Abstract
Hepatic encephalopathy (HE) is a cognitive disturbance characterized by neuropsychiatric alterations. It occurs in acute and chronic hepatic disease and also in patients with portosystemic shunts. The presence of these portosystemic shunts allows the passage of nitrogenous substances from the intestines through systemic veins without liver depuration. Therefore, the embolization of these shunts has been performed to control HE manifestations, but the presence of portal vein thrombosis is considered a contraindication. In this presentation we show a cirrhotic patient with severe HE and portal vein thrombosis who was submitted to embolization of a large portosystemic shunt. Case report: a 57 years-old cirrhotic patient who had been hospitalized many times for persistent HE and hepatic coma, even without precipitant factors. She had a wide portosystemic shunt and also portal vein thrombosis. The abdominal angiography confirmed the splenorenal shunt and showed other shunts. The larger shunt was embolized through placement of microcoils, and the patient had no recurrence of overt HE. There was a little increase of esophageal and gastric varices, but no endoscopic treatment was needed. Since portosystemic shunts are frequent causes of recurrent HE in cirrhotic patients, portal vein thrombosis should be considered a relative contraindication to perform a shunt embolization. However, in particular cases with many shunts and severe HE, we found that one of these shunts can be safely embolized and this procedure can be sufficient to obtain a good HE recovery. In conclusion, we reported a case of persistent HE due to a wide portosystemic shunt associated with portal vein thrombosis. As the patient had other shunts, she was successfully treated by embolization of the larger shunt.
Keywords: Recurrent hepatic encephalopathy, Liver cirrhosis, Port systemic shunt, Shunt embolization, Portal vein thrombosis
Core tip: Portosystemic shunts are a cause of hepatic encephalopathy and decreased survival of cirrhotic patients. The embolization of these shunts has been performed to control hepatic encephalopathy manifestations, but the presence of portal vein thrombosis is considered a contraindication. We presented a cirrhotic patient with persistent hepatic encephalopathy and hepatic coma. She had a wide splenorenal shunt and also portal vein thrombosis. As she had other shunts, we performed the embolization of the largest shunt and she achieved a good recovery. In conclusion, for patients with many shunts and severe hepatic encephalopathy, one of these shunts can be successfully embolized.
INTRODUCTION
According to the increase of portal pressure, many cirrhotic patients develop decompensated liver disease, and many of these complications are still a challenge. Hepatic encephalopathy (HE) is a severe neuropsychiatric illness that occurs in acute and chronic liver diseases, often in cirrhotic patients. HE is not always reversible, and can lead to hepatic coma and death[1]. Furthermore, multiple bouts of hepatic coma are the only known risk factor for hepatocerebral degeneration[2]. The latter disease can be distinguished from HE because it leads to typical motor disturbances, varying from Parkinsonism to chorea.
Overt HE can afflict 30%-40% of cirrhotic patients, and after one episode, the overall survival is only 15% after three years[3,4]. HE is classified as type A when it occurs in acute liver failure, type B when there are portosystemic bypasses without intrinsic hepatocellular disease and type C in the presence of cirrhosis and portal hypertension with portosystemic shunts[5]. Another classification divides HE presentation into overt (episodic or persistent) and covert (minimal)[6]. HE intensity is graded according to the West Haven criteria. Cerebral toxicity in HE is caused by many factors, but the lack of hepatic conversion of ammonia to urea and glutamine is still considered a key point. The blood ammonia reaches the central nervous system and increases glutamine formation inside the astrocytes, leading to some HE manifestations.
Many patients with episodic HE can be treated only by correcting the trigger factor. Patients with persistent HE usually need medications that prevent the ammonia formation from the gut and kidneys. For this purpose, antibiotics, non-absorbable disaccharides and l-ornithine-l-aspartate (LOLA) are frequently employed. The most severe cases are commonly associated with portosystemic shunts, which can be embolized to improve HE manifestations, hepatic function, quality of life and survival[7-13].
From 46% to 70% of patients with refractory HE present with large spontaneous portosystemic shunts, which represent a therapeutic target for their neurological condition[7]. Even so, portal vein thrombosis (PVT) is considered a contraindication for embolization of these shunts, and cases with PVT are excluded from studies aimed to evaluate the results of embolization procedures[7].
