Abstract
Several clinical trials have demonstrated the potent antiviral efficacy of entecavir (ETV), and this relatively new nucleoside analogue drug has rapidly become a frequently prescribed therapy for chronic hepatitis B (CHB) worldwide. While the studies have also shown a good overall safety profile for ETV, adverse drug reactions (ADRs) in patients with advanced cirrhosis have been reported and represent a broad spectrum of drug-induced injuries, including lactic acidosis, myalgia, neuropathy, azotemia, hypophosphatemia, muscular weakness, and pancreatitis, as well as immune-mediated responses (i.e., allergic reactions). Cutaneous ADRs associated with ETV are very rare, with only two case reports in the publicly available literature; both of these cases were classified as unspecified hypersensitivity allergic (type I) ADR, but neither were reported as pathologically proven or as evaluated by cytokine release analysis. Here, we report the case of a 45-year-old woman who presented with a generalized maculopapular rash after one week of ETV treatment for lamivudine-resistant CHB. The patient reported having experienced a similar skin eruption during a previous three-month regimen of ETV, for which she had self-discontinued the medication. Histopathological analysis of a skin biopsy showed acanthotic epidermis with focal parakeratosis and a perivascular lymphocytic infiltrate admixed with interstitial eosinophils in the papillary and reticular dermis, consistent with a diagnosis of drug sensitivity. A lymphocyte stimulation test showed significantly enhanced IL-4, indicating a classification of type IVb delayed hypersensitivity. The patient was switched to an adefovir-lamivudine combination regimen and the skin eruption resolved two weeks after the ETV withdrawal. This case represents the first pathologically and immunologically evidenced ETV-induced delayed type hypersensitivity skin reaction reported to date. Physicians should be aware of the potential, although rare, for cutaneous ADRs associated with ETV treatment.
Keywords: Entecavir, Delayed type hypersensitivity, Maculopapular drug eruption, Dermatology, Adverse drug reaction, Chronic hepatitis B
Core tip: A potent efficacy and good overall safety profile has supported the rapid establishment of entecavir as a frequently prescribed antiviral nucleoside for treatment of chronic hepatitis B (CHB). Among the adverse drug reactions that have been reported in CHB patients, allergic dermatitis is especially rare, with only two cases in the literature to date. This report describes a pathologically and immunologically evidenced case of entecavir (ETV)-induced type IV delayed hypersensitivity reaction, which resolved upon ETV withdrawal.
INTRODUCTION
Entecavir (ETV), a nucleoside analogue, is a highly potent antiviral agent. While the clinical trials of ETV in chronic hepatitis B (CHB) patients have demonstrated a good overall safety profile, cases of adverse drug reactions (ADRs) have been reported both during the licensing period and after market introduction. An ETV-901 rollover study of a large group of CHB patients treated with ETV for at least five years showed that most adverse drug events were mild (grade 1) to moderate (grade 2); the relatively small portion of severe (grade 3) or potentially life-threatening (grade 4) adverse events (accounting for 19%, with only 4% considered ETV-related) included myalgias, neuropathy, increased lipase, increased serum creatinine, hypophosphatemia, muscular weakness, pancreatitis, and creatinine phosphokinase elevation[1]. Other studies showed that the rates of adverse events most commonly reported in ETV-treated patients (e.g., headache, fatigue, dizziness and nausea) were comparable to rates in patients treated with the longer established nucleoside analogue lamivudine (LAM)[2,3]. Unlike LAM, however, ETV appears to rarely induce cutaneous ADRs. To date, only two cases of ETV-related cutaneous ADRs have been reported in the publicly available literature[4,5], and very little is known about the manifestation and clinical course of these rare events. Here, we describe the presentation, diagnosis and management of a case of delayed hypersensitivity reaction to ETV that manifested as a maculopapular eruption after one week of treatment in a CHB patient.
