Abstract
The repeated cycles of cessation of consumption and relapse remain the major clinical concern in treating drug addiction. The endogenous opioid system is a crucial component of the reward circuit that participates in the adaptive changes leading to relapse in the addictive processes. We have used genetically modified mice to evaluate the involvement of μ-opioid receptor (MOR) and δ-opioid receptor (DOR) and their main endogenous ligands, the enkephalins derived from proenkephalin (PENK) and prodynorphin (PDYN), in the reinstatement of cocaine-seeking behavior. Constitutive knockout mice of MOR, DOR, PENK, and PDYN, and their wild-type littermates were trained to self-administer cocaine or to seek for palatable food, followed by a period of extinction and finally tested on a cue-induced reinstatement of seeking behavior. The four lines of knockout mice acquired operant cocaine self-administration behavior, although DOR and PENK knockout mice showed less motivation for cocaine than wild-type littermates. Moreover, cue-induced relapse was significantly decreased in MOR and DOR knockout mice. In contrast, PDYN knockout mice showed a slower extinction and increased relapse than wild-type littermates. C-Fos expression analysis revealed differential activation in brain areas related with memory and reward in these knockout mice. No differences were found in any of the four genotypes in operant responding to obtain palatable food, indicating that the changes revealed in knockout mice were not due to unspecific deficit in operant performance. Our results indicate that MOR, DOR, and PDYN have a differential role in cue-induced reinstatement of cocaine-seeking behavior.
INTRODUCTION
Addiction is a chronic brain disease characterized by the compulsive use of drugs in spite of their adverse consequences, loss of control over drug taking, and relapse even after long periods of abstinence according to the ‘Diagnostic and Statistical Manual of Mental Disorders (5th edition; DSM–5; American Psychiatric Association, 2013), the most widely accepted nomenclature used by clinicians and researchers for the classification of mental disorders. The repeated cycles of relapse after cessation of consumption remain the major clinical concern in the treatment of drug addiction (Miller and Gold, 1994; Weiss, 2010). In our laboratory, we have recently validated novel models of reinstatement of drug and food-seeking behavior (Martin-Garcia et al, 2011; Soria et al, 2008) in mice that allow to study the neurobiological mechanisms of relapse through the use of genetically modified mice.
Complex adaptive changes within the brain reward circuits occurring during the addictive processes are responsible of drug relapse. Several neurotransmitters, including the endogenous opioid system are involved in these changes (Bodnar, 2008; Volkow et al, 2009). Chronic exposure to the different prototypical drugs of abuse, including opioids, alcohol, nicotine, psychostimulants, and cannabinoids has been reported to produce significant alterations within the endogenous opioid system, which seem to have an important role in the development of the addictive process (Trigo et al, 2010). The endogenous opioid system is integrated by different families of endogenous opioid peptides, and three different opioid receptors, μ (MOR), δ (DOR), and κ (KOR), widely distributed in the central nervous system and peripheral tissues. The activation of these opioid receptors leads to different intracellular responses that produce an inhibition of neuronal activity and a reduction of neurotransmitter release (Law et al, 2000).
Three families of endogenous peptides derived from either proopiomelanocortin (POMC), proenkephalin (PENK), or prodynorphin (PDYN) generate several final active peptides including β-endorphin, met- and leu-enkephalin, dynorphins, and neo-endorphins, respectively, that exhibit different affinities for each opioid receptor (Kieffer and Gaveriaux-Ruff, 2002). The opioid peptides derived from PENK represent the main endogenous ligands that activate MOR and DOR in multiple brain areas, although endogenous enkephalins also derive from PDYN (Kieffer and Gaveriaux-Ruff 2002). These receptors have an important role in regulating mood and reward, and are key components in the control of drug reinforcing effects leading to addictive behavior. Multiple studies have suggested an important role for opioid receptors and their endogenous ligands in cocaine addiction (Charbogne et al, 2014). Administration of MOR antagonists attenuates cocaine-induced conditioned place preference and reduces cocaine self-administration and reinstatement in rats (Soderman and Unterwald, 2008; Tang et al, 2005; Ward et al, 2003), and cocaine reinforcement was reduced in MOR knockout mice (Mathon et al, 2005), suggesting the involvement of MOR in cocaine addiction. DOR antagonists can increase or decrease cocaine self-administration in rats depending on the brain area microinjected (Ward et al, 2003). The role of endogenous opioid peptides derived from PENK in cocaine responses is still unknown, although these peptides participate in the reinforcing effects of other drugs of abuse (Berrendero et al, 2005; Marinelli et al, 2005). Several opioid peptides have opposite roles in the control of behavioral responses such as dynorphins and Leu-enkephalin derived from PDYN (Butelman et al, 2012), and their specific involvement in cocaine reinforcement remains unclaryfied.
Both drug and food reward has in common the involvement of similar neurochemical pathways within the mesolimbic system (Lutter and Nestler, 2009). Indeed, the endogenous opioid system also has an important role in the mechanisms underlying the behavioral responses directed to obtain food (Kelley, 2004; Shippenberg et al, 2007). In agreement, pharmacological agonism or antagonism of MOR and DOR increased or decreased, respectively, food intake (Bodnar, 2004; Zhang et al, 1998).
The aim of this study is to investigate the participation of the two main opioid receptors involved in drug reinforcing effects, MOR and DOR, and enkephalins derived from PENK and PDYN that represent their main endogenous ligands, in the reinstatement of cocaine-seeking behavior by using knockout mice deficient in these four components of the endogenous opioid system and their wild-type littermates. We have also evaluated the impact of the deletion of the components of the opioid system on cue-induced reinstatement using c-Fos expression as a marker of neuronal activity in brain areas involved in addiction. The use of knockout mice is an essential tool for understanding the role of the opioid system in drug reinforcement and relapse and complement the information previously obtained on pharmacological studies (Lutz and Kieffer, 2013).
MATERIALS AND METHODS
Animals
Homozygous knockout mice deficient in MOR, DOR, PENK and PDYN on a C57BL/6J background and their respective wild-type littermates were used (Matthes et al, 1996; Filliol et al, 2000; König et al, 1996; Galeote et al, 2009). Previous studies have shown that the genetic ablation of a specific opioid receptor did not result in major changes in other opioid receptor sites (Kieffer and Gaveriaux-Ruff, 2002). Mice were housed individually in controlled laboratory conditions with the temperature maintained at 21±1 °C and humidity at 55±10%. Mice were tested during the first hours of the dark phase of a reversed light/dark cycle (lights off at 08:00 h and on at 20:00 h). Food and water were available ad libitum in mice used in the cocaine experiment. For operant behavior maintained by food, mice were food deprived (85% of the initial weight) and water was available ad libitum. Animal procedures were conducted in strict accordance with the guidelines of the European Communities Directive 86/609/EEC regulating animal research and were approved by the local ethical committee (CEEA-PRBB).
Cocaine Self-Administration Apparatus
Cocaine self-administration training was performed in operant chambers (Model ENV-307A-CT, Med Associates, Georgia, VT, USA) equipped with two holes, one randomly selected as the active hole and the other as the inactive (see Supplementary Information).
Surgery
Mice were anaesthetized with a ketamine/xylazine mixture (20 ml/kg of body weight) and then implanted with indwelling i.v. silastic catheters as previously described (Soria et al, 2005) (see Supplementary Information). The success rate for maintaining patency of the catheter (mean duration of 13 days) until the end of the cocaine self-administration training was 88%.
Drugs
Cocaine hydrochloride was obtained from Ministerio de Sanidad y Consumo (Spain) and dissolved in sterile 0.9% physiological saline. Ketamine hydrochloride (100 mg/kg) (Imalgène 1000; Rhône Mérieux, Lyon, France) and xylazine hydrochloride (20 mg/kg) (Sigma, Madrid, Spain) were mixed and dissolved in ethanol (5%) and distilled water (95%). This anesthetic mixture was administered intraperitoneally in an injection volume of 20 ml/kg of body weight. Thiopental sodium (5 mg/ml) (Braun Medical S.A, Barcelona, Spain) was dissolved in distilled water and delivered by infusion of 0.1 ml through the i.v. catheter.
Food-Maintained Operant Behavior Apparatus
Operant responding maintained by food was performed in mouse operant chambers (Model ENV-307A-CT, Med Associates, Georgia, VT, USA) equipped with two retractable levers, one randomly selected as the active lever and the other as the inactive (see Supplementary Information).