Additionally, there is no doubt that liver cirrhosis is a major cause of PVT. The reported rates of PVT are in the range of 0.6%-15.8% in patients with liver cirrhosis, but these rates increase in relation to the patients’ age and their liver disease severity, reaching 15% in patients awaiting liver transplantation[14]. Unfortunately, liver transplantation for patients with advanced PVT (grades 3-4 according to the Yerdel classification) is a big challenge, and even in specialized centers the results are not as good as in patients without PVT[15]. Thus, there is a significant amount of cirrhotic patients who cannot be included in the liver transplantation list because they have PVT. Since the prevalence of portal vein thrombosis rises in proportion to the liver disease severity, even among patients who are already waiting for liver transplantation there is a considerable amount who need to be removed from the waiting list.
Once PVT occurs, these patients suffer an important raise on their portal pressure and this is a stimulus to the development of portosystemic shunts, which are a further predictor of poor prognosis and a cause of hepatic encephalopathy[8]. Therefore, the question is: how these patients should be managed when the liver transplantation is not an option and they have PVT and hepatic encephalopathy? In some selected cases, the embolization of the large portosystemic shunt seems to be an effective and safe option. We report herein a case of a large spontaneous portosystemic shunt associated with PVT which was successfully treated by embolization of this shunt.
CASE REPORT
A 57-year-old woman with hepatitis-C-related cirrhosis, diabetes and arterial hypertension had been hospitalized many times for multiple episodes of HE (grades 2-4) in 2010. Of note, in most of these episodes no trigger factor was found. Her MELD (Model of End-Stage Liver Disease) score was 14 and the hepatitis C virus relapsed after she finished the antiviral treatment. During the hospitalizations the patient received antibiotics, LOLA, lactulose and branched-chain amino acids (BCAA). Even so, these medications were not sufficient to avoid new episodes of HE and hepatic coma. In January of 2011, an abdominal ultrasonography exam showed that she had hepatofugal flux into her portal and splenic veins, associated with portal vein thrombosis. In February of 2011, a computed tomography scan confirmed the portal vein thrombosis with cavernomatous transformation and showed the presence of a large spontaneous splenorenal shunt.
After obtaining informed consent from the patient and her family, we decided to perform an angiographic exam to evaluate the possibility of embolization of her splenorenal shunt, even in the presence of the portal vein thrombosis. Prior to that, we did an upper endoscopy, which showed thin esophageal varices, mild portal hypertensive gastropathy and gastric varices in the stomach fundus. None of these findings required prophylactic procedures. Venous catheterization was performed in March of 2011 while the patient was again hospitalized with hepatic coma (HE grade 4), showing that even though the splenorenal shunt was occluded by a balloon, the contrast injected still left the shunt. This signal indicated that there were other shunts that could maintain the hepatofugal portal flow even without the larger shunt. Given this context, 8 microcoils were placed in a narrowing location into the splenorenal shunt (Figure 1). After the procedure the splenic artery was catheterized to show the indirect flow in the splenic vein (Figure 2).
Figure 1.
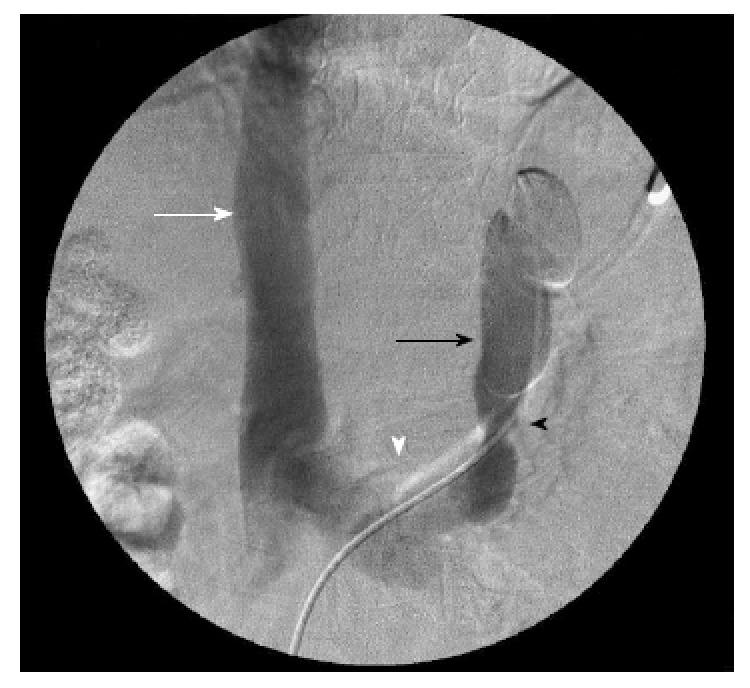
Venographic images of the splenorenal shunt before the embolization, showing a narrowing point where the 8 microcoils would be placed. Inferior vena cava (white arrow); left renal vein (white arrowhead); splenorenal shunt with the catheter inside it (black arrow); narrowing location inside the splenorenal shunt (black arrowhead).