CASE REPORT
A 45-year-old woman was referred to our dermatology department (College of Medicine and Medical Research Institute at the Chungbuk National University, Cheongju, South Korea) for evaluation of a generalized erythema involving the lower back and extremities. History taking revealed that the patient had taken a seven-day course of ETV (1.0 mg/d, prescribed for CHB) prior to the development of the skin rash. Further inquiry of the CHB status revealed the following ten-year history of antiviral therapy: LAM therapy (100 mg) was first initiated in 2004, when (according to medical records) the patient showed compromised liver function [125 U/L aspartate aminotransferase (AST); 88 U/L alanine aminotransferase (ALT)], positivity for hepatitis B e antigen (HBeAg), negativity for hepatitis B e antibody (anti-HBe), and high viral load [3.38 × 106 IU/mL hepatitis B virus (HBV) DNA]. After 4 years of the LAM therapy, the patient developed viral breakthrough and genetic analysis confirmed the presence of two LAM-resistance-associated mutations (L180M and M204V). The drug therapy was switched to ETV (1.0 mg/d) on February 4 2008. The patient reported that she took the prescribed ETV for three months, but developed a disseminated, itchy rash (fitting the clinical description of a maculopapular eruption). The patient could not remember the exact timeline of the rash onset, but reported having noticed the rash “after about one month of treatment”. The patient stated that she continued to take the medication over the next two months because she did not consider the possible relation to the drug. On April 4 2008, the patient visited the outpatient clinic at our hospital and obtained a three-month refill of the ETV, but she self-discontinued the medication after one month. The patient did, however, request a follow-up visit and presented to the outpatient clinic for her next assigned visit on July 4 2008, when she reported to the attending staff that she had not taken the prescribed ETV since the self-discontinuation and refused a prescription refill. In both months, the liver enzyme analyses showed levels of AST and ALT within the normal range (April: 23 and 24 U/L; July: 20 and 18 U/L, respectively) and elevated HBV DNA (April: 3.55 × 103 IU/mL; July: 7.11 × 102 IU/mL), indicating viral breakthrough had occurred.
At the patient’s next visit on September 29 2008, she accepted re-initiation of the ETV treatment (corresponding to seven months after the initial ETV treatment) due to the staff’s urging to address her continued elevated HBV DNA levels (6.76 × 102 IU/mL; AST and ALT, 23 and 16 U/L, respectively). However, when the patient revisited our clinic a little over one month later (on November 10 2008), she reported that after one week of restarting the medication, the same disseminated, itchy rash again developed on the lower back and extremities (Figure 1). At this time, the patient also chose to visit a private dermatology clinic as well as our hospital’s dermatology outpatient clinic. In our clinic, the attending staff performed a skin biopsy for histopathological analysis; the findings were consistent with a diagnosis of drug sensitivity (Figure 2) and the patient was switched to adefovir-LAM combination therapy. The skin eruption resolved within two weeks of the ETV withdrawal, and after one month, the HBV DNA level remarkably increased (from 6.76 × 102 IU/mL measured on September 29 2008 to 1.50 × 103 IU/mL on December 29 2008). The patient also reported that in early September 2008, she had started consuming an over-the-counter extract of the Garcinia gummi-gutta fruit (former scientific name: garcinia cambogia; common name: tamarind), by the brand name of “Fat-Down” (Cheil Jedang, Seoul, South Korea) as a weight loss supplement; our review of the literature indicated that the active component of G. gummi-gutta is hydroxycitric acid, which has potential influencing effects on lipid metabolism[6,7]. The patient was withdrawn from the ETV regimen and prescribed antihistamines (azelastine at 2 mg bid and olopatadine at 10 mg bid) and a low-dose steroid (prednisone at 10 mg qd) until the symptom resolved. Testings on February 4 and March 4 2009 showed an increasing trend in viral load (1.49 × 104 and 1.74 × 104 IU/mL, respectively) and the patient was switched back to the adefovir-LAM therapy, which appeared to effectively control the viral breakthrough as the tests showed lower viral load on April 1 2009 (1.10 × 102 IU/mL).