Experimental Design
A first group of mice (n=101) was trained for cocaine self-administration experiments, during 2-h daily sessions to acquire operant responding maintained by cocaine (0.5 mg/kg/infusion, i.v.) under fixed ratio 1 (FR1) (5 consecutive days) and FR3 (7 consecutive days). The criteria for acquisition of operant responding were achieved when mice maintained a stable responding with less than 20% deviation from the mean of the total number of infusions earned in three consecutive sessions, with at least 75% responding on the reinforced nose-poke, and a minimum of 10 reinforcements per session (Martin-Garcia et al, 2011; Soria et al, 2008). After the 12 FR sessions, animals were tested in a progressive ratio (PR) schedule where the response requirement to earn infusions escalated according to the following series: 1–2–3–5–12–18–27–40–60–90–135–200–300–450–675–1000. The maximum duration of the PR session was 4 h or until mice did not respond on any hole within 1 h, and was performed only once. After PR session, the thiopental test was applied and only mice that showed patency of catheter were moved to the extinction and relapse phases. The first extinction session occurred 48 h after the thiopental tests to avoid any possible influence of thiopental residual effects.
During the extinction phase, the experimental conditions were similar to the acquisition sessions except that cocaine was not available and the cue light was not presented after active responding. Mice were given 2 h daily extinction sessions, during 15 consecutive days until the criteria for extinction was achieved ie, during three consecutive sessions, mice responded on the active lever less than 30% of the responses reached in the three last acquisition days, and made less than 15 active responses per session. After extinction, mice were tested for reinstatement. Cues-induced reinstatement was conducted under the same conditions used in the acquisition phase except that cocaine was not delivered. Each response on the active manipulandum in this phase led to the presentation of the cue light for 2 s. The reinstatement criterion was achieved when responding in the nose-poke doubled with respect to extinction responding.
For food-maintained operant behavior experiments, a second group of mice (n=63) was trained during 1 h for 10 consecutive days to lever press for highly caloric and banana-flavored food pellets (14% protein, 60% fat, 26% carbohydrate, with a caloric value of 5.32 kcal/g) (Bio-Serv, Frenchtown, NJ, USA) paired with the presentation of a cue light on a FR1 schedule followed by 10 sessions under FR5, using the same criteria for the acquisition of operant behavior previously described. After the 20 FR sessions, animals were trained in one single PR schedule session where the response requirement to earn pellets escalated according to the following series: 1–5–12–21–33–51–75–90–120–155–180–225–260–300–350–410–465–540–630–730–850–1000–1200–1500–1800–2100–2400–2700–3000–3400–3800–4200–4600–5000–5500. The maximum duration of the PR session was 5 h or until mice did not respond on any lever within 1 h. This second group of mice was food deprived during the whole experiment at 85% of their ad libitum initial weight adjusted for growth.
Immunohistochemistry Studies
See Supplementary Information for tissue preparation, immunofluorescence and c-Fos quantification.
Statistical Analysis
Analysis of the data obtained during the acquisition and extinction phase was conducted using two-way ANOVA with manipulandum (active/inactive) as within-subjects factor and genotype as between-subjects factor. Post hoc analysis (Newman–Keuls) was performed when required. Progressive ratio, day of extinction, cues-induced reinstatement, and immunohistochemistry results were compared using one-way ANOVA.
All results are expressed as mean±SEM. Differences were considered significant at p<0.05. The statistical analysis was performed using the Statistical Package for Social Science program SPSS 19.0 (SPSS, Chicago, USA).
RESULTS
Acquisition and Maintenance of Cocaine Self-Administration
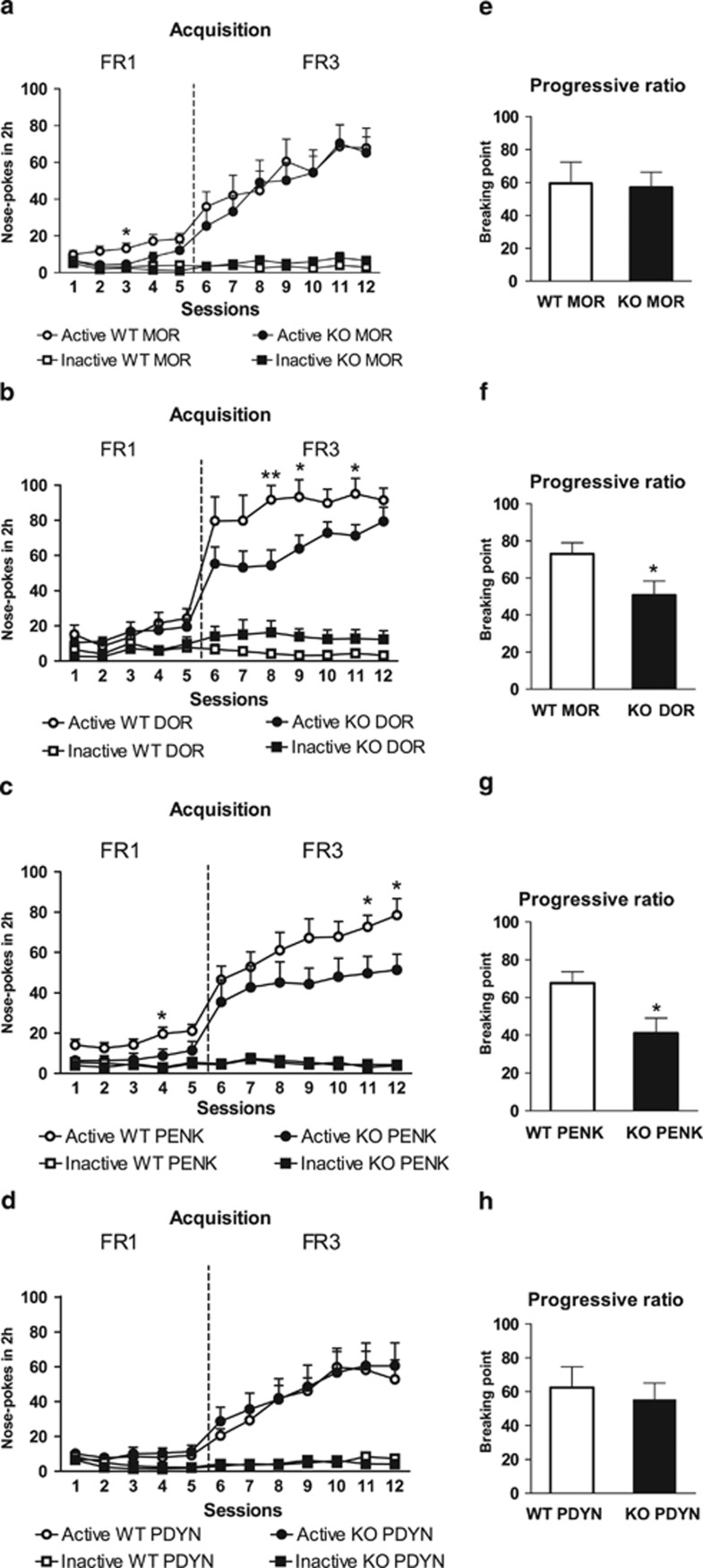
MOR knockout mice (n=11) and wild-type littermates (n=13) were trained to self-administer cocaine under FR1 (5 days), and FR3 (7 days) schedule of reinforcement. Two-way ANOVA revealed significant main effects of hole during the training period indicating a continuous operant responding for cocaine and discrimination between holes. Significant effects of genotype on day 2, 3 and 4, and a significant interaction between genotype and hole were revealed on day 3 (Table 1A). Subsequent post hoc analysis (Newman–Keuls) showed significant differences between genotypes on day 3 (p<0.05) (Figure 1a). The acquisition criteria were achieved by 90% of the MOR knockout mice and 77% of wild-type littermates. No significant difference was revealed between the breaking point achieved by MOR knockout and wild-type littermates in the PR session [F(1,22)=0.652; NS] (Figure 1e).