Figure 2.
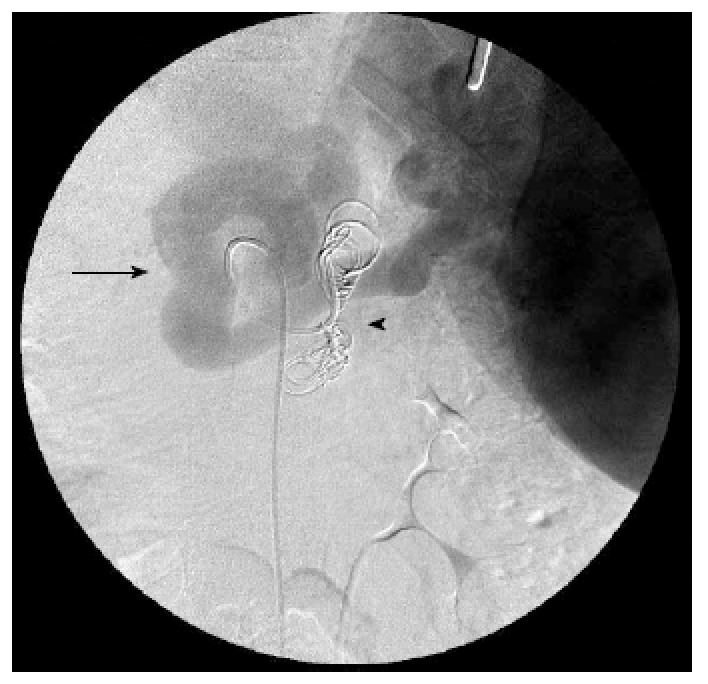
Angiographic images of the splenic vein after the shunt embolization, showing the 8 microcoils placed in the splenorenal shunt. Splenic vein (black arrow); microcoils (black arrowhead). The catheter is inside the splenic artery, and the image is obtained in a late phase after the contrast injection. The metallic tip of the nasoenteral tube can also be seen in the right upper quadrant.
Further upper gastrointestinal endoscopic exams were carried out in April and June of 2011, revealing the same pattern of the gastroesophageal varices. A new abdominal ultrasonography was performed in June of 2011, showing the same profile of hepatofugal portal vein flow of 5 cm/s. The patient continued with high serum ammonia levels, indicating that the other portosystemic shunts kept their flow (Figure 3). She maintained only minor HE (grade 0) and therefore has not been hospitalized since 2011. Her MELD score changed from 14 to 12 points.
Figure 3.
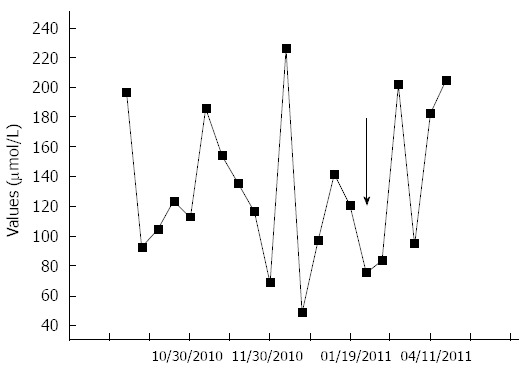
Ammonia values and their respective dates obtained from venous samples before and after the shunt embolization (arrow), showing that there were no major differences between the values before and after the procedure, due to the other shunts that the patient already had before the embolization. The black arrow indicates the date of the splenorenal shunt embolization.
DISCUSSION
Portosystemic shunts can be a cause of HE even when the patient still presents preserved hepatic function, while more than 46% of cirrhotic patients with these shunts will develop HE[3-5]. In a study of percutaneous transhepatic portography carried out in 460 patients with portal hypertension, the authors observed that the most frequent collateral route involved two veins: the left gastric vein, which was connected to the esophageal varices, and the “posterior gastric vein” (a distinct vein located between the left gastric vein and the short gastric vein). This latter vein constituted a major collateral route in 42% of patients, and also formed renal shunts in 23% of them. The relative frequency of renal shunt formation by this vein was significantly greater than that by the left gastric vein (12%) and the short gastric vein (18%)[16].