Figure 1.
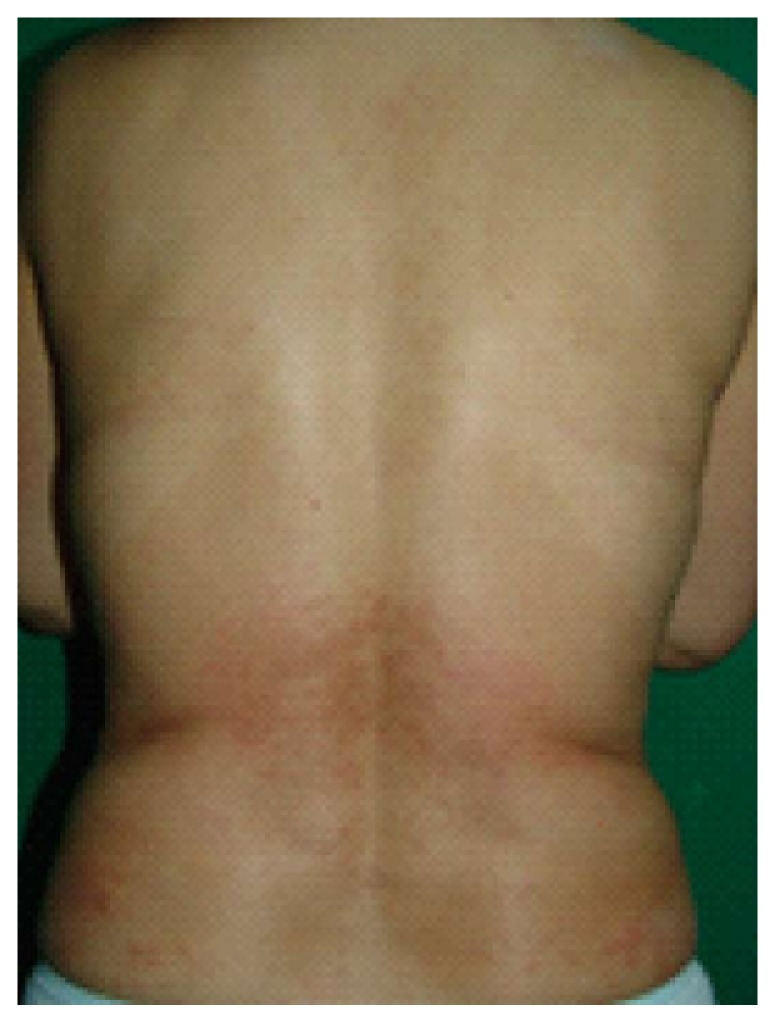
Cutaneous eruption associated with entecavir that manifested after one week of therapy in a non-naïve patient with chronic hepatitis B. Coalescent erythema, intermingled with papules, was observed on the lower back.
Figure 2.
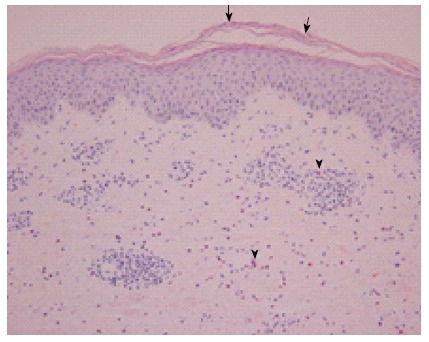
Hematoxylin and eosin stained erupted skin biopsy sample. The epidermis is acanthotic with focal parakeratosis (arrows). A perivascular lymphocytic infiltrate admixed with interstitial eosinophils is present in the papillary and reticular dermis (arrowheads). Magnification: 200 ×.
