Abstract
Dopamine D3 receptors are implicated in cue-induced relapse to drug seeking. We have previously shown that systemic administration of a selective D3 antagonist reduces cue-induced reinstatement of nicotine seeking in rats. The current study sought to investigate potential neural substrates mediating this effect. The D3 antagonist SB-277011-A (0.01–1 μg/0.5 μl/side) infused into the basolateral amygdala or the lateral habenula, but not the nucleus accumbens, significantly attenuated cue-induced reinstatement of nicotine seeking. Moreover, infusion of SB-277011-A (1 μg/0.5 μl/side) into the basolateral amygdala or lateral habenula had no effect on food self-administration. Together with the finding that systemic SB-277011-A had no effect on extinction responding, this suggests that the effects observed here were on reinstatement and cue seeking, and not due to nonspecific motor activation or contextual-modified residual responding. The further finding of binding of [125I]7-OH-PIPAT to D3 receptors in the lateral habenula and in the basolateral amygdala is consistent with an important role of D3 receptors in these areas in nicotine seeking. It was also found that systemic administration of the selective D2 antagonist L741626 decreased cue-induced reinstatement, consistent with a role of D2 and D3 receptors in modulating this behavior. The current study supports an important role for D3 receptors in the basolateral amygdala and lateral habenula in cue-induced reinstatement.
INTRODUCTION
Cigarette smoking has been termed a global epidemic by the World Health Organization (WHO, 2006). In animal models of substance abuse, cue-induced reinstatement has high predictive validity and has been used extensively to study the neurobiology of drug seeking (Epstein and Preston, 2003; Shaham et al, 2003). There are five subtypes of dopamine receptor, of which the dopamine D3 receptor has received attention as a possible therapeutic target for addictions, including nicotine dependence (Joyce and Millan, 2005). This is due to the localization of D3 receptors in areas such as the amygdala, nucleus accumbens, and habenula (Bouthenet et al, 1991; Diaz et al, 2000), which are implicated in addiction processes (Heidbreder, 2005). Indeed, systemic administration of the selective D3 antagonist, SB-277011-A, attenuated cue-induced reinstatement of nicotine seeking (Khaled et al, 2010). In addition, repeated systemic administration of nicotine to animals produced an elevation in D3 binding sites (Le Foll et al, 2003a) and D3 antagonism reduced the response to a conditioned stimulus (Le Foll et al, 2003b; Le Foll et al, 2005). Furthermore, recent imaging studies revealed elevations in dopamine binding to D3 receptors in the brains of smokers after smoking a single cigarette (Le Foll et al, 2014). Despite converging evidence that D3 receptors are involved in (relapse to) nicotine use, the underlying neural circuit remains to be determined.
The amygdala, the nucleus accumbens, and their dopaminergic innervations are viewed as key structures underlying drug relapse, including relapse to nicotine (Everitt et al, 1999). In particular, D3 receptors in the basolateral amygdala have been implicated in cocaine seeking maintained by conditioned stimuli. Infusion of the selective antagonist, SB-277011-A, into the basolateral amygdala disrupted responding for cocaine under a second-order schedule of reinforcement (Di Ciano, 2008), a behavior that, like cue-induced reinstatement, is maintained by the earned presentations of drug-paired-conditioned stimuli (Di Ciano and Everitt, 2005; Goldberg et al, 1975). By contrast, infusion of the D3 antagonist into the nucleus accumbens shell was without effect (Di Ciano, 2008). Although suggestive as to the role, or lack thereof, of D3 receptors in the basolateral amygdala and nucleus accumbens in conditioned stimulus-maintained behavior, it is not known whether D3 receptors are involved in nicotine-seeking maintained by conditioned stimuli.
Another area that has received recent attention is the habenula. The habenula can be divided into two main subdivisions, the medial habenula and the lateral habenula. Chronic nicotine produced selective degeneration in the medial habenula (Carlson et al, 2001), whereas aversion to nicotine is also regulated in the medial habenula (Fowler et al, 2011; Frahm et al, 2011). By comparison, it is the lateral habenula that appears to code reward-relevant information and was the only one of a number of limbic structures studied to show c-fos immunoreactivity in rats that were sensitized to amphetamine (Hamamura and Ichimaru, 1997). A potential role for the lateral habenula in cue-maintained behavior was identified by findings of c-fos immunoreactivity in the lateral habenula during cue-induced reinstatement of heroin seeking (Zhang et al, 2005) and during the expression of conditioned locomotion to a cocaine-paired environment (Brown et al, 1992). The lateral habenula has been implicated in the mechanisms underlying reward and learning (Lecourtier and Kelly, 2007). However, effects of lesions of the lateral habenula, or manipulations of D3 receptors therein, on cue-induced reinstatement of nicotine seeking remains to be determined.