Table 1A. Two-Way ANOVA of the Operant Responses During the Acquisition Phase.
| Dependent variables |
Two-way ANOVA |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cocaine self-administration |
Food-maintained operant behaviour |
||||||||||||||||
|
MOR |
DOR |
PENK |
PDYN |
MOR |
DOR |
PENK |
PDYN |
||||||||||
| F(1.44) | Sig. | F(1.46) | Sig. | F(1.52) | Sig. | F(1.46) | Sig. | F(1. 28) | Sig. | F(1.28) | Sig. | F(1.28) | Sig. | F(1.26) | Sig. | ||
| Genotype | Day 1 | 1.55 | NS | 1.92 | NS | 5.97 | p<.05 | 2.30 | NS | 2.54 | NS | .07 | NS | .00 | NS | .49 | NS |
| Day 2 | 6.47 | p<.05 | .05 | NS | 4.17 | p<.05 | 1.56 | NS | .06 | NS | 1.12 | NS | .93 | NS | .35 | NS | |
| Day 3 | 6.71 | p<.05 | .00 | NS | 1.93 | NS | .00 | NS | 1.34 | NS | 1.96 | NS | 1.23 | NS | .19 | NS | |
| Day 4 | 5.5 | p<.05 | .2 | NS | 3.95 | NS | .18 | NS | .01 | NS | .27 | NS | .17 | NS | .01 | NS | |
| Day 5 | 3.51 | NS | .1 | NS | 1.88 | NS | .20 | NS | .86 | NS | .81 | NS | .00 | NS | 1.05 | NS | |
| Day 6 | .72 | NS | .93 | NS | .6 | NS | .47 | NS | .15 | NS | .18 | NS | .00 | NS | .2 | NS | |
| Day 7 | .32 | NS | .79 | NS | .53 | NS | .37 | NS | .86 | NS | .01 | NS | .01 | NS | 4.82 | NS | |
| Day 8 | .29 | NS | 2.94 | NS | 1.10 | NS | .01 | NS | .16 | NS | 1.18 | NS | .04 | NS | 1.04 | NS | |
| Day 9 | .31 | NS | 1.94 | NS | 2.96 | NS | .00 | NS | 1.00 | NS | 2.55 | NS | .10 | NS | 9.7 | p<.01 | |
| Day 10 | .05 | NS | .45 | NS | 3.00 | NS | .01 | NS | .98 | NS | 1.74 | NS | .16 | NS | 5.88 | p<.05 | |
| Day 11 | .13 | NS | 1.57 | NS | 4.23 | p<.05 | .01 | NS | .00 | NS | 2.29 | NS | .27 | NS | 2.29 | NS | |
| Day 12 | .01 | NS | .05 | NS | 5.28 | p<.05 | .07 | NS | 0.00 | NS | 3.91 | NS | .19 | NS | .25 | NS | |
| Day 13 | 1.16 | NS | 2.28 | NS | .41 | NS | .22 | NS | |||||||||
| Day 14 | .35 | NS | 1.28 | NS | .34 | NS | .64 | NS | |||||||||
| Day 15 | .60 | NS | 1.24 | NS | .01 | NS | .84 | NS | |||||||||
| Day 16 | .02 | NS | .59 | NS | .05 | NS | 3.7 | NS | |||||||||
| Day 17 | .33 | NS | .31 | NS | .61 | NS | 1.12 | NS | |||||||||
| Day 18 | .23 | NS | .50 | NS | .80 | NS | .04 | NS | |||||||||
| Day 19 | .53 | NS | .72 | NS | .29 | NS | .7 | NS | |||||||||
| Day 20 | .01 | NS | .58 | NS | 1.2 | NS | 1.08 | NS | |||||||||
| Hole / lever | Day 1 | 1.66 | NS | 7.21 | p<.05 | 7.94 | p<.01 | .37 | NS | 8.7 | p<.01 | 11.95 | p<.01 | 2.9 | NS | .13 | NS |
| Day 2 | 10.51 | p<.01 | 10.59 | p<.01 | 7.38 | p<.01 | 4.91 | p<.05 | 13.45 | p<.001 | 38.28 | p<.001 | 8.82 | p<.01 | 7.10 | p<.05 | |
| Day 3 | 12.36 | p<.01 | 2.21 | NS | 5.48 | p<.05 | 9.19 | p<.01 | 41.68 | p<.001 | 54.36 | p<.001 | 41.59 | p<.001 | 31.30 | p<.001 | |
| Day 4 | 19.71 | p<.001 | 10.55 | p<.01 | 19.00 | p<.001 | 16.87 | p<.001 | 49.72 | p<.001 | 55.55 | p<.001 | 43.95 | p<.001 | 42.40 | p<.001 | |
| Day 5 | 25.82 | p<.001 | 8.36 | p<.01 | 11.15 | p<.01 | 13.73 | p<.01 | 102.46 | p<.001 | 104.11 | p<.001 | 41.31 | p<.001 | 63.60 | p<.001 | |
| Day 6 | 19.19 | p<.001 | 41.93 | p<.001 | 29.72 | p<.001 | 17.75 | p<.001 | 35.79 | p<.001 | 216.37 | p<.001 | 41.08 | p<.001 | 106.30 | p<.001 | |
| Day 7 | 21.32 | p<.001 | 34.00 | p<.001 | 36.61 | p<.001 | 25.08 | p<.001 | 83.16 | p<.001 | 399.66 | p<.001 | 70.01 | p<.001 | 146.80 | p<.001 | |
| Day 8 | 27.24 | p<.001 | 73.53 | p<.001 | 45.4 | p<.001 | 24.51 | p<.001 | 48.12 | p<.001 | 152.71 | p<.001 | 39.92 | p<.001 | 106.70 | p<.001 | |
| Day 9 | 39.72 | p<.001 | 107.6 | p<.001 | 64.02 | p<.001 | 28.69 | p<.001 | 68.61 | p<.001 | 170.91 | p<.001 | 72.28 | p<.001 | 227.70 | p<.001 | |
| Day 10 | 41.03 | p<.001 | 157.13 | p<.001 | 75.22 | p<.001 | 34.35 | p<.001 | 64.01 | p<.001 | 301.18 | p<.001 | 36.81 | p<.001 | 310.00 | p<.001 | |
| Day 11 | 62.00 | p<.001 | 149.36 | p<.001 | 120.44 | p<.001 | 35.94 | p<.001 | 54.55 | p<.001 | 125.64 | p<.001 | 48.78 | p<.001 | 174.50 | p<.001 | |
| Day 12 | 74.35 | p<.001 | 152.87 | p<.001 | 108.45 | p<.001 | 32.39 | p<.001 | 45.35 | p<.001 | 135.59 | p<.001 | 64.97 | p<.001 | 247.10 | p<.001 | |
| Day 13 | 83.22 | p<.001 | 249.98 | p<.001 | 44.83 | p<.001 | 170.00 | p<.001 | |||||||||
| Day 14 | 97.59 | p<.001 | 325.17 | p<.001 | 63.06 | p<.001 | 127.20 | p<.001 | |||||||||
| Day 15 | 79.42 | p<.001 | 182.57 | p<.001 | 53.31 | p<.001 | 81.20 | p<.001 | |||||||||
| Day 16 | 83.50 | p<.001 | 146.48 | p<.001 | 40.37 | p<.001 | 107.60 | p<.001 | |||||||||
| Day 17 | 99.72 | p<.001 | 134.11 | p<.001 | 45.75 | p<.001 | 120.30 | p<.001 | |||||||||
| Day 18 | 108.65 | p<.001 | 137.28 | p<.001 | 52.95 | p<.001 | 86.20 | p<.001 | |||||||||
| Day 19 | 144.22 | p<.001 | 209.91 | p<.001 | 58.54 | p<.001 | 283.80 | p<.001 | |||||||||
| Day 20 | 135.97 | p<.001 | 249.66 | p<.001 | 55.56 | p<.001 | 171.20 | p<.001 | |||||||||
| Genotype * Hole / lever | Day 1 | .23 | NS | .03 | NS | 2.64 | NS | .19 | NS | 3.13 | NS | .01 | NS | .58 | NS | .22 | NS |
| Day 2 | 3.6 | NS | 1.05 | NS | 1.02 | NS | .14 | NS | .10 | NS | 1.12 | NS | 1.37 | NS | .10 | NS | |
| Day 3 | 5.39 | p<.05 | .50 | NS | 2.42 | NS | .54 | NS | 1.11 | NS | .14 | NS | .90 | NS | .41 | NS | |
| Day 4 | 1.82 | NS | .25 | NS | 4.63 | p<.05 | .89 | NS | .22 | NS | .9 | NS | 2.11 | NS | 6.76 | p<.05 | |
| Day 5 | .41 | NS | .54 | NS | 2.38 | NS | .28 | NS | .31 | NS | .10 | NS | 3.86 | NS | 5.22 | p<.05 | |
| Day 6 | .71 | NS | 3.24 | NS | .74 | NS | .93 | NS | .27 | NS | .27 | NS | .04 | NS | 1.18 | NS | |
| Day 7 | .43 | NS | 3.51 | NS | .63 | NS | .26 | NS | .00 | NS | .47 | NS | .07 | NS | .27 | NS | |
| Day 8 | .00 | NS | 11.38 | p<.01 | 1.51 | NS | .00 | NS | .27 | NS | 2.32 | NS | 1.86 | NS | .08 | NS | |
| Day 9 | .52 | NS | 8.84 | p<.01 | 3.52 | NS | .06 | NS | 1.00 | NS | 3.23 | NS | 1.16 | NS | .00 | NS | |
| Day 10 | .06 | NS | 4.94 | p<.05 | 2.32 | NS | .06 | NS | 5.95 | p<.05 | 1.53 | NS | .07 | NS | 2.46 | NS | |
| Day 11 | .02 | NS | 6.96 | p<.05 | 5.49 | p<.05 | .14 | NS | .22 | NS | .86 | NS | .58 | NS | 3.01 | NS | |
| Day 12 | .18 | NS | 2.86 | NS | 5.56 | p<.05 | .39 | NS | .19 | NS | 1.71 | NS | .11 | NS | .13 | NS | |
| Day 13 | 2.53 | NS | 1.2 | NS | .58 | NS | 2.22 | NS | |||||||||
| Day 14 | 1.08 | NS | .83 | NS | .10 | NS | .00 | NS | |||||||||
| Day 15 | .18 | NS | .24 | NS | .00 | NS | .11 | NS | |||||||||
| Day 16 | .00 | NS | .28 | NS | .03 | NS | 2.12 | NS | |||||||||
| Day 17 | .03 | NS | .14 | NS | .21 | NS | .32 | NS | |||||||||
| Day 18 | .00 | NS | .15 | NS | .48 | NS | .62 | NS | |||||||||
| Day 19 | .43 | NS | .49 | NS | .20 | NS | .14 | NS | |||||||||
| Day 20 | .00 | NS | .16 | NS | .88 | NS | .55 | NS | |||||||||
Figure 1.