A physical examination of the patient during the skin ETV-associated eruption revealed pruritic, erythematous macules, intermingled with papules on the anterior chest, lower abdomen and lower back, as well as on the extensor side of the right thigh and the tibial side of the lower leg. Initial laboratory tests showed the patient had a normal white blood cell count (4990 cells/L, with 3.2% eosinophil content), hemoglobin level (12.6 g/dL), and platelet count (190000 cells/L). Liver enzyme tests indicated normal function (AST and ALT, 16 and 23 U/L, respectively), and the patient’s total bilirubin level was also within normal range (0.4 mg/dL). The changing pattern of ALT and HBV DNA levels is plotted in Figure 3, in relation to the course of the antiviral treatments. Serological testing for the HBV surface antigen at this time showed positive results, while testing for the HBV surface antibody showed negative results; the patient also tested positive for HBeAg and negative for anti-HBe. Other viral serological testing yielded negative results for hepatitis viruses A, C and E, Epstein-Barr virus, and cytomegalovirus. The serological testing results for antibodies against influenza A and B and human immunodeficiency virus type 1 were also negative, and all tests carried out in the Venereal Disease Research Laboratory yielded “non-reactive” test results. Similarly, all of the tests for anti-mitochondrial, antinuclear and anti-smooth muscle antibodies were negative.
Figure 3.
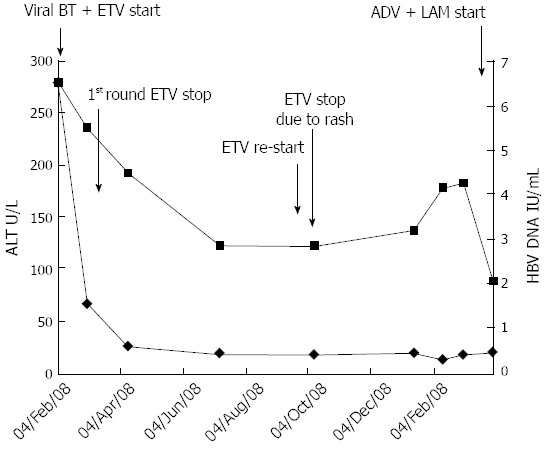
Alanine aminotransferase and hepatitis B virus DNA levels following the course of various antiviral treatments. Entecavir (1 mg) treatment could not be continued past the 15th day due to an adverse drug reaction (skin rash). ALT: Alanine aminotransferas; HBV: Hepatitis B virus; ADV: Adverse drug reaction; BT: Breakthrough; ETV: Entecavir; LAM: Lamivudine.
Allergy skin tests (skin prick and patch test) were ordered by the treating staff, but the patient refused both procedures. The patient did, however, allow for a peripheral blood draw to determine the cytokine release profile of the peripheral blood mononuclear cells (PBMCs) (Figure 4). The lymphocyte stimulation test was carried out as follows[8]: the patient’s heparinized blood sample, alongside a sample from an anonymized normal control (a volunteer healthy patient who took entecavir for five days and experienced no ADRs, with blood drawn on day-7 after the treatment was initiated, corresponding to the appropriate drug washout period), was centrifuged through the Ficoll-Paque PLUS medium (GE Healthcare Bio-Sciences AB, Uppsala, Sweden); the layer of isolated PBMCs was collected and washed twice with sterile phosphate-buffered saline; the prepared PBMCs were then plated at 1 × 106 cells/mL per well in a 6-well plate in the presence of ETV at 2.0 × 10-4 mol/L; the plate was incubated for 72 h at 37 °C in a humidified atmosphere with 5% CO2; the cell culture supernatants were harvested at 6, 18, 24, 48 and 72 h (data not shown) and applied to the Bio-Plex 200 System with Luminex xMap Technology (Bio-Rad Laboratories, Hercules, CA, United States) for automated detection and quantification of the cytokine release profile; the data were analyzed using the accompanying Bio-Plex Manager Software, v4.1 and mean values of the cytokines’ concentrations (calculated as results from triplicate wells) were used to assess statistically significant changes in response to ETV over time. The levels of interleukin (IL)-4 were found to be significantly enhanced at post-ETV treatment hours 18 and 24, indicating a classification of type IVb delayed hypersensitivity (Figure 4). None of the other tested cytokines (interferon-γ, tumor necrosis-α, granulocyte-macrophage colony-stimulating factor, IL-2, IL-6, IL-8, and IL-10) showed a significant change in response to the ETV treatment.