The purpose of the present study was to investigate the effects of infusions of the selective D3 antagonist, SB-277011-A, which has 100-fold selectivity over D2 receptors (Reavill et al, 2000), into the basolateral amygdala, nucleus accumbens, and lateral habenula on cue-induced reinstatement of nicotine seeking. It was hypothesized that antagonism of D3 receptors in all three areas would attenuate reinstatement, thus underscoring their important functions in this behavior and potential interactions between these areas. Control experiments were conducted to determine whether the effects were due to any impairments of operant behavior. In addition, SB-277011-A was administered systemically to examine effects on responding in extinction. The D2-selective antagonist, L741626, was administered before cue-induced reinstatement to further delineate whether effects observed are selective to D3 receptor. That is, previous studies have shown that eticlopride, a D2 antagonist that has equal affinity for all D2-like receptors (D2, D3, D4), decreased cue-induced reinstatement of nicotine seeking (Liu et al, 2010), but it is not known whether this effect is due to an effect at the level of D2 or D3. A further aim of this study was to evaluate [125I]7-OH-PIPAT binding in the basolateral amygdala and lateral habenula to demonstrate that D3 receptors are indeed present in these areas. Binding in the NAcc has been extensively studied and demonstrated in the past (Bouthenet et al, 1991; Levant, 1998; Levesque et al, 1992; Stanwood et al, 2000), but binding in the basolateral amygdala and lateral habenula has not been studied a great deal.
MATERIALS AND METHODS
Animals
Behavioral studies were conducted in 46 male, Long–Evans rats (Charles River, Canada; 250–275grams) individually housed in a temperature-controlled environment on a 12 h reversed light/dark cycle and maintained on 18–20 g of chow/day. Experimental procedures were carried out in compliance with the guidelines of the Canadian Council on Animal Care, and were approved by the institutional Animal Care Committee.
Autoradiography studies were conducted in young adult male Sprague Dawley rats (200–230 g, Iffa Credo, France) that were maintained under a 12 h light/dark cycle with constant temperature and humidity. Food and water were available ad libitum. The experimental procedures were performed in strict accordance with European Communities Council Directive of 24 November 1986 (89/609/EEC) and were approved by the regional ethical committee for animal experimentation, Ile-de-France-Paris Descartes (France).
Drugs
Nicotine hydrogen tartrate (Sigma-Aldrich, St Louis, MO, USA) was dissolved in saline, the pH was adjusted to 7.0 (±0.2), and the solution was filtered through a 0.22-μm syringe filter (Fisher Scientific, Pittsburgh, PA, USA). Nicotine (30 μg/kg/infusion) was administered intravenously in a volume of 0.1 ml/kg/infusion. SB-277011-A (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide) was provided by Dr Steven Goldberg (NIH, NIDA, IRP), as part of an ongoing research collaboration. For local infusion, SB-277011-A was dissolved in 10% DMSO in 10% w/v hydroxypropyl–β–cyclodextrin in sterile water and was locally infused into the specified areas in the brain, 5 min before testing, in a volume of 0.5 μl/side. For systemic injections, the vehicle was 10% cyclodextrin in sterile water. The 0, 1, and 3 mg/kg doses were given in a volume of 1 ml/kg, whereas the 10 mg/kg dose was given in a volume of 2 ml/kg. For systemic L741626 (Sigma-Aldrich), the vehicle was saline with one drop of Tween 80.
Apparatus and Food Training
Methods are as previously reported (Forget et al, 2010; Gamaleddin et al, 2011; Khaled et al, 2010). Briefly, rats were trained to lever press for food such that each lever press resulted in a 45 mg food pellet (Bioserv, Frenchtown, NJ, USA). During the food training sessions, the house light was on and pressing for food resulted in food. As the purpose of this training was to establish operant responding, no cue light was presented at this stage to prevent an association being formed between food and a cue light (associations between nicotine and cue were formed in the sections detailed below). One hour sessions were conducted for 5 days or until animals reached criterion, defined as at least 100 reinforcements within a 20 minute interval. Following food training, animals were assigned to either nicotine self-administration conditions (with an intravenous catheter) or food control experiments.
