Tse CK, Newman B, Conway K. Polychlorinated biphenyls, cytochrome P450 1A1 (CYP1A1) polymorphisms, and breast cancer risk among African American women and white women in North Carolina: a population-based case-control study. Breast Cancer Res. 2005;7:R12–R18. doi: 10.1186/bcr941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laden F, Ishibe N, Hankinson SE, Wolff MS, Gertig DM, Hunter DJ, Kelsey KT. Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev. 2002;11:1560–1565. [PubMed] [Google Scholar]
- 18.Schaum J, Schuda L, Wu C, Sears R, Ferrario J, Andrews K. A national survey of persistent, bioaccumulative, and toxic (PBT) pollutants in the United States milk supply. J Expo Anal Environ Epidemiol. 2003;13:177–186. doi: 10.1038/sj.jea.7500269. [DOI] [PubMed] [Google Scholar]
- 19.Larsson SC, Orsini N, Wolk A. Milk, milk products and lactose intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Int J Cancer. 2006;118:431–441. doi: 10.1002/ijc.21305. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97:1768–1777. doi: 10.1093/jnci/dji402. [DOI] [PubMed] [Google Scholar]
- 21.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 22.Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, Norat T. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:37–45. doi: 10.1093/annonc/mdr269. [DOI] [PubMed] [Google Scholar]
- 23.Narisawa T, Reddy BS, Weisburger JH. Effect of bile acids and dietary fat on large bowel carcinogenesis in animal models. Gastroenterol Jpn. 1978;13:206–212. doi: 10.1007/BF02773665. [DOI] [PubMed] [Google Scholar]
- 24.Mettlin CJ, Schoenfeld ER, Natarajan N. Patterns of milk consumption and risk of cancer. Nutr Cancer. 1990;13:89–99. doi: 10.1080/01635589009514049. [DOI] [PubMed] [Google Scholar]
- 25.Li WQ, Park Y, Wu JW, Ren JS, Goldstein AM, Taylor PR, Hollenbeck AR, Freedman ND, Abnet CC. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol. 2013;11:1130–1136.e2. doi: 10.1016/j.cgh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res. 1985;76:705–716. [PubMed] [Google Scholar]
- 27.La Vecchia C, Negri E, Decarli A, D’Avanzo B, Franceschi S. A case-control study of diet and gastric cancer in northern Italy. Int J Cancer. 1987;40:484–489. doi: 10.1002/ijc.2910400409. [DOI] [PubMed] [Google Scholar]
- 28.Kono S, Ikeda M, Tokudome S, Kuratsune M. A case-control study of gastric cancer and diet in northern Kyushu, Japan. Jpn J Cancer Res. 1988;79:1067–1074. doi: 10.1111/j.1349-7006.1988.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 30.Galpin OP, Whitaker CJ, Whitaker R, Kassab JY. Gastric cancer in Gwynedd. Possible links with bracken. Br J Cancer. 1990;61:737–740. doi: 10.1038/bjc.1990.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HH, Wu HY, Chuang YC, Chang AS, Chao HH, Chen KY, Chen HK, Lai GM, Huang HH, Chen CJ. Epidemiologic characteristics and multiple risk factors of stomach cancer in Taiwan. Anticancer Res. 1990;10:875–881. [PubMed] [Google Scholar]
- 32.González CA, Sanz JM, Marcos G, Pita S, Brullet E, Saigi E, Badia A, Riboli E. Dietary factors and stomach cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49:513–519. doi: 10.1002/ijc.2910490407. [DOI] [PubMed] [Google Scholar]
- 33.Tuyns AJ, Kaaks R, Haelterman M, Riboli E. Diet and gastric cancer. A case-control study in Belgium. Int J Cancer. 1992;51:1–6. doi: 10.1002/ijc.2910510102. [DOI] [PubMed] [Google Scholar]
- 34.Ramón JM, Serra L, Cerdó C, Oromí J. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer. 1993;71:1731–1735. doi: 10.1002/1097-0142(19930301)71:5<1731::aid-cncr2820710505>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 35.Xibin S, Moller H, Evans HS, Dixing D, Wenjie D, Jianbang L. Residential Environment, Diet and Risk of Stomach Cancer: a Case-control Study in Linzhou, China. Asian Pac J Cancer Prev. 2002;3:167–172. [PubMed] [Google Scholar]