Figure 4.
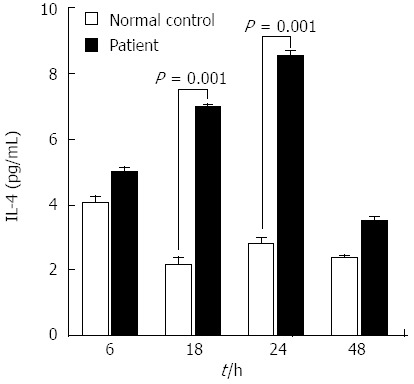
Cytokine release profile of peripheral blood mononuclear cells isolated from the patient during the cutaneous eruption associated with entecavir treatment. The level of secreted interleukin (IL)-4 was significantly elevated at 18 and 24 h after entecavir treatment. IL-4 is the representative cytokine of type 4b hypersensitivity.
DISCUSSION
This case report describes a type IVb delayed hypersensitivity reaction to ETV in a 45-year-old woman with an over ten-year history of CHB and various antiviral therapies. The cutaneous eruption from the drug hypersensitivity developed within one week of the ETV treatment, which had previously occurred within one week to one month of initiation of prior ETV treatments. The patient’s diagnosis was confirmed by both skin biopsy findings and lymphocyte stimulation test results.
Two other cases of cutaneous ADRs associated with ETV have been reported in the medical literature. The first of those cases was diagnosed as cutaneous immediate allergy induced by ETV according to a scratch test showing a positive reaction for ETV[4]. The second case was diagnosed as a delayed hypersensitivity to ETV, with the eruption considered to be T-cell-mediated, according to results from the lymphocyte stimulation test; specifically, tritiated-thymidine incorporation was significantly increased by the addition of ETV to peripheral lymphocyte culture, with a stimulation index of 7.2 compared to a normal control[5]. However, neither of these cases of ETV sensitivity was histopathologically proven, and our case described herein represents the first reported case of delayed hypersensitivity to ETV based on a more comprehensive panel of clinical observations (i.e., detailed clinical course, pathological findings and lymphocyte stimulation testing).
The workup for diagnosing drug sensitivity is very cumbersome, and it can be categorized as a wastebasket diagnosis. While there are no specific histopathological findings that can be used to diagnose drug sensitivity[9], histopathology can be used to rule out other diagnoses; although this approach provides limited insights, if any at all, into the pathognomonic features related to a particular drug sensitivity. During the history taking for the current case, the patient recalled noticing the eruption at about one month after the first ETV treatment was initiated. The patient was unable to provide any more precise information on the interval between the initial administration of ETV and the development of the symptoms. Moreover, the patient reported a history of non-adherence to the prescribed regimen and of similar skin eruptions occurring shortly after the times that the ETV regimen would be re-initiated to address viral breakthrough and failure of other antivirals. The symptoms described during this clinical course of treatments were compatible with the signs of a delayed hypersensitivity reaction occurring in response to the ETV drug.
The skin biopsy findings of this patient included hyperkeratosis with parakeratosis and mild acanthosis in the epidermis and a mild to moderate superficial, perivascular lymphocytic infiltrate admixed with numerous eosinophils in the upper dermis. Inflammatory cell infiltration confined to the superficial dermis and the perivascular and interstitial pattern of dermal infiltration are common findings among cutaneous drug eruption cases; a review by Gerson et al[9] calculated that 80%-82% of reported cases showed these findings. Moreover, the immune cell-type composition of the inflammatory cell infiltrate is frequently either lymphocytes or lymphocytes and eosinophils[9]. Histological analysis of the eruption biopsy sample from the current case revealed that the inflammatory cell infiltrate included both lymphocytes and eosinophils, and that the dermal infiltrates were distributed both perivascularly and in the interstitium. Thus, the histopathological findings of this patient were compatible with those of other drug sensitivity cases reported in the literature.