The closure of portosystemic shunts has been performed as a way to improve the neurological condition of patients with severe HE. In 1981, Hanna et al[17] retrospectively evaluated 7 patients after portosystemic shunt occlusion (surgical or spontaneous), and found that all of them achieved improvement of HE; but 2 patients died after gastroesophageal bleeding and worsening of hepatic function. In 1987, Uflacker et al[18] performed direct shunt embolization through radiological techniques in 5 patients. Of note, four of them had their symptoms controlled but 1 died after intra-abdominal bleeding. This and many other studies showed that shunt embolization is useful and less harmful than the surgical closure, although both procedures are associated with such complications as ascites and enlargement of esophageal varices[13,19]. In 2004, Mezawa et al[20] carried out embolization of the splenic vein, instead of the direct occlusion of the splenorenal shunts. None of the six patients submitted to the splenic vein embolization had complications and most achieved control of symptoms. The authors concluded that splenic vein embolization is as effective as the direct occlusion of the splenorenal shunts and leads to a lesser degree of elevation in the portal vein pressure, making this procedure safer than the first ones. Until now, no direct comparisons of these two techniques have been published. These previous studies were limited to reports and case series whose aim was to evaluate the HE symptoms after shunt closure. However, other authors had shown that shunt occlusion also improves the hepatic function as soon as the procedure is performed[21-23].
Therefore, Kumamoto et al[8] decided to analyze the impact of the shunt closing in time, evaluating 59 cirrhotic patients divided into three groups: patients without splenorenal shunt (SRS), SRS patients not embolized and SRS patients submitted to embolization. After three years, the patients with SRS had a large increase in the Child-Pugh classification and a shorter survival time than the two other groups. More prophylactic rubber band ligation was needed in the SRS group submitted to embolization, but the rate of gastrointestinal bleeding did not differ significantly between the groups. The authors concluded that large SRS results in progressive liver dysfunction and worsens the survival rates among cirrhotic patients. Laleman et al[7] performed a multicenter retrospective study evaluating the efficacy of portosystemic embolization in 37 cirrhotic patients with refractory HE. Two years after the procedure, 48.6% of patients had become free of HE whereas 78.4% presented improved autonomy. The number and the length of hospitalizations were also reduced. There were no deaths and the authors did not find any signs of significant worsening of gastroesophageal varices, portal hypertensive gastropathy or ascites. They concluded that the embolization of these shunts is safe and effective.
Another aspect to be discussed is that even patients with portal venous thrombosis without liver cirrhosis have signs of hepatic encephalopathy, and the proposed physiopathology is the presence of portal-systemic shunting, showing the relevance of the hepatic circulatory disturbances[24]. Thus, the mechanism of hepatic encephalopathy improvement after the splenorenal shunt embolization seems to be directly linked to the hepatic venous flow.
Although in our patient both the portal vein thrombosis and the serum ammonia persisted after the shunt embolization, the hepatic blood flow enhancement is the most conceivable reason to the hepatic encephalopathy improvement. As the hepatic blood flow depends mostly of the portal vein, before the embolization she had a serious limitation in her hepatic circulation, not only due to the portal vein thrombosis but mainly because the splenorenal shunt turning aside the portal flow. A partial correction of this flow steal was possible due to the fact that she had portal vein thrombosis with cavernomatous transformation, so even after the shunt embolization there were other veins that kept part of the venous flow around the portal vein. Since Doppler abdominal ultrasonography can measure the blood flow only into the portal vein and not in other small veins that keep the flow to the liver, on cases like this patient’s the imaging exams performed could not provide evidence to this partial increase in the hepatic flow, but the decrease in the MELD score (without changes in creatinine levels) is a good marker of liver function improvement. The MELD score decreasing demonstrates that we re-established part of the hepatic venous flow by closing the shunt and increasing the flow into the other veins that kept the hepatic flow. Despite that, there is not a clear recommendation to perform portosystemic shunt embolization in patients with portal thrombosis, and this case was indeed an exception.