- 36.Buiatti E, Palli D, Bianchi S, Decarli A, Amadori D, Avellini C, Cipriani F, Cocco P, Giacosa A, Lorenzini L. A case-control study of gastric cancer and diet in Italy. III. Risk patterns by histologic type. Int J Cancer. 1991;48:369–374. doi: 10.1002/ijc.2910480310. [DOI] [PubMed] [Google Scholar]
- 37.Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80:1021–1028. [PubMed] [Google Scholar]
- 38.Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol. 2011;35:e91–e99. doi: 10.1016/j.canep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoshiyama Y, Sasaba T. A case-control study of single and multiple stomach cancers in Saitama Prefecture, Japan. Jpn J Cancer Res. 1992;83:937–943. doi: 10.1111/j.1349-7006.1992.tb02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palli D, Russo A, Ottini L, Masala G, Saieva C, Amorosi A, Cama A, D’Amico C, Falchetti M, Palmirotta R, et al. Red meat, family history, and increased risk of gastric cancer with microsatellite instability. Cancer Res. 2001;61:5415–5419. [PubMed] [Google Scholar]
- 41.Nan HM, Song YJ, Yun HY, Park JS, Kim H. Effects of dietary intake and genetic factors on hypermethylation of the hMLH1 gene promoter in gastric cancer. World J Gastroenterol. 2005;11:3834–3841. doi: 10.3748/wjg.v11.i25.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risch HA, Jain M, Choi NW, Fodor JG, Pfeiffer CJ, Howe GR, Harrison LW, Craib KJ, Miller AB. Dietary factors and the incidence of cancer of the stomach. Am J Epidemiol. 1985;122:947–959. doi: 10.1093/oxfordjournals.aje.a114199. [DOI] [PubMed] [Google Scholar]
- 43.Chow WH, Swanson CA, Lissowska J, Groves FD, Sobin LH, Nasierowska-Guttmejer A, Radziszewski J, Regula J, Hsing AW, Jagannatha S, et al. Risk of stomach cancer in relation to consumption of cigarettes, alcohol, tea and coffee in Warsaw, Poland. Int J Cancer. 1999;81:871–876. doi: 10.1002/(sici)1097-0215(19990611)81:6<871::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Woo HD, Kim J. Nutritional epidemiology of cancer in Korea: recent accomplishments and future directions. Asian Pac J Cancer Prev. 2011;12:2377–2383. [PubMed] [Google Scholar]
- 45.Yassibaş E, Arslan P, Yalçin S. Evaluation of dietary and life-style habits of patients with gastric cancer: a case-control study in Turkey. Asian Pac J Cancer Prev. 2012;13:2291–2297. [PubMed] [Google Scholar]
- 46.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 47.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 48.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura A, Grove JS, Stemmermann GN, Severson RK. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res. 1990;50:627–631. [PubMed] [Google Scholar]
- 50.Kneller RW, McLaughlin JK, Bjelke E, Schuman LM, Blot WJ, Wacholder S, Gridley G, CoChien HT, Fraumeni JF. A cohort study of stomach cancer in a high-risk American population. Cancer. 1991;68:672–678. doi: 10.1002/1097-0142(19910801)68:3<672::aid-cncr2820680339>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173–180. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 52.Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer. 2002;87:37–42. doi: 10.1038/sj.bjc.6600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, Sakauchi F, Washio M, Mori M. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev. 2004;5:58–65. [PubMed] [Google Scholar]
- 54.Tokui N, Yoshimura T, Fujino Y, Mizoue T, Hoshiyama Y, Yatsuya H, Sakata K, Kondo T, Kikuchi S, Toyoshima H, et al. Dietary habits and stomach cancer risk in the JACC Study. J Epidemiol. 2005;15 Suppl 2:S98–108. doi: 10.2188/jea.15.S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Pols JC, Bain C, Gunnell D, Smith GD, Frobisher C, Martin RM. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr. 2007;86:1722–1729. doi: 10.1093/ajcn/86.5.1722. [DOI] [PubMed] [Google Scholar]