Operant behavior to obtain cocaine in MOR, DOR, PENK, and PDYN knockout mice. Mean number of nose-pokes in the active and inactive hole during the acquisition training in fixed ratio 1 (FR1) and FR3 schedule of reinforcement to obtain cocaine (0.5 mg/kg/infusion) (a) MOR knockout mice (n=11) and wild-type littermates (n=13). (b) DOR knockout mice (n=14) and wild-type littermates (n=10). (c) PENK knockout mice (n=14) and wild-type littermates (n=14). (d) PDYN knockout mice (n=14) and wild-type littermates (n=11). (e) Breaking point achieved in the progressive ratio session in MOR knockout mice and wild-type littermates. (f) Breaking point in DOR knockout mice and wild-type littermates. (g) Breaking point in PENK knockout mice and wild-type littermates. (h) Breaking point in PDYN knockout mice and wild-type littermates. Data are expressed as mean±SEM. *p<0.05, **p<0.01 vs knockout group (Newman–Keuls test (acquisition) or one-way ANOVA (progressive ratio)).
For DOR knockout (n=14) and wild-type littermates (n=10), two-way ANOVA revealed significant main effects of hole during the training period, indicating a continuous operant responding for cocaine and discrimination between holes. No main genotype effects but significant interactions between genotype and hole on days 8, 9, 10, and 11 were revealed (Table 1A). Subsequent post hoc analysis (Newman–Keuls) showed significant differences between genotypes on day 8 (p<0.01), 9 (p<0.05) and 11 (p<0.05) (Figure 1b). The acquisition criteria were achieved by 93% of the DOR knockout mice and 100% of wild-type littermates. One-way ANOVA showed a significant decrease of the breaking point achieved by DOR knockout mice when compared with wild-type littermates [F(1,23)=5.673; p<0.05] (Figure 1f).
For PENK knockout (n=14) and wild-type littermates (n=14), two-way ANOVA revealed a significant effect of hole during the whole training period, indicating a continuous operant responding for cocaine and discrimination between holes. Significant effects of genotype on day 1, 2, 11, and 12, and a significant interaction between genotype and hole on day 4, 11 and 12 were revealed (Table 1A). Subsequent post hoc analysis (Newman–Keuls) showed significant differences between genotypes on day 4 (p<0.05), 11 (p<0.05) and 12 (p<0.05) (Figure 1c). The acquisition criteria were achieved by 76% of the PENK knockout mice and 100% of wild-type littermates. One-way ANOVA showed a significant decrease of the breaking point achieved by PENK knockout mice when compared with wild-type littermates [F(1,26)=7.123; p<0.05] (Figure 1g).
For PDYN knockout (n=14) and wild-type littermates (n=11), two-way ANOVA revealed significant main effects of hole during the whole training period, indicating a continuous operant responding for cocaine and discrimination between holes. Two-way ANOVA did not reveal main effects of genotype nor interaction between genotype and hole through the entire acquisition phase (Table 1A; Figure 1d). All PDYN KO mice and wild-type littermates achieved the acquisition criteria in the last experimental sequence. One-way ANOVA showed no significant differences in the breaking point achieved by PDYN knockout mice and wild-type littermates [F(1,23)=0.213; NS] (Figure 1h).
Extinction and Cues-Induced Reinstatement of Cocaine-Seeking Behavior
The extinction criteria were achieved by all mice. Two-way ANOVA revealed significant main effects in hole during the whole extinction phase in the four experiments (Table 1B).
Table 1B. Two-Way ANOVA of the Operant Responses During the Extinction Phase.
| Dependent variables |
Two-way ANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Extinction |
|||||||||
|
MOR |
DOR |
PENK |
PDYN |
||||||
| F(1.38) | Sig. | F(1.32) | Sig. | F(1.40) | Sig. | F(1.36) | Sig. | ||
| Genotype | Day 1 | .47 | NS | .01 | NS | .49 | NS | 6.54 | p<0.05 |
| Day 2 | .04 | NS | .00 | NS | .01 | NS | 8.11 | p<0.05 | |
| Day 3 | .01 | NS | .54 | NS | .06 | NS | 3.94 | NS | |
| Day 4 | .52 | NS | .73 | NS | .13 | NS | 8.49 | p<0.01 | |
| Day 5 | .01 | NS | .30 | NS | 6.47 | p<0.05 | 1.73 | NS | |
| Day 6 | 3.33 | NS | .47 | NS | .01 | NS | 11.97 | p<0.01 | |
| Day 7 | 5.79 | p<0.05 | .08 | NS | .92 | NS | 3.12 | NS | |
| Day 8 | 3.43 | NS | .04 | NS | .11 | NS | .95 | NS | |
| Day 9 | 2.57 | NS | .21 | NS | .79 | NS | 3.31 | NS | |
| Day 10 | 5.85 | p<0.05 | 2.13 | NS | .98 | NS | 5.23 | p<0.05 | |
| Day 11 | 3.96 | NS | .08 | NS | 2.08 | NS | 12.11 | p<0.01 | |
| Day 12 | .33 | NS | 1.75 | NS | .66 | NS | 6.86 | p<0.05 | |
| Day 13 | 1.26 | NS | 3.11 | NS | .18 | NS | 2.94 | NS | |
| Day 14 | 1.26 | NS | .71 | NS | .00 | NS | .14 | NS | |
| Day 15 | 4.21 | p<0.05 | 1.39 | NS | .14 | NS | 1.10 | NS | |