Another interesting issue is the possibility of prior prophylaxis and treatment of portal vein thrombosis as a modality of prophylaxis and treatment of hepatic encephalopathy. First, regarding the treatment, as liver cirrhosis is a major cause of portal venous thrombosis, we need to be aware to confirm the presence of acute portal vein thrombosis in cirrhotic patients to begin the anticoagulation in order to re-establish the hepatic venous flow as soon as possible, because it is a marker of decompensated disease[25,26]. When the portal venous thrombosis is only diagnosed at the routine imaging exams, the evidence to indicate the anticoagulation is not so clear, since patients with cavernomatous transformation are excluded from the studies of anticoagulation treatment[25]. Unfortunately, most of these imaging exams are indicated just as a screening strategy to find hepatocellular carcinoma nodules at an early stage, so they are performed no more than twice a year. As a result, many diagnoses of portal thrombosis in these patients are confirmed only when the acute phase of thrombus formation was missed and the complications are already installed. To avoid these late diagnoses, doctors who attend cirrhotic patients must be aware of the possibility of portal venous thrombosis in any kind of cirrhosis decompensation with no evident trigger factor. Therefore, cirrhotic patients with abdominal pain, hepatic encephalopathy, esophageal variceal bleeding or newly diagnosed ascites without a comprehensible trigger factor should be submitted to abdominal ultrasonography before the hospital discharge. Doing this simple and non-invasive exam at time, much more cases of acute and subacute thrombosis could be found earlier, avoiding the late diagnosis and its complications. We also could reflect on the anticoagulation treatment to some selected patients when the time of the portal thrombosis is not clear enough and they already have some signs of late diagnosis, but the better strategy in this setting is controversial.
The role of prophylactic treatment to cirrhotic patients aiming to avoid portal venous thrombosis is still more controversial because they have a significant risk of bleeding. There is not a clear indication for this prophylaxis, but it also could be considered in selected patients who already have some kind of procoagulation disturbances. However, this is a complex issue that was not well evaluated yet. We hope that further studies can access this topic to raise more information about it.
It is important to note that almost all the studies about portosystemic shunts embolization excluded patients who had portal vein thrombosis. We found only one case report of a patient with portal vein thrombosis having been previously submitted to thrombolysis before the shunt embolization[27]. But until now we have not found cases of patients who, like ours, had already undergone partial recanalization of the portal vein and other portosystemic shunts that maintain the gastrointestinal flow even after the closure of the SRS.
Given that the main complication of the SRS embolization is the worsening of gastroesophageal varices, the majority of the authors considered endoscopic exams to be a key indicator to keep the patients free from gastroesophageal bleeding after this procedure. In our case, still in the presence of portal vein thrombosis, this effect was insignificant because she had other shunts. Even so, endoscopic surveillance was performed to certify she would not suffer bleeding complications, as other authors suggested[7,8].
In addition to the endoscopic exams, abdominal ultrasonography must be carried out before the shunt embolization[11]. Portal vein thrombosis must be carefully evaluated, but in our opinion it should not be considered an absolute contraindication for this procedure. The presence of other portosystemic shunts should be recognized as a clue that the patient still can be submitted to the embolization of the largest one. In our patient the procedure was done with metallic microcoils. Sclerosing agents could also be used, such as ethanolamine and cyanoacrylate. Portal venous gradient pressure may be measured before and after the shunt embolization to obtain more information about the portal venous hemodynamic changes of each patient; but its unavailability in most hospitals should not prevent the embolization of a splenorenal shunt in comatose patients as in our case.
In conclusion, we reported a case of persistent HE due to a large portosystemic shunt associated with portal vein thrombosis. As the patient had other shunts, she was successfully treated through embolization of the largest shunt. Portal vein thrombosis still should be considered a relative contraindication to perform a shunt embolization. However, in particular cases with many shunts and severe HE, we show that the largest shunt can be safely closed and that this procedure can be sufficient to achieve good HE recovery. Endoscopic follow-up was performed and detected no complications.
COMMENTS
Case characteristics
The patient had been hospitalized many times presenting cognitive decline, memory loss, attention deficit, behavioral disorders, psychomotor slowing and recurrent episodes of coma, even without precipitant factors.
Clinical diagnosis
The patient had splenomegaly, cutaneous vascular spiders, drowsiness, mental confusion and flapping tremor without neurological focal deficits, leading to the diagnosis of portal hypertension and hepatic encephalopathy.