We also investigated the cytokine release assay profile of our case, using PBMCs isolated during an ETV-associated eruption event. A type IVb hypersensitivity reaction is characterized by enhanced cytokine release of IL-5, IL-4 and IL-13, which activate eosinophils (the main cells that modulate this type of reaction). The clinical manifestations of a type IVb reaction include maculopapular exanthema accompanied by eosinophilia[10]. According to the cytokine release assay performed in our study, only the level of IL-4 in the supernatant of PBMCs isolated from our patient was significantly enhanced at 18 and 24 h after ETV treatment. While other cytokines (IL-2, IL-6, IL-8 and TNF-α; data not shown) were also found to be elevated in the patient, the differences from the controls were not significant; we currently do not understand why only IL-4 showed a significant elevation and we intend to further investigate the cytokine profile in our future studies.
The drug eruption was not severe in our case, but the symptom of itchiness was affecting her quality of life. The patient self-discontinued the ETV medical treatment and refused further medications at times throughout the course of her CHB disease management for personal reasons. By tracing the history of her taking the ETV, we recognized that the skin eruption corresponded with the ETV medication and was reproduced sooner with re-treatment of ETV; this may indicate sensitization causing a more rapid response. It is also intriguing to consider that the patient consistently experienced high levels of HBV DNA in serum during her drug withdrawal periods, and it is possible that a drug-resistant strain may have developed in this high viral load environment. Indeed, the serum level of HBV DNA sharply decreased during her treatment period with the adefovir-LAM combination therapy, which was first prescribed as a rescue therapy for LAM resistance. Certainly ADRs, such as skin eruption, can affect a patient’s compliance with a drug regimen, but patients rarely consider that their behavior of poor drug compliance may further worsen their disease condition by promoting drug resistance.
The patient described herein also undertook a non-medically prescribed intake of an unproven health food supplement. The limited research on a primary constituent of this supplement, hydroxycitric acid, has yet to show any evidence that it can induce any kind of skin rashes or that it can interact with concomitantly taken pharmacologic agents.
In conclusion, we have reported the first pathologically and immunologically evidenced case of ETV-induced type IV delayed hypersensitivity reaction manifested as a cutaneous eruption. The long history of this patient’s experiences with ETV medication (consisting of short treatment periods of three months and one week) that consistently corresponded to a skin reaction also hinted at a progressive drug sensitivity (re-manifestation in shorter time periods). Furthermore, this case demonstrates how periods of non-compliance with antiviral drug regimens may produce a high viral load environment that can support development of drug-resistance mutations.
COMMENTS
Case characteristics
A 45-year-old woman with an over ten-year history of chronic hepatitis B and various antiviral treatment strategies presented with a generalized maculopapular rash that developed after entecavir (ETV) treatment.
Clinical diagnosis
Delayed hypersensitivity reaction associated with ETV and manifested as cutaneous eruption.
Differential diagnosis
A causal relationship existed between the drug administered and the symptom onset. The patient had no history of skin rash before the initial ETV treatment.
Laboratory diagnosis
Liver function markers were normalized and hepatitis B virus (HBV) DNA level was decreased following the ETV treatment. However, the HBV DNA level rose during periods of antiviral withdrawal.
Pathological diagnosis
Histopathological analysis of the erupted skin biopsy showed perivascular lymphocytic infiltration and accumulated interstitial eosinophils.
Treatment
In cases of suspected drug-induced skin eruption, the corresponding drug should be withdrawn. Following withdraw of ETV, the patient was switched to another appropriate antiviral (adefovir-lamivudine combination) and given an antihistamine and low dose steroid until the symptom resolved.
Related reports
Two case reports of unspecified hypersensitivity allergic (type I) cutaneous eruption related to ETV exist in the medical literature and describe diagnoses by skin test and lymphocyte stimulation test.
Term explanation
Type IV hypersensitivity (known as a delayed hypersensitivity reaction) manifests as an inflammatory reaction within days of exposure to the causative agent. All drug eruptions are classified as either type I or type IV.
Experiences and lessons
Drug eruption is a bothersome adverse drug reaction involving the cutaneous tissues, and it can affect a patient’s compliance with a necessary medication regimen. Poor compliance with antivirals for treating chronic hepatitis B can lead to viral breakthrough and may promote development of a drug-resistant phenotype.
Peer review
This report describes a rare case of ETV-induced type IV delayed hypersensitivity reaction with manifestation as a cutaneous eruption that was diagnosed according to evidence obtained during evaluation of the patient’s clinical course, histopathological analysis and cytokine release profiling. The unclear causal relationship between drug administration and symptom onset in the first round of ETV treatment and concomitant drug can represent an obstacle to obtaining the correct diagnosis of an adverse drug reaction.
Footnotes
Supported by Grant from the Chungbuk National University Hospital (2013)
P- Reviewer: Quarleri J, Ye XG S- Editor: Ma N L- Editor: A E- Editor: Zhang DN
References
- 1.Manns MP, Akarca US, Chang TT, Sievert W, Yoon SK, Tsai N, Min A, Pangerl A, Beebe S, Yu M, Wongcharatrawee S. Long-term safety and tolerability of entecavir in patients with chronic hepatitis B in the rollover study ETV-901. Expert Opin Drug Saf. 2012;11:361–368. doi: 10.1517/14740338.2012.653340. [DOI] [PubMed] [Google Scholar]
- 2.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 3.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 4.Yamada S, Sawada Y, Nakamura M. Maculopapular-type drug eruption caused by entecavir. Eur J Dermatol. 2011;21:635–636. doi: 10.1684/ejd.2011.1411. [DOI] [PubMed] [Google Scholar]
- 5.Sugiura K, Sugiura M, Takashi T, Naoki H, Itoh A. Immediate allergy, drug-induced eruption, by entecavir. J Eur Acad Dermatol Venereol. 2009;23:487–489. doi: 10.1111/j.1468-3083.2008.02932.x. [DOI] [PubMed] [Google Scholar]
- 6.Shara M, Ohia SE, Yasmin T, Zardetto-Smith A, Kincaid A, Bagchi M, Chatterjee A, Bagchi D, Stohs SJ. Dose- and time-dependent effects of a novel (-)-hydroxycitric acid extract on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation and histopathological data over a period of 90 days. Mol Cell Biochem. 2003;254:339–346. doi: 10.1023/a:1027358106407. [DOI] [PubMed] [Google Scholar]
- 7.Asghar M, Monjok E, Kouamou G, Ohia SE, Bagchi D, Lokhandwala MF. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol Cell Biochem. 2007;304:93–99. doi: 10.1007/s11010-007-9489-3. [DOI] [PubMed] [Google Scholar]
- 8.Wolf B, Morgan H, Krieg J, Gani Z, Milicov A, Warncke M, Brennan F, Jones S, Sims J, Kiessling A. A whole blood in vitro cytokine release assay with aqueous monoclonal antibody presentation for the prediction of therapeutic protein induced cytokine release syndrome in humans. Cytokine. 2012;60:828–837. doi: 10.1016/j.cyto.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Gerson D, Sriganeshan V, Alexis JB. Cutaneous drug eruptions: a 5-year experience. J Am Acad Dermatol. 2008;59:995–999. doi: 10.1016/j.jaad.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Noguera-Morel L, Hernández-Martín Á, Torrelo A. Cutaneous drug reactions in the pediatric population. Pediatr Clin North Am. 2014;61:403–426. doi: 10.1016/j.pcl.2013.12.001. [DOI] [PubMed] [Google Scholar]