| Hole | Day 1 | 37.84 | p<0.001 | 33.34 | p<0.001 | 154.23 | p<0.001 | 59.82 | p<0.001 |
| Day 2 | 26.69 | p<0.001 | 14.23 | p<0.01 | 112.36 | p<0.001 | 32.37 | p<0.001 | |
| Day 3 | 24.65 | p<0.001 | 7.61 | p<0.01 | 95.73 | p<0.001 | 27.62 | p<0.001 | |
| Day 4 | 34.55 | p<0.001 | 4.52 | p<0.05 | 84.69 | p<0.001 | 21.87 | p<0.001 | |
| Day 5 | 34.27 | p<0.001 | 26.34 | p<0.001 | 60.60 | p<0.001 | 37.38 | p<0.001 | |
| Day 6 | 16.40 | p<0.001 | 17.05 | p<0.001 | 53.00 | p<0.001 | 54.52 | p<0.001 | |
| Day 7 | 20.08 | p<0.001 | 6.12 | p<0.05 | 48.03 | p<0.001 | 54.62 | p<0.001 | |
| Day 8 | 11.35 | p<0.01 | 14.22 | p<0.01 | 35.49 | p<0.001 | 22.76 | p<0.001 | |
| Day 9 | 10.78 | p<0.01 | 11.49 | p<0.01 | 46.10 | p<0.001 | 30.39 | p<0.001 | |
| Day 10 | 21.1 | p<0.001 | 13.99 | p<0.01 | 50.17 | p<0.001 | 27.27 | p<0.001 | |
| Day 11 | 16.4 | p<0.001 | 10.84 | p<0.01 | 28.30 | p<0.001 | 32.26 | p<0.001 | |
| Day 12 | 29.16 | p<0.001 | 8.94 | p<0.01 | 15.66 | p<0.001 | 26.02 | p<0.001 | |
| Day 13 | 10.95 | p<0.01 | 4.06 | NS | 33.83 | p<0.001 | 35.23 | p<0.001 | |
| Day 14 | 6.94 | p<0.05 | 7.34 | p<0.05 | 31.23 | p<0.001 | 12.70 | p<0.01 | |
| Day 15 | 8.65 | p<0.01 | 3.26 | NS | 25.51 | p<0.001 | 24.46 | p<0.001 | |
| Genotype* | Day 1 | .78 | NS | 2.46 | NS | 1.93 | NS | 5.61 | p<0.05 |
| Hole | Day 2 | .65 | NS | 1.79 | NS | 1.90 | NS | 7.80 | p<0.01 |
| Day 3 | .66 | NS | .09 | NS | 1.84 | NS | 8.84 | p<0.01 | |
| Day 4 | .92 | NS | 2.05 | NS | 2.95 | NS | 5.24 | p<0.05 | |
| Day 5 | .2 | NS | 7.76 | p<0.01 | .09 | NS | 2.69 | NS | |
| Day 6 | .23 | NS | 4.05 | NS | .51 | NS | 8.66 | p<0.01 | |
| Day 7 | .14 | NS | 2.14 | NS | .01 | NS | 8.52 | p<0.01 | |
| Day 8 | .00 | NS | 3.81 | NS | .67 | NS | 5.86 | p<0.05 | |
| Day 9 | .45 | NS | 2.68 | NS | .04 | NS | 4.09 | NS | |
| Day 10 | .51 | NS | 4.94 | p<0.05 | .07 | NS | 5.29 | p<0.05 | |
| Day 11 | .03 | NS | 4.1 | NS | .85 | NS | 4.66 | p<0.05 | |
| Day 12 | .05 | NS | .14 | NS | 2.02 | NS | 7.61 | p<0.01 | |
| Day 13 | .49 | NS | .05 | NS | 1.23 | NS | 2.48 | NS | |
| Day 14 | .00 | NS | 2.18 | NS | 1.16 | NS | .46 | NS | |
| Day 15 | .12 | NS | .21 | NS | .94 | NS | .81 | NS | |
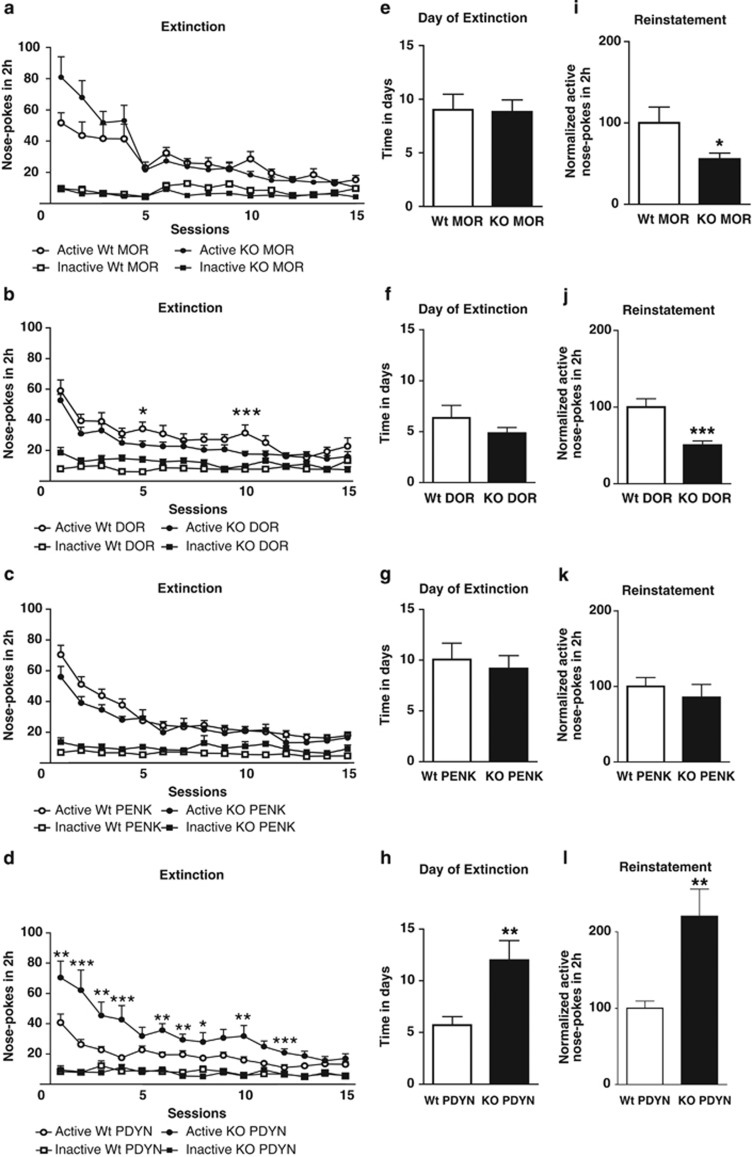
In MOR experiment, two-way ANOVA showed significant effects of genotypes on day 7, 10, and 15 without interaction between genotype and hole (Table 1B; Figure 2a). One-way ANOVA did not reveal significant differences in the time required to achieve the extinction criteria [F(1,19)=0.012; NS] (Figure 2e). After 15 daily sessions of extinction, one-way ANOVA demonstrated a significant decrease in cues-induced reinstatement of cocaine-seeking behavior in MOR knockout mice compared with wild-type littermates [F(1,19)=5.255; p<0.05] (Figure 2i).
Figure 2.
Operant behavior in extinction phase, the day of extinction and cues-induced reinstatement of cocaine-seeking behavior in MOR, DOR, PENK, and PDYN knockout mice. Mean number of nose-pokes in the active and inactive hole during the extinction training. (a) MOR knockout mice (n=10) and wild-type littermates (n=11). (b) DOR knockout mice (n=13) and wild-type littermates (n=9). (c) PENK knockout mice (n=11) and wild-type littermates (n=14). (d) PDYN knockout mice (n=9) and wild-type littermates (n=11). (e) Time in days necessary to accomplish extinction criteria in MOR KO mice (n=10) and wild-type littermates (n=11), (f) DOR KO mice (n=13) and wild-type littermates (n=9), (g) PENK KO mice (n=11) and wild-type littermates (n=14), (h) PDYN KO (n=9) mice and wild-type littermates (n=11). (i) Cues-induced reinstatement of cocaine-seeking behavior shown as normalized nose-pokes in the active hole in MOR KO mice (n=10) and wild-type littermates (n=11), (j) DOR KO mice (n=13) and wild-type littermates (n=9), (k) PENK KO mice (n=11) and wild-type littermates (n=14), (l) PDYN KO mice (n=9) and wild-type littermates (n=11). Data are expressed as mean±SEM. *p<0.05, **p<0.01, ***p<0.001 vs KO group; (Newman–Keuls test (extinction) or one-way ANOVA (day of extinction and cues-induced reinstatement)).
In DOR experiment, no significant main effects of genotype were obtained (two-way ANOVA), although a significant interaction between genotype and hole was revealed on day 5 and 10 (Table 1B). Subsequent post hoc analysis (Newman–Keuls) showed significant differences between genotypes on day 5 (p<0.05) and 10 (p<0.001) (Figure 2b). One-way ANOVA did not reveal significant differences in the time required to achieve the extinction criteria [F(1,21)=1.492; NS] (Figure 2f). After extinction, one-way ANOVA showed a significant decrease in cues-induced reinstatement in cocaine-seeking behavior in knockout mice compared with wild-type littermates [F(1,21)=17.581; p<0.001] (Figure 2j).
In PENK experiment, two-way ANOVA revealed significant main effects of genotype on day 5 without significant interaction between genotype and hole (Table 1B; Figure 2c). One-way ANOVA did not show significant differences in the time required to achieve extinction [F(1.23)=0.172; NS] (Figure 2g) nor in cues-induced reinstatement [F(1,23)=0.55; NS] (Figure 2k).
In PDYN experiment, two-way ANOVA revealed significant main effects of genotype on day 1, 2, 4, 6, 10, 11, and 12, as well as a significant interaction between genotype and hole on day 1, 2, 3, 4, 6, 7, 8, 10, 11, and 12 (Table 1B). Post hoc analysis (Newman–Keuls) showed significant differences between genotypes on day 1 (p<0.01), 2 (p<0.001), 3 (p<0.01), 4 (p<0.001), 6 (p<0.01), 7 (p<0.01), 8 (p<0.05), 10 (p<0.01) and 12 (p<0.001) (Figure 2d). One-way ANOVA revealed a significant increase in the time required to achieve extinction [F(1,18)=10.618; p<0.01] (Figure 2h) and cues-induced reinstatement of cocaine-seeking behavior in PDYN knockout mice compared with wild-type littermates [F(1,18)=12.892; p<0.01] (Figure 2l).
C-Fos Expression
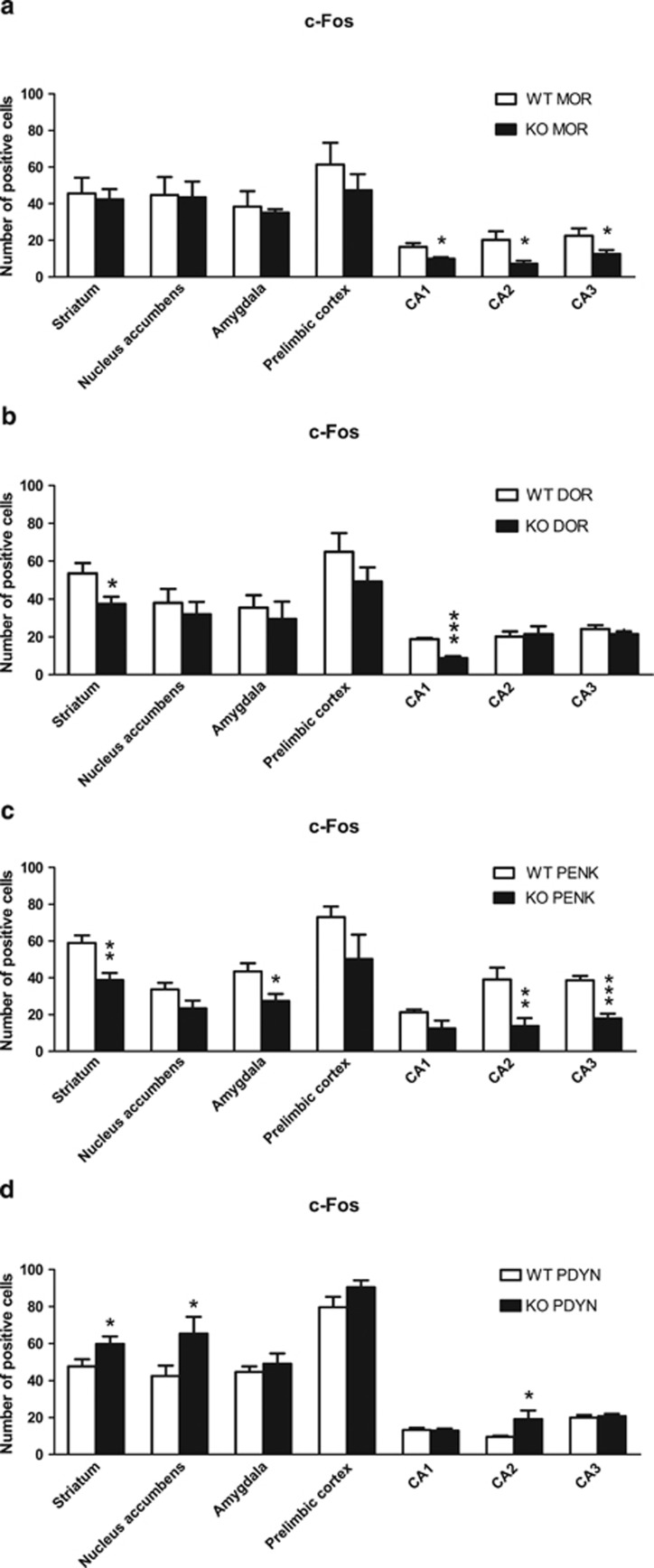
Fos protein levels were evaluated in brain areas involved in drug operant reinstatement (striatum, nucleus accumbens core, amygdala, prelimbic cortex, and CA1, CA2, CA3 regions of the hippocampus) in different knockout mice and wild-type littermates after the cues-induced reinstatement session. One-way ANOVA showed significant decreases in c-Fos levels in MOR knockout mice compared with wild-type littermates in CA1 [F(1,11)=9.48; p<0.05], CA2 [F(1,11)=7.60; p<0.05] and CA3 [F(1,11)=5.42; p<0.05] regions of the hippocampus. No significant differences were observed in other brain areas (Figures 3a and 4).
Figure 3.
Levels of c-Fos expression in different brain areas after cues-induced reinstatement of cocaine-seeking behavior in MOR, DOR, PENK, and PDYN knockout mice. Number of positive immunostained cells in the striatum, nucleus accumbens core, amygdala, prelimbic cortex, CA1 of the hippocampus, CA2 of the hippocampus, and CA3 of the hippocampus. (a) MOR knockout mice (n=7) and wild-type littermates (n=6). (b) DOR knockout mice (n=7) and wild-type littermates (n=6). (c) PENK knockout mice (n=7) and wild-type littermates (n=6). (d) PDYN knockout mice (n=8) and wild-type littermates (n=10). Data are expressed as mean±SEM. *p<0.05, **p<0.01, ***p<0.001 vs wild-type (one-way ANOVA).
Figure 4.
Representative images of c-Fos immunoreactivity in different brain areas after cues-induced reinstatement of cocaine-seeking behavior in MOR, DOR, PENK, and PDYN knockout mice. Striatum, nucleus accumbens core, amygdala, prelimbic cortex, CA1 of the hippocampus, CA2 of the hippocampus, and CA3 of the hippocampus.
One-way ANOVA showed a significant decrease in c-Fos levels in DOR knockout mice compared with wild-type littermates in the striatum [F(1,11)=6.04; p<0.05] and CA1 region of the hippocampus [F(1,11)=57.24; p<0.001]. No significant differences were revealed in other brain areas (Figures 3b and 4). Significant decreases in c-Fos levels in PENK knockout mice compared with wild-type littermates were shown in the striatum [F(1,11)=13.12; p<0.01], amygdala [F(1,11)=7.53; p<0.05], CA2 [F(1,11)=10.45; p<0.01] and CA3 [F(1,11)=37.46; p<0.001] region of the hippocampus, without significant differences in other brain areas (Figures 3c and 4).
One-way ANOVA showed a significant increase in c-Fos levels in PDYN knockout mice compared with wild-type littermates in the striatum [F(1,16)=4.51; p<0.05], nucleus accumbens core [F(1,16)=5,12; p<0.05] and CA2 region of the hippocampus [F(1,16)=5,42; p<0.05]. No significant differences were revealed in other brain areas (Figures 3d and 4).
Acquisition and Maintenance of Operant Conditioning Maintained by Food
MOR knockout (n=8) and wild-type littermates (n=8) mice were trained to acquire an operant responding maintained by high palatable food pellets under FR1 (10 days), and FR5 (10 days) schedule. Two-way ANOVA revealed significant main effects of lever during the whole training period indicating a continuous operant responding for food pellets and discrimination between levers. No significant effects of genotype were revealed (Tables 1A and B). The mean number of active lever presses during the 3 days when the acquisition criteria were achieved was 216.17±11.96 in MOR knockout group and 226.83±21.08 in wild-type littermates (number of inactive lever presses 13.67±3.63 and 16.25±4.43, respectively). The breaking point achieved by MOR knockout mice in the PR session was 248.13±33.57 and 213.13±20.72 in wild-type littermates [F(1,14)=0.787; NS] (data not shown).