Differential diagnosis
The abdominal ultrasonography exam showed signs of cirrhosis, portal vein thrombosis and a large splenorenal shunt, thus confirming the clinical diagnosis.
Laboratory diagnosis
Serum ammonia and total bilirubins levels were increased, while platelets count was markedly decreased and blood electrolytes were normal.
Imaging diagnosis
The venous catheterization verified the splenorenal shunt and showed other shunts that could maintain the hepatofugal portal flow even without the larger shunt.
Treatment
Antibiotics, lactulose and branched-chain amino acids were not sufficient to avoid new episodes of hepatic coma, therefore the embolization of the larger splenorenal shunt was done and after this procedure the patient maintained only minor hepatic encephalopathy.
Related reports
Since the prevalence of portal vein thrombosis rises in proportion to the liver disease severity, many cirrhotic patients who are waiting for liver transplantation need to be removed from the waiting list, because advanced portal vein thrombosis impairs the liver transplantation. Once the portal vein thrombosis occurs, these patients suffer an important raise on their portal pressure and this is a stimulus to the development of portosystemic shunts, which are a further predictor of poor prognosis and a cause of hepatic encephalopathy. If the liver transplantation is no more possible and the patient has severe hepatic encephalopathy associated to portal vein thrombosis, new treatment options are needed, and the splenorenal shunt embolization could be one of them. Conversely, the presence of portal vein thrombosis is considered a contraindication to portosystemic shunt embolization. In this article we performed the splenorenal shunt embolization even in the presence of portal vein thrombosis, showing that it is feasible and safe in selected patients.
Experiences and lessons
In severe hepatic encephalopathy, splenorenal shunt embolization can be the best treatment, and in selected cases it can be done even in the presence of portal vein thrombosis.
Peer review
By the first time a patient with portal vein thrombosis and hepatic coma was submitted to embolization of a splenorenal shunt without the thrombosis treatment. Therefore, this paper shows a new perspective to severe hepatic encephalopathy cases, even in the presence of portal vein thrombosis. However, the article is based in just one patient, so the findings should be cautiously analyzed and further articles are needed to confirm them.
Footnotes
P- Reviewer: Greco L, Massimo Tiberio GA, Mihaila RG S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M, Bell DE, Sterling RK, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijdicks EF, Wiesner RH. Acquired (non-Wilsonian) hepatocerebral degeneration: complex management decisions. Liver Transpl. 2003;9:993–994. doi: 10.1053/jlts.2003.50107. [DOI] [PubMed] [Google Scholar]
- 3.Lewis M, Howdle PD. The neurology of liver failure. QJM. 2003;96:623–633. doi: 10.1093/qjmed/hcg110. [DOI] [PubMed] [Google Scholar]
- 4.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 5.Wright G, Jalan R. Management of hepatic encephalopathy in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:95–110. doi: 10.1016/j.bpg.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 7.Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M, Jalan R, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57:2448–2457. doi: 10.1002/hep.26314. [DOI] [PubMed] [Google Scholar]
- 8.Kumamoto M, Toyonaga A, Inoue H, Miyakoda K, Morita Y, Emori K, Sakamoto Y, Oho K, Sata M. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25:1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]
- 9.Crespo L, Graus J, García-Hoz F, Bárcena R, Gil Grande L, Moreira VF, Milicua JM, Sánchez J, Blázquez J. [Hepatic encephalopathy secondary to porto-systemic shunt satisfactorily treated with interventionist radiology] Rev Esp Enferm Dig. 2007;99:667–670. doi: 10.4321/s1130-01082007001100010. [DOI] [PubMed] [Google Scholar]
- 10.Carrión JA, Bellot P, Colmenero J, Garcia Pagan JC. Large spontaneous splenorenal shunt as a cause of chronic hepatic encephalopathy. J Hepatol. 2004;40:868. doi: 10.1016/j.jhep.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Tanoue S, Kiyosue H, Komatsu E, Hori Y, Maeda T, Mori H. Symptomatic intrahepatic portosystemic venous shunt: embolization with an alternative approach. AJR Am J Roentgenol. 2003;181:71–78. doi: 10.2214/ajr.181.1.1810071. [DOI] [PubMed] [Google Scholar]