- 56.Pham TM, Fujino Y, Kikuchi S, Tamakoshi A, Matsuda S, Yoshimura T. Dietary patterns and risk of stomach cancer mortality: the Japan collaborative cohort study. Ann Epidemiol. 2010;20:356–363. doi: 10.1016/j.annepidem.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Buckland G, Agudo A, Luján L, Jakszyn P, Bueno-de-Mesquita HB, Palli D, Boeing H, Carneiro F, Krogh V, Sacerdote C, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91:381–390. doi: 10.3945/ajcn.2009.28209. [DOI] [PubMed] [Google Scholar]
- 58.Ko KP, Park SK, Yang JJ, Ma SH, Gwack J, Shin A, Kim Y, Kang D, Chang SH, Shin HR, et al. Intake of soy products and other foods and gastric cancer risk: a prospective study. J Epidemiol. 2013;23:337–343. doi: 10.2188/jea.JE20120232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Correa P, Fontham E, Pickle LW, Chen V, Lin YP, Haenszel W. Dietary determinants of gastric cancer in south Louisiana inhabitants. J Natl Cancer Inst. 1985;75:645–654. [PubMed] [Google Scholar]
- 60.Wu-Williams AH, Yu MC, Mack TM. Life-style, workplace, and stomach cancer by subsite in young men of Los Angeles County. Cancer Res. 1990;50:2569–2576. [PubMed] [Google Scholar]
- 61.Boeing H, Frentzel-Beyme R, Berger M, Berndt V, Göres W, Körner M, Lohmeier R, Menarcher A, Männl HF, Meinhardt M. Case-control study on stomach cancer in Germany. Int J Cancer. 1991;47:858–864. doi: 10.1002/ijc.2910470612. [DOI] [PubMed] [Google Scholar]
- 62.Boeing H, Jedrychowski W, Wahrendorf J, Popiela T, Tobiasz-Adamczyk B, Kulig A. Dietary risk factors in intestinal and diffuse types of stomach cancer: a multicenter case-control study in Poland. Cancer Causes Control. 1991;2:227–233. doi: 10.1007/BF00052138. [DOI] [PubMed] [Google Scholar]
- 63.Yu GP, Hsieh CC. Risk factors for stomach cancer: a population-based case-control study in Shanghai. Cancer Causes Control. 1991;2:169–174. doi: 10.1007/BF00056210. [DOI] [PubMed] [Google Scholar]
- 64.Memik F, Nak SG, Gulten M, Ozturk M. Gastric carcinoma in northwestern Turkey: epidemiologic characteristics. J Environ Pathol Toxicol Oncol. 1992;11:335–338. [PubMed] [Google Scholar]
- 65.Hansson LE, Nyrén O, Bergström R, Wolk A, Lindgren A, Baron J, Adami HO. Diet and risk of gastric cancer. A population-based case-control study in Sweden. Int J Cancer. 1993;55:181–189. doi: 10.1002/ijc.2910550203. [DOI] [PubMed] [Google Scholar]
- 66.Inoue M, Tajima K, Hirose K, Kuroishi T, Gao CM, Kitoh T. Life-style and subsite of gastric cancer--joint effect of smoking and drinking habits. Int J Cancer. 1994;56:494–499. doi: 10.1002/ijc.2910560407. [DOI] [PubMed] [Google Scholar]
- 67.Falcao JM, Dias JA, Miranda AC, Leitao CN, Lacerda MM, da Motta LC. Red wine consumption and gastric cancer in Portugal: a case-control study. Eur J Cancer Prev. 1994;3:269–276. doi: 10.1097/00008469-199403030-00005. [DOI] [PubMed] [Google Scholar]
- 68.Cornée J, Pobel D, Riboli E, Guyader M, Hémon B. A case-control study of gastric cancer and nutritional factors in Marseille, France. Eur J Epidemiol. 1995;11:55–65. doi: 10.1007/BF01719946. [DOI] [PubMed] [Google Scholar]
- 69.Watabe K, Nishi M, Miyake H, Hirata K. Lifestyle and gastric cancer: a case-control study. Oncol Rep. 1998;5:1191–1194. doi: 10.3892/or.5.5.1191. [DOI] [PubMed] [Google Scholar]
- 70.Ward MH, López-Carrillo L. Dietary factors and the risk of gastric cancer in Mexico City. Am J Epidemiol. 1999;149:925–932. doi: 10.1093/oxfordjournals.aje.a009736. [DOI] [PubMed] [Google Scholar]
- 71.Muñoz N, Plummer M, Vivas J, Moreno V, De Sanjosé S, Lopez G, Oliver W. A case-control study of gastric cancer in Venezuela. Int J Cancer. 2001;93:417–423. doi: 10.1002/ijc.1333. [DOI] [PubMed] [Google Scholar]
- 72.Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer. 2002;97:531–535. doi: 10.1002/ijc.10111. [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, Russell RM, Weisenburger DD, Tucker KL. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137–144. doi: 10.1093/ajcn/75.1.137. [DOI] [PubMed] [Google Scholar]
- 74.Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga S. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol. 2003;13:24–31. doi: 10.1016/s1047-2797(02)00269-7. [DOI] [PubMed] [Google Scholar]
- 75.Lissowska J, Gail MH, Pee D, Groves FD, Sobin LH, Nasierowska-Guttmejer A, Sygnowska E, Zatonski W, Blot WJ, Chow WH. Diet and stomach cancer risk in Warsaw, Poland. Nutr Cancer. 2004;48:149–159. doi: 10.1207/s15327914nc4802_4. [DOI] [PubMed] [Google Scholar]
- 76.De Stefani E, Correa P, Boffetta P, Deneo-Pellegrini H, Ronco AL, Mendilaharsu M. Dietary patterns and risk of gastric cancer: a case-control study in Uruguay. Gastric Cancer. 2004;7:211–220. doi: 10.1007/s10120-004-0295-2. [DOI] [PubMed] [Google Scholar]
- 77.Huang XE, Hirose K, Wakai K, Matsuo K, Ito H, Xiang J, Takezaki T, Tajima K. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pac J Cancer Prev. 2004;5:419–427. [PubMed] [Google Scholar]
- 78.Fei SJ, Xiao SD. Diet and gastric cancer: a case-control study in Shanghai urban districts. Chin J Dig Dis. 2006;7:83–88. doi: 10.1111/j.1443-9573.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 79.Lucenteforte E, Scita V, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Food groups and alcoholic beverages and the risk of stomach cancer: a case-control study in Italy. Nutr Cancer. 2008;60:577–584. doi: 10.1080/01635580802054512. [DOI] [PubMed] [Google Scholar]
- 80.Chen MJ, Wu DC, Lin JM, Wu MT, Sung FC. Etiologic factors of gastric cardiac adenocarcinoma among men in Taiwan. World J Gastroenterol. 2009;15:5472–5480. doi: 10.3748/wjg.15.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pourfarzi F, Whelan A, Kaldor J, Malekzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran--a population based study. Int J Cancer. 2009;125:1953–1960. doi: 10.1002/ijc.24499. [DOI] [PubMed] [Google Scholar]
- 82.Lazarevic K, Nagorni A, Rancic N, Milutinovic S, Stosic L, Ilijev I. Dietary factors and gastric cancer risk: hospital-based case control study. J BUON. 2010;15:89–93. [PubMed] [Google Scholar]
- 83.Icli F, Akbulut H, Yalcin B, Ozdemir F, Isıkdogan A, Hayran M, Unsal D, Coskun S, Buyukcelik A, Yamac D. Education, economic status and other risk factors in gastric cancer: "a case-control study of Turkish Oncology Group". Med Oncol. 2011;28:112–120. doi: 10.1007/s12032-009-9406-6. [DOI] [PubMed] [Google Scholar]
- 84.Pakseresht M, Forman D, Malekzadeh R, Yazdanbod A, West RM, Greenwood DC, Crabtree JE, Cade JE. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 2011;22:725–736. doi: 10.1007/s10552-011-9744-5. [DOI] [PubMed] [Google Scholar]
- 85.Ericksen RE, Rose S, Westphalen CB, Shibata W, Muthupalani S, Tailor Y, Friedman RA, Han W, Fox JG, Ferrante AW, et al. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut. 2014;63:385–394. doi: 10.1136/gutjnl-2013-305092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cousin FJ, Jouan-Lanhouet S, Dimanche-Boitrel MT, Corcos L, Jan G. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PLoS One. 2012;7:e31892. doi: 10.1371/journal.pone.0031892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cousin FJ, Foligné B, Deutsch SM, Massart S, Parayre S, Le Loir Y, Boudry G, Jan G. Assessment of the probiotic potential of a dairy product fermented by Propionibacterium freudenreichii in piglets. J Agric Food Chem. 2012;60:7917–7927. doi: 10.1021/jf302245m. [DOI] [PubMed] [Google Scholar]
- 88.Kelley NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J Nutr. 2007;137:2599–2607. doi: 10.1093/jn/137.12.2599. [DOI] [PubMed] [Google Scholar]
- 89.Haug A, Christophersen OA, Høstmark AT, Harstad OM. [Milk and health] Tidsskr Nor Laegeforen. 2007;127:2542–2545. [PubMed] [Google Scholar]
- 90.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]