In DOR knockout (n=8) and wild-type littermates (n=8), two-way ANOVA revealed significant main effects of lever during the whole training period, indicating a continuous operant responding for food pellets and discrimination between levers. No significant effects of genotype were revealed (Table 1A). The mean number of active lever presses during the 3 days when the acquisition criteria were achieved was 333.5±33.21 in DOR knockout group and 364.13±42.73 in wild-type littermates (number of inactive lever presses 12.42±3.25 and 19.75±6.17, respectively). The breaking point achieved by DOR knockout mice in the PR session was 305±21.46 and 426.88±63.21 in wild-type littermates [F(1,14)=3.333; NS] (data not shown).
In PENK knockout mice (n=8) and wild-type littermates (n=8), two-way ANOVA revealed significant main effects of lever during the whole training period indicating a continuous operant responding for food pellets and discrimination between levers. No significant effects of genotype were revealed (Table 1A). The mean number of active lever presses during the 3 days when the acquisition criteria were achieved was 249.88±43.62 in PENK knockout group and 304.25±39.87 in wild-type littermates (number of inactive lever presses 19.25±5.32 and 24.25±3.97, respectively). The breaking point achieved by PENK knockout mice in the PR session was 237.5±33.74 and 275±37.86 in wild-type littermates [F(1,14)=0.547; NS] (data not shown).
In PDYN knockout mice (n=5) and wild-type littermates (n=10), two-way ANOVA revealed significant main effects of lever during the whole training period indicating a continuous operant responding for food pellets and discrimination between levers. Significant effects of genotype were revealed only on day 9 and 10 (Tables 1A and B). The mean number of active lever presses during the 3 days when the acquisition criteria were achieved was 324.87±37.38 in PDYN knockout group and 334.6±23.63 in wild-type littermates (number of inactive lever presses 16.2±7.22 and 36.63±7.9, respectively). The breaking point achieved by PDYN knockout mice in the PR session was 248±27.14 and 251.5±23.66 in wild-type littermates [F(1,13)=0.008; NS] (data not shown).
DISCUSSION
The present study shows the specific involvement of four components of the endogenous opioid system in the acquisition and relapse of cocaine self-administration in mice. We have used a reliable operant model of reinstatement validated in our laboratory (Martin-Garcia et al, 2011; Soria et al, 2008) to demonstrate that the constitutive deletion of MOR, DOR, PENK, and PDYN differentially modifies the acquisition and reinstatement of cocaine-seeking behavior, but has no significant consequences in a similar operant training for palatable food. Indeed, a similar performance in operant responding maintained by food was obtained in all knockout mice and wild-type littermates under our experimental conditions, which ruled out a potential learning impairment for operant training in these four lines of knockout mice. This experimental control was mandatory considering that MOR knockout mice displayed learning impairment in the radial-maze task (Jamot et al, 2003) and DOR knockout mice showed impaired place conditioning (Le Merrer et al, 2011). These spatial memory impairments have no consequences in the performance of an operant training to obtain a rewarding stimulus, such as palatable food.
Previous studies have reported that selective MOR antagonists attenuate cocaine-conditioned place preference (Schroeder et al, 2007) and self-administration in rats (Ward et al, 2003), while the deletion of MOR in knockout mice reduced oral ethanol self-administration and intravenous cocaine self-administration (Becker et al, 2002; Mathon et al, 2005). In our experimental conditions, no major differences in the acquisition of cocaine self-administration were revealed in MOR knockout mice, as only a single significant reduction of active nose-poking was observed on day 3, and no differences in cocaine motivation was shown in the PR. This discrepancy could be due to differences in experimental protocol used in terms of cocaine dose, and time of conditioning sessions, as the previous study only found significant differences at high cocaine doses in shorter session times. Our results reveal that MOR is involved in cocaine relapse, as reinstatement of cocaine-seeking behavior was attenuated in MOR knockout mice. In agreement, pharmacological studies have shown that MOR antagonists reduced cocaine relapse in rats (Tang et al, 2005). Furthermore, the non-selective opioid antagonist naltrexone reduced cue-induced cocaine-seeking behavior (Burattini et al, 2008), and had no effects on cocaine priming-induced reinstatement in rats (Comer et al, 1993), although repeated naltrexone treatment suppressed this priming-induced reinstatement (Gerrits et al, 2005). Accordingly, microinjection of selective MOR agonists into the nucleus accumbens reinstated cocaine-seeking behavior in rats (Simmons and Self, 2009). Interestingly, the number of positive c-Fos-immunostained cells was lower in MOR knockout mice after cue-induced cocaine reinstatement than in wild-type mice in CA1, CA2 and CA3 regions of the hippocampus. This result reflects a decreased neuronal activation in this brain structure closely involved in memory processing after the exposure to the cocaine-associated cues when the activity of MOR is absent. These behavioral and neurochemical results suggest that MOR is involved in cocaine reinstatement by modifying the neuronal activity in brain areas involved in memory.
Cocaine self-administration was significantly attenuated in DOR knockout mice when trained in FR3, but not in FR1, suggesting that the response is impaired only when the effort required to obtain the reward is enhanced. Other studies showed that DOR knockout mice acquired morphine self-administration similarly to wild-type mice (Le Merrer et al, 2011), although these mutants showed a decreased operant responding when trained to obtain high doses of intravenous nicotine (Berrendero et al, 2012). In agreement, with our acquisition data, the breaking point achieved by DOR knockout mice was significantly lower than wild-type littermates during the PR session. In contrast, DOR knockout were reported to achieve a similar breaking point for morphine than wild-type mice under a PR schedule (Le Merrer et al, 2011). The reinstatement of cocaine-seeking behavior was also significantly reduced in DOR knockout mice. In agreement, pharmacological studies have suggested the participation of DOR in particular brain areas in cocaine-reinforcing effects (Le Merrer et al, 2009), and microinjection of selective DOR agonists in the nucleus accumbens reinstated cocaine-seeking behavior in rats (Simmons and Self, 2009). Furthermore, the enhancement of positive c-Fos-immunostained cells induced by cocaine reinstatement was attenuated in DOR knockout mice in the striatum, and the CA1 region of the hippocampus. These results revealed a decreased neuronal activation in these brain structures involved in motor and motivation control, and memory processing after the exposure to cocaine-associated cues in the absence of DOR activity. Our findings suggest that DOR modulates the motivation to obtain cocaine and cocaine reinstatement by modifying neuronal activity in brain areas involved in motor, motivation, and memory processing.
Cocaine self-administration was attenuated in PENK knockout mice, mainly when animals were trained in FR3. In agreement, the breaking point achieved by PENK knockout mice was also reduced during the PR session suggesting that opioid peptides derived from PENK have an important role in cocaine-reinforcing properties. PENK has been postulated to mediate the reinforcing effects of other drugs of abuse (Berrendero et al, 2005; Marinelli et al, 2005; Shoblock and Maidment, 2007), and changes in PENK gene expression have been revealed after long-term cocaine self-administration (Crespo et al, 2001). In contrast, cue-induced reinstatement of cocaine-seeking behavior was not modified in PENK knockout mice, which suggests that other opioid peptides different from those derived from PENK must be involved in the reinstatement of cocaine-seeking behavior. However, the number of positive c-Fos-immunostained cells was decreased in PENK knockout in the striatum, amygdala, CA2, and CA3 regions of the hippocampus after cue-induced reinstatement. Therefore, the absence of PENK decreases neuron activation in several brain structures during cue-induced reinstatement session, although these changes were not associated with a significant modification of cues-induced reinstatement of cocaine-seeking behavior.