- 12.Takayama Y, Moriura S, Nagata J, Akutagawa A, Hirano A, Ishiguro S, Matsumoto T, Sato T. Embolization of the left portal vein to inferior vena cava shunts for chronic recurrent hepatic encephalopathy via the mesenteric vein. J Gastroenterol Hepatol. 2001;16:1425–1428. doi: 10.1046/j.1440-1746.2001.02508.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Uematsu T, Nishigaki Y, Sugihara J, Tomita E, Moriwaki H. Therapeutic effect of balloon-occluded retrograde transvenous obliteration on portal-systemic encephalopathy in patients with liver cirrhosis. Intern Med. 2001;40:688–691. doi: 10.2169/internalmedicine.40.688. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo N, Kawanaka H, Akahoshi T, Matsumoto Y, Kamori M, Nagao Y, Hashimoto N, Uehara H, Tomikawa M, Shirabe K, et al. Portal vein thrombosis in liver cirrhosis. World J Hepatol. 2014;6:64–71. doi: 10.4254/wjh.v6.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponziani FR, Zocco MA, Senzolo M, Pompili M, Gasbarrini A, Avolio AW. Portal vein thrombosis and liver transplantation: implications for waiting list period, surgical approach, early and late follow-up. Transplant Rev (Orlando) 2014;28:92–101. doi: 10.1016/j.trre.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Kimura K, Ohto M, Matsutani S, Furuse J, Hoshino K, Okuda K. Relative frequencies of portosystemic pathways and renal shunt formation through the “posterior” gastric vein: portographic study in 460 patients. Hepatology. 1990;12:725–728. doi: 10.1002/hep.1840120417. [DOI] [PubMed] [Google Scholar]
- 17.Hanna SS, Smith RS, Henderson JM, Millikan WJ, Warren WD. Reversal of hepatic encephalopathy after occlusion of total portasystemic shunts. Am J Surg. 1981;142:285–289. doi: 10.1016/0002-9610(81)90294-4. [DOI] [PubMed] [Google Scholar]
- 18.Uflacker R, Silva Ade O, d’Albuquerque LA, Piske RL, Mourão GS. Chronic portosystemic encephalopathy: embolization of portosystemic shunts. Radiology. 1987;165:721–725. doi: 10.1148/radiology.165.3.3685350. [DOI] [PubMed] [Google Scholar]
- 19.Condat B, Dusoleil A, Bernardeau M, Roche A, Pelletier G, Buffet C. [Chronic acquired hepatocerebral degeneration: the role of manganese and treatment by endovascular occlusion of a porto-systemic shunt] Gastroenterol Clin Biol. 1999;23:268–270. [PubMed] [Google Scholar]
- 20.Mezawa S, Homma H, Akiyama T, Katsuki S, Murakami K, Hirata K, Kogawa K, Takahashi S, Sato T, Doi T, et al. Selective embolization of the splenic vein in patients with hepatic encephalopathy and splenorenal shunt. J Vasc Interv Radiol. 2004;15:1475–1481. doi: 10.1097/01.RVI.0000141447.87966.D7. [DOI] [PubMed] [Google Scholar]
- 21.Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4:109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12:327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- 23.Akahane T, Iwasaki T, Kobayashi N, Tanabe N, Takahashi N, Gama H, Ishii M, Toyota T. Changes in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol. 1997;92:1026–1030. [PubMed] [Google Scholar]
- 24.Mínguez B, García-Pagán JC, Bosch J, Turnes J, Alonso J, Rovira A, Córdoba J. Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatology. 2006;43:707–714. doi: 10.1002/hep.21126. [DOI] [PubMed] [Google Scholar]
- 25.Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García-Criado A, Abraldes JG, de la Peña J, Bañares R, Albillos A, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–783. doi: 10.1016/j.cgh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Qi X, Bai M, Yang Z, Yuan S, Zhang C, Han G, Fan D. Occlusive portal vein thrombosis as a new marker of decompensated cirrhosis. Med Hypotheses. 2011;76:522–526. doi: 10.1016/j.mehy.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Nakai M, Sato M, Sahara S, Kawai N, Kimura M, Maeda Y, Ibata Y, Higashi K. Transhepatic catheter-directed thrombolysis for portal vein thrombosis after partial splenic embolization in combination with balloon-occluded retrograde transvenous obliteration of splenorenal shunt. World J Gastroenterol. 2006;12:5071–5074. doi: 10.3748/wjg.v12.i31.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]


