Abstract
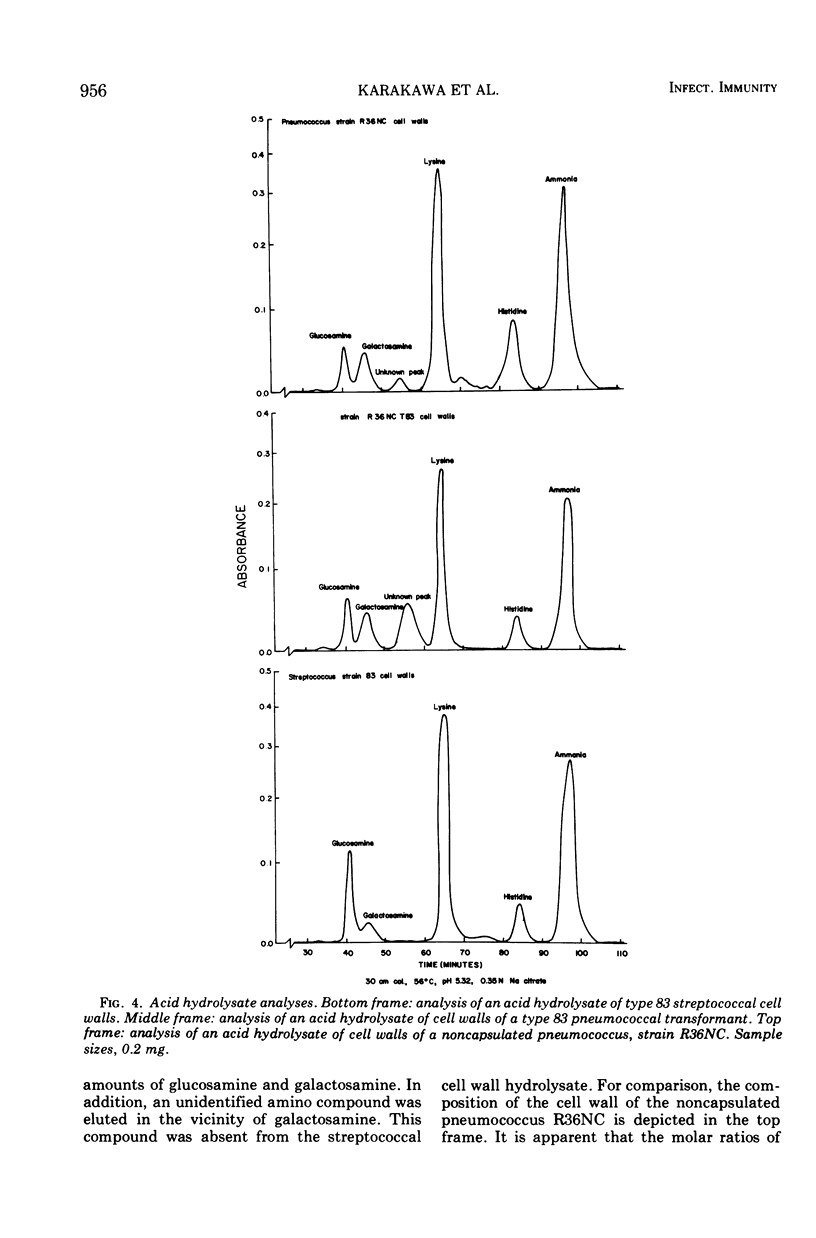
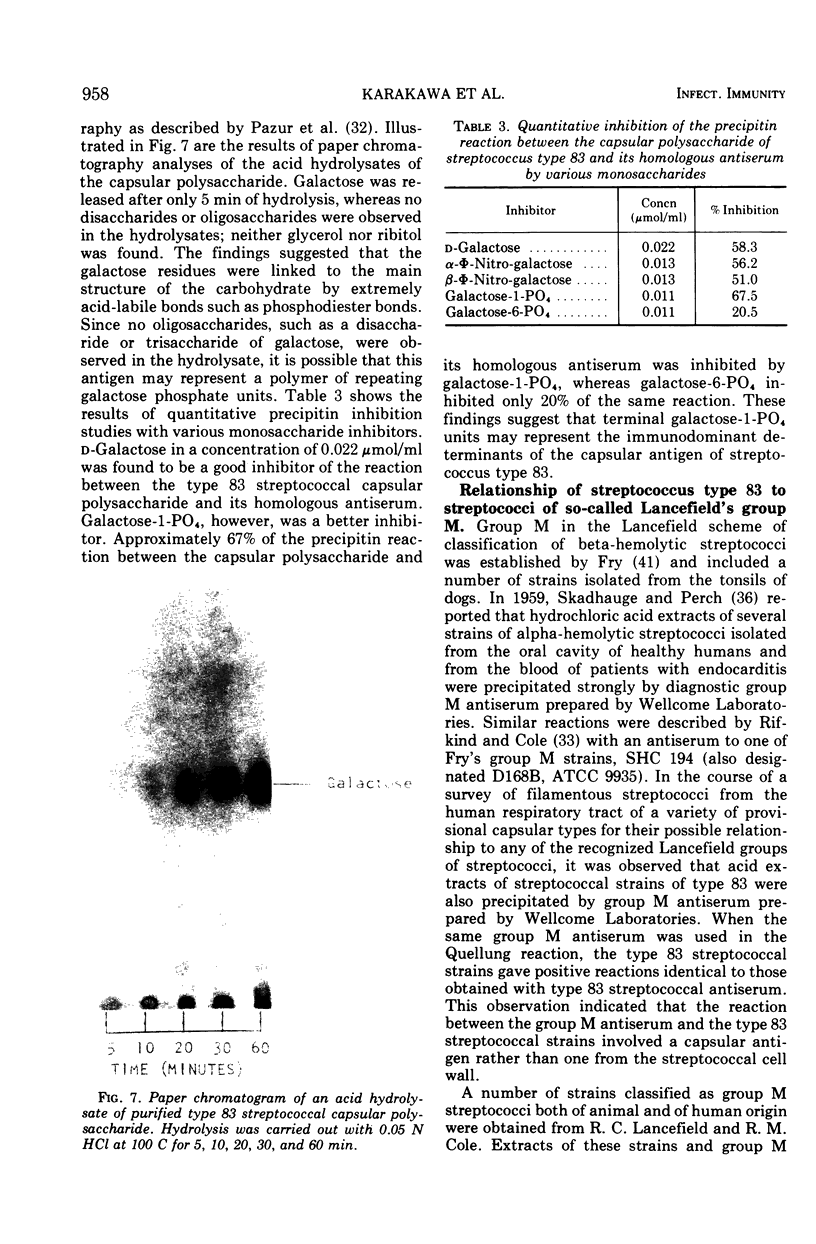
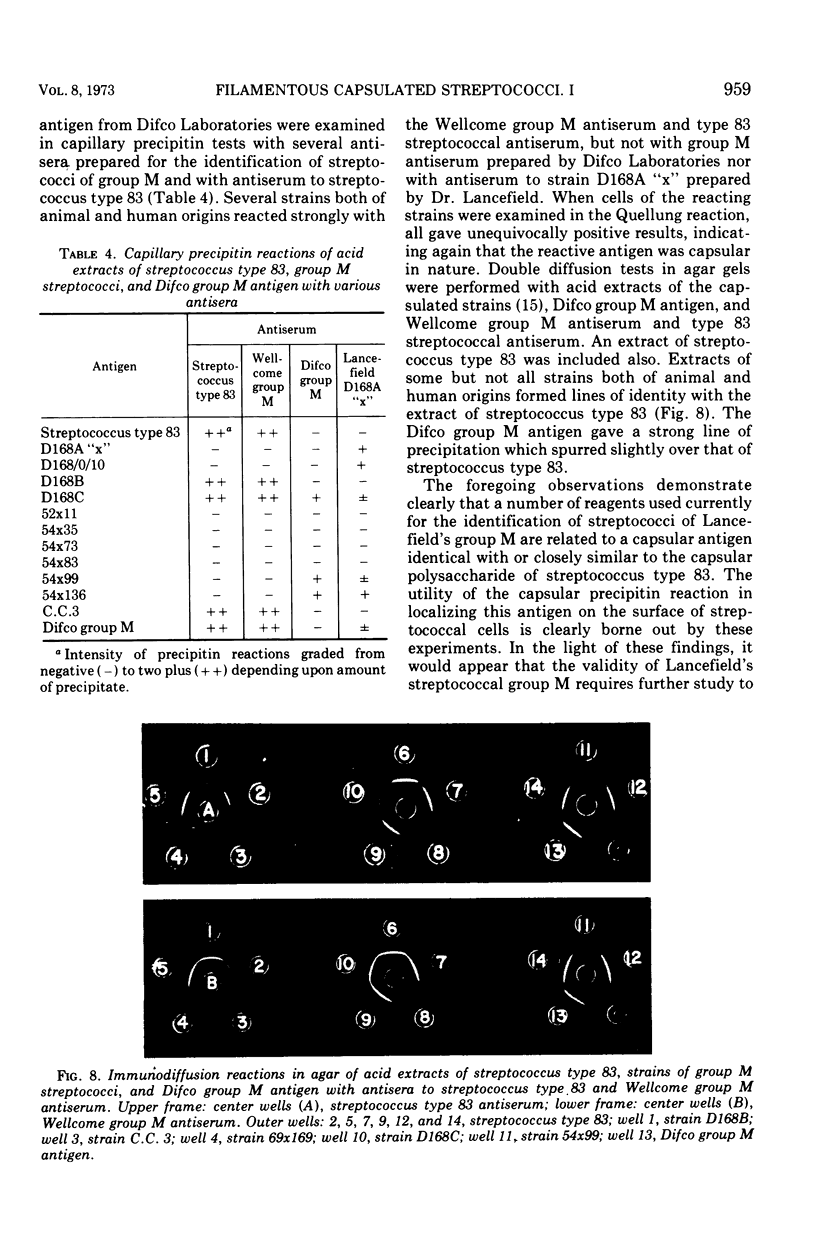
Two immunologically reactive polysaccharides have been isolated from the cell walls and from culture filtrates of a filamentous alpha-hemolytic streptococcus provisionally designated capsular type 83. Both polysaccharides were purified by diethylaminoethyl-cellulose chromatography. Analysis indicates that the capsular polysaccharide consists of galactose and phosphorus, whereas the cell wall polysaccharide contains galactosamine, glucosamine, glucose, and phosphorus. On the basis of immunochemical experiments, it is suggested that the capsular polysaccharide is composed of galactose-phosphate units with terminal galactose residues at the nonreducing end. It has also been found that the capsular antigen of streptococcus type 83 is shared by a number of streptococcal strains classified in Lancefield's group M. The cell wall polysaccharide of streptococcus type 83 cross-reacts with antibody to the Cs, or cell wall-like capsular, polysaccharide of Diplococcus pneumoniae, and this cross-reactivity may be a reflection that the streptococcal antigen possesses certain structural features which are similar to those of pneumococcal C and Cs polysaccharides.