The acquisition of cocaine self-administration and the motivation to obtain cocaine in the PR schedule were not modified in PDYN knockout mice. However, PDYN knockouts showed slower extinction and increased cues-induced reinstatement of cocaine-seeking behavior when compared with wild-type littermates, the opposite result to that obtained in MOR and DOR knockout mice on cocaine reinstatement. Opioids derived from PDYN include the MOR and DOR agonist leu-enkephalin as well as other opioid peptides with preferential KOR agonist properties, such as dynorphins (Kieffer and Gaveriaux-Ruff 2002). Considering the opposite role on the control of the rewarding pathways of KOR with regards to MOR and DOR (Trigo et al, 2010), and the opposite responses on cocaine reinstatement of our lines of knockout mice, we can postulate that the enhanced reinstatement of cocaine seeking revealed in PDYN knockout mice would be related to opioid peptides acting on KOR, such as dynorphins. In agreement, the DYN/KOR system appears to participate in the aversive effects related to cocaine exposure. Thus, KOR reduces the effects of stress on the reinstatement of cocaine-seeking behavior in mice (McLaughlin et al, 2006) and rats (Beardsley et al, 2005). Furthermore, repeated cocaine administration increases levels of dynorphins and prePDYN mRNA in animals and humans (Trifilieff and Martinez, 2013). In support of our hypothesis, c-fos mapping reveals an opposite result to other lines of opioid knockout in PDYN knockouts after cocaine reinstatement. Indeed, the number of positive c-Fos-immunostained cells induced by cocaine reinstatement was enhanced in PDYN knockout mice in the striatum, the core of nucleus accumbens and CA2 region of the hippocampus, revealing an increased neuronal activation in these brain structures related to motor, motivation, and memory processing. The findings suggest that PDYN modulates cocaine reinstatement by modifying neuronal activity in these brain areas.
Our behavioral and neurochemical results suggest that DOR and PENK are involved in the motivation to obtain cocaine, and the absence of these opioid components reduces cocaine self-administration mainly when the effort to obtain the reward is increased. Moreover, cocaine reinstatement is reduced in MOR and DOR knockout mice, whereas it is not modified in the absence of PENK and results increased in the absence of PDYN. Therefore, the reduced cocaine reinstatement revealed in MOR and DOR was not mediated by the main endogenous ligand of these receptors, enkephalins, as the deletion of the two precursors of these endogenous opioid peptides, PENK and PDYN, did not mimic this behavioral response. In agreement, we have previously demonstrated that another MOR and DOR endogenous ligand, β-endorphin, has a crucial role in the rewarding properties of other drugs of abuse such as nicotine (Trigo et al, 2009).
The genetic deletion of MOR, DOR, PENK, and PDYN did not modify the acquisition and motivation to maintain operant responding to obtain palatable food in deprived mice. In agreement, opioid receptor antagonists did not significantly modify food-seeking behavior (Abdoullaye et al, 2010) nor preference for high-caloric food in rats (Dela Cruz et al, 2012). However, the enhancement of opioid activity by administration of opioid agonists increased preferentially high-caloric food intake (Taha, 2010). In addition, rats with chronic access to highly palatable food increased their mRNA expression of POMC in the medial prefrontal cortex (Blasio et al, 2013). Taken together, these results suggest that the absence of the basal tone of MOR, DOR, PENK, and PDYN did not modify food-seeking behavior in agreement with previous pharmacological studies, whereas opioid system activation promotes this behavior.
In conclusion, our results suggest that opioid peptides derived from PENK acting on DOR have an important role in cocaine-reinforcing properties. MOR and DOR, and endogenous opioid peptides different from enkephalins are crucial for cue-induced reinstatement of cocaine-seeking behavior by modulating neuronal activation of brain areas involved in the control of motor, motivation, and memory processes. Opioid peptides derived from PDYN have an opposite role to MOR and DOR in the control of cocaine reinstatement. The elucidation of these neurobiological mechanisms involved in cocaine-reinforcing effects and relapse opens new possibilities for developing new therapeutic strategies targeting the endogenous opioid system.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the DG Research of the European Commission (PHECOMP, no. LHSM-CT-2007-037669, the Spanish ‘Instituto de Salud Carlos III' (RTA, no. RD06/001/001), the Spanish ‘Ministerio de Ciencia e Innovación' (no. SAF2007-64062, no. SAF2011-29864), the Catalan Government (SGR2009-00131), the ICREA Foundation (ICREA Academia-2008). JG-C was supported by a ‘Juan de la Cierva' post-doctoral fellowship from the Spanish ‘Ministerio de Ciencia e Innovación', A.B. and S.M. were supported by a FI predoctoral fellowships of the Catalan Government, and E. M-G. was supported by a ‘Sara Borrell' post-doctoral fellowship from the Spanish ‘Instituto de Salud Carlos III'. Partial support from FEDER funds is also acknowledged. We thank Alberto Allica Abellan for invaluable technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Author Contributions
J. G-C., A.B., S.M., S.K., and E. M-G. conducted the behavioral studies and participated in the interpretation and manuscript writing. R.M. participated in the experimental design, interpretation and manuscript writing and funded the project.
Supplementary Material
References
- Abdoullaye D, Acevedo I, Adebayo AA, Behrmann-Godel J, Benjamin RC, Bock DG, et al. Permanent Genetic Resources added to Molecular Ecology Resources Database 1 August 2009-30 September 2009. Mol Ecol Res. 2010;10:232–236. doi: 10.1111/j.1755-0998.2009.02796.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 2013Diagnostic and Statistical Manual of Mental Disorders(5th editionDSM-5) and proposed draft revisions (DSM-V)American Psychiatric Press: Washington, DC [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, et al. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Plaza-Zabala A, Galeote L, Flores A, Bura SA, Kieffer BL, et al. Influence of delta-opioid receptors in the behavioral effects of nicotine. Neuropsychopharmacology. 2012;37:2332–2344. doi: 10.1038/npp.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, Cottone P.2013Opioid system in the medial prefrontal cortex mediates binge-like eating Addict Bioldoi: 10.1111/adb.12033(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2007. Peptides. 2008;29:2292–2375. doi: 10.1016/j.peptides.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Burattini C, Burbassi S, Aicardi G, Cervo L. Effects of naltrexone on cocaine- and sucrose-seeking behavior in response to associated stimuli in rats. Int J Neuropsychopharmacol. 2008;11:103–109. doi: 10.1017/S1461145707007705. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. Kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76:204–217. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Curtis LK, Carroll ME. Effects of buprenorphine and naltrexone on reinstatement of cocaine-reinforced responding in rats. J Pharmacol Exp Ther. 1993;267:1470–1477. [PubMed] [Google Scholar]
- Crespo JA, Manzanares J, Oliva JM, Corchero J, Palomo T, Ambrosio E. Extinction of cocaine self-administration produces a differential time-related regulation of proenkephalin gene expression in rat brain. Neuropsychopharmacology. 2001;25:185–194. doi: 10.1016/S0893-133X(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Dela Cruz JA, Bae VS, Icaza-Cukali D, Sampson C, Bamshad D, Samra A, et al. Critical role of NMDA but not opioid receptors in the acquisition of fat-conditioned flavor preferences in rats. Neurobiol Learn Mem. 2012;98:341–347. doi: 10.1016/j.nlm.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Kuzmin AV, van Ree JM. Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. Eur Neuropsychopharmacol. 2005;15:297–303. doi: 10.1016/j.euroneuro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav. 2003;2:80–92. doi: 10.1034/j.1601-183x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- König M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, et al. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29:1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, Burokas A, Kostrzewa E, Gieryk A, Korostynski M, Ziolkowska B, et al. New operant model of reinstatement of food-seeking behavior in mice. Psychopharmacology. 2011;215:49–70. doi: 10.1007/s00213-010-2110-6. [DOI] [PubMed] [Google Scholar]
- Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, et al. Increased gabaergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience. 2005;130:359–367. doi: 10.1016/j.neuroscience.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Gold MS. Dissociation of ‘conscious desire' (craving) from and relapse in alcohol and cocaine dependence. Ann Clin Psychiatry. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology. 2007;195:265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–649. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience. 2008;154:1506–1516. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology. 2008;199:593–603. doi: 10.1007/s00213-008-1184-x. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Taha SA. Preference or fat? Revisiting opioid effects on food intake. Physiol Behav. 2010;100:429–437. doi: 10.1016/j.physbeh.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Kappa-opioid receptor signaling in the striatum as a potential modulator of dopamine transmission in cocaine dependence. Front Psychiatry. 2013;4:44. doi: 10.3389/fpsyt.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108:183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Zimmer A, Maldonado R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology. 2009;56:1147–1153. doi: 10.1016/j.neuropharm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56 Suppl 1:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Martin TJ, Roberts DC. Beta-funaltrexamine affects cocaine self-administration in rats responding on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2003;75:301–307. doi: 10.1016/s0091-3057(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Weiss F.2010Advances in animal models of relapse for addiction researchIn: Kuhn CM, Koob GF, (eds).Advances in the Neuroscience of Addiction2nd edn, Boca Raton (FL): London; [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.