Self-Administration Procedures
Techniques for surgical implantation of an intravenous catheter were similar to those previously reported (Khaled et al, 2010). At least 1 week after surgery (for nicotine experiments), self-administration training was carried out in daily 1 h sessions. The acquisition phase was conducted under a fixed ratio (FR) schedule of reinforcement where schedule requirements gradually increased from FR-1 (one active lever press resulted in the delivery of an infusion of 30 μg/kg/infusion of nicotine base, or a 45 mg food pellet in food control groups) to FR-5 (5 days under FR-1, 3 days under FR-2, then FR-5 until stability). A time-out period of 60 s followed each reinforcement during which lever pressing had no consequences and the cue light above the active lever remained illuminated. This illumination of the cue light served as the conditioned stimulus. Self-administration was allowed to stabilize over a total of at least 12 days of training under FR-5. The criterion for stability was a minimum of 10 infusions of nicotine and a ratio of active : inactive lever presses of 2 : 1 for 3 consecutive days. Following criterion, animals were implanted with intracranial cannulae.
Intracranial Cannulation Surgery
Guide cannulae (22 gauge, Plastics One) were bilaterally implanted 2.0 mm above the target site according to the following coordinates: basolateral amygdala: −2.5AP, ±5ML, –6.6DV; nucleus accumbens: +1.6AP, ±1.7ML, −4.7DV (with 6° off-vertical angle of approach; convergent), and lateral habenula: –3.6AP, ±2.5ML, –3.1DV (with 20° off-vertical angle of approach; convergent; Paxinos and Watson, 1997; incisor bar at −3.3). Cannula occluders (28 gauge, Plastics One) were inserted into the guide cannulae immediately after the surgery. Following recovery, self-administration sessions were resumed for a minimum of 2–3 days under an FR-5 schedule, to ensure the surgeries had no effect on the operant behavior. Once stable, animals entered the extinction and reinstatement phase of testing.
Extinction Training and Cue-Induced Reinstatement of Nicotine Seeking
During extinction, responses on the active or inactive levers had no consequence. Extinction criteria were met when the number of presses on the active lever was <20 and <15% of the average number of responses made on the last 3 days of self-administration. During reinstatement tests, responses on the active lever (on a FR-5 schedule) resulted in contingent presentation of the cue (60 s illumination of the cue light above the active lever) without nicotine availability. The session began with a single 60-s non-contingent presentation of the cue light.
Local Infusion of SB-277011-A
Rats received intracranial microinfusions of the vehicle or SB-277011-A according to a counterbalanced within-subjects design. Microinjectors (28 gauge, Plastics One) were inserted into the guide cannulae and extended to the target site. A volume of 0.5 μl of SB-277011-A (0.01, 0.1 or 1 μg/0.5 μl) or vehicle (10% DMSO in 10% w/v hydroxyl-propyl-β-cyclodextrin in sterile water) was infused into each side over the course of 1 min using a microinfusion pump (Harvard Apparatus), 5–10 min before a test session. The microinjectors were left in place for 1 min after the infusion. Testing sessions were separated by at least 2 days of responding in extinction.
Effects of Intracerebral Infusion of SB-277011-A on Cue-Induced Reinstatement
Following extinction criterion, reinstatement was conducted as described above. Each rat received SB-277011-A infused into the basolateral amygdala (n=7), the nucleus accumbens (n=8), or the lateral habenula (n=7). Each rat received doses in a counterbalanced order. At least 2 days of responding in extinction separated each test day with infusions. Infusions were given on the day after extinction criterion was reached. Doses were selected based on a previously published report (Xi et al, 2004).