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P. Simultaneous production of two capsular polysaccharides by pneumococcus. I. Properties of a pneumococcus manifesting binary capsulation. J Exp Med. 1959 Oct 1;110:571–584. doi: 10.1084/jem.110.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEIWEIS A. S., KARAKAWA W. W., KRAUSE R. M. IMPROVED TECHNIQUE FOR THE PREPARATION OF STREPTOCOCCAL CELL WALLS. J Bacteriol. 1964 Oct;88:1198–1200. doi: 10.1128/jb.88.4.1198-1200.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein D. L., Schiffman G., Bernheimer H. P., Austrian R. Capsulation of pneumococcus with soluble C-like (Cs) polysaccharide. I. Biological and genetic properties of Cs pneumococcal strains. J Exp Med. 1968 Dec 1;128(6):1385–1400. doi: 10.1084/jem.128.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. N., KRAUSE R. M. ANTIGENIC RELATIONSHIPS BETWEEN GROUPS B AND G STREPTOCOCCI. J Exp Med. 1964 Oct 1;120:629–637. doi: 10.1084/jem.120.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G. The application of computers to the classification of streptococci. J Gen Microbiol. 1968 Jan;50(1):149–158. doi: 10.1099/00221287-50-1-149. [DOI] [PubMed] [Google Scholar]
- HORSFALL F. L., Jr Studies on non-hemolytic streptococci isolated from the respiratory tract of man; the antigenic basis for type specific reactions with streptococcus salivarius and non-levan-forming streptococci. J Exp Med. 1951 Mar;93(3):229–245. doi: 10.1084/jem.93.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. A., Karakawa W. W., Pazur J. H. Glycans from streptococcal cell walls: structural features of a diheteroglycan isolated from the cell wall of Streptococcus bovis. J Immunol. 1972 May;108(5):1218–1226. [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Characterization of the surface antigens of Staphylococcus aureus, strain K-93M. J Immunol. 1972 May;108(5):1199–1208. [PubMed] [Google Scholar]
- Karakawa W. W., Krause R. M. Studies on the immunochemistry of streptococcal mucopeptide. J Exp Med. 1966 Aug 1;124(2):155–171. doi: 10.1084/jem.124.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUND E. Antigenic relationship between pneumococci and non-hemolytic streptococci. Acta Pathol Microbiol Scand. 1950;27(1):110–118. doi: 10.1111/j.1699-0463.1950.tb05200.x. [DOI] [PubMed] [Google Scholar]
- LUND E. POLYVALENT, DIAGNOSTIC PNEUMOCOCCUS SERA. Acta Pathol Microbiol Scand. 1963;59:533–536. doi: 10.1111/j.1699-0463.1963.tb01256.x. [DOI] [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. C., Karakawa W. W. Immunochemical analysis of a uronic acid polymer of Staphylococcus epidermidis, strain 53. J Immunol. 1970 Aug;105(2):389–395. [PubMed] [Google Scholar]
- Michel M. F., Krause R. M. Immunochemical studies on the group and type antigens of group F streptococci and the identification of a grouplike carbohydrate in a type II strain with an undesignated group antigen. J Exp Med. 1967 Jun 1;125(6):1075–1089. doi: 10.1084/jem.125.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- OTTENS H., WINKLER K. C. Indifferent and haemolytic streptococci possessing group-antigen F. J Gen Microbiol. 1962 Apr;28:181–191. doi: 10.1099/00221287-28-1-181. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
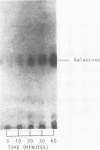
- Pazur J. H., Anderson J. S., Karakawa W. W. Glycans from streptococcal cell walls. Immunological and chemical properties of a new diheteroglycan from Streptococcus faecalis. J Biol Chem. 1971 Mar 25;246(6):1793–1798. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D., Cole R. M. NON-BETA-HEMOLYTIC GROUP M-REACTING STREPTOCOCCI OF HUMAN ORIGIN. J Bacteriol. 1962 Jul;84(1):163–168. doi: 10.1128/jb.84.1.163-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKADHAUGE K., PERCH B. Studies on the relationship of some alpha-hemolytic streptococci of human origin to the Lancefield group M. Acta Pathol Microbiol Scand. 1959;46:239–250. doi: 10.1111/j.1699-0463.1959.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Swift H. F. Sharp Interfacial Precipitin Reactions in Capillary Pipettes. Science. 1947 Jan 10;105(2715):49–50. doi: 10.1126/science.105.2715.49-a. [DOI] [PubMed] [Google Scholar]