Effects of Intracerebral Infusion of SB-277011-A on Food-Maintained Responding
Following stable responding for food, separate groups of rats were implanted with guide cannulae into either the basolateral amygdala (n=5) or the lateral habenula (n=6) and received counterbalanced infusions of either SB-277011-A (1 μg) or vehicle. These areas were selected due to the significant effects obtained in the cue-induced reinstatement test for nicotine. Control experiments on responding for food were conducted to verify that effects obtained were not due to alterations in general motivation produced by infusion of SB-277011-A into the brain; these studies allowed for the interpretation of the effects in terms of changes in responding for the cue per se. Thus, these experiments were a control for the potential effects of SB-27011-A on motor activation, behavioral activation, or nonspecific reward. Animals were trained using the same parameters as those for nicotine self-administration, namely, training progressed from a FR-1 to a FR-5 schedule of reinforcement (5 days of FR-1, 3 days of FR-2, and 12 days of FR-5). Following stable acquisition of food administration, defined as <20% variability in the number of pellets self-administered for 2 consecutive days, rats were implanted with intracranial cannulae into either the basolateral amygdala or lateral habenula using the procedures and coordinates given above. Following surgery, rats were stabilized again on food self-administration. Rats were infused with either 0 or 1 μg of SB-277011-A in a counterbalanced order as described above. At least 2 days of food self-administration separated each infusion day.
Effects of Systemic SB-277011-A on Extinction
On reaching the extinction criterion, rats were given four counterbalanced (Latin Square design) i.p. injections of SB-277011-A (0, 1, 3, and 10 mg/kg) in 10% cyclodextrin vehicle 30 min before extinction sessions. At least 2 days of extinction baseline separated each test with SB-277011-A. These doses were selected based on previous reports (Di Ciano et al, 2003). These studies allowed for the demonstration that the effects of intracerebral SB-277011-A was not due to motor activation or activity levels in general.
Effects of Systemic L741626 on Cue-Induced Reinstatement of Nicotine Seeking
Once the animals reached the extinction criterion, they were given four counterbalanced (Latin Square) s.c. injections of the selective D2 antagonist L741626 (0, 0.1, 0.3, and 1 mg/kg in 1 ml/kg, 30 min pretreatment time) before reinstatement tests. At least 2 days at extinction baseline separated each test with L741626. These doses were selected based on a previously published report (Collins et al, 2012).
Histological Examination
Rats were overdosed with pentobarbital (350 mg/kg, i.p.) and 0.5 μl of cresyl violet dye was infused bilaterally into the target area using microinjectors inserted into the guide cannulae. The rats were then decapitated. Brains were dissected, flash frozen in methyl-butane on dry ice, and stored at −65 to −80 °C until sectioning. Serial coronal sections (25-μm thick) were obtained using a cryostat at –25 °C thaw mounted on pre-cleaned glass slides, and examined for verification of the placement.
Data Analysis of Effects of SB-277011-A or L741626
A two-way dose (extinction, vehicle, three doses of SB-277011-A) × lever (active, inactive) analysis of variance (ANOVA) with repeated measurements was used to analyze the effects of SB-277011-A or L741,626 on cue-induced reinstatement. The effects of SB-277011-A on food-taking behavior or extinction were analyzed with one-way ANOVAs on the effects of dose (five levels: extinction, vehicle, and three doses of SB-277011-A). Tukey's post hoc test was used to assess the differences between individual means (extinction baseline vs vehicle and vehicle vs each dose of SB-277011-A). To assess baseline during extinction, the two extinction days before each reinstatement test were averaged. Data were analyzed with GraphPad Prism 6.
Receptor Autoradiography
Rats were decapitated, brains were rapidly removed and immediately frozen by isopentane cooled by dry ice (−40 °C), and conserved at −75 °C until sectioning. Coronal brain sections 10-μm thick were prepared on a cryostat (JUNG3000, Leica), thaw mounted onto slides (SuperFrost-Plus Menzel-Glaser), and kept at −75 °C until use for autoradiography.
Unfixed sections were thawed by bringing slides to 4 °C and then washed at room temperature three times for 5 min in 50 mM sodium-HEPES buffer (pH 7.5) containing 1 mM EDTA and 0.1% BSA. For specific binding, sections were incubated for 45 min at room temperature in the same buffer containing 0.2 nM [1251]R(+)trans-7-hydroxy-2-[N-propyl-N-(3′-iodo-2′-propenyl)amino]tetralin ([125I]trans-7-0H-PIPAT, 2200 Ci/mmol, Amersham Life Science, Arlington Heights, IL), a selective D3 receptor radioligand. Nonspecific binding was determined by incubating adjacent brain sections in the same medium in the presence of 1 μM dopamine. Following incubation, slices were washed four times (2 min each) in ice-cold sodium-HEPES buffer containing 100 mM NaCl, dipped in ice-cold distilled water, and then dried under a stream of cold air. Autoradiograms were generated by apposing sections and autoradiographic [125I] microscale standards (Amersham Life Science, Arlington Heights, IL) to films BIO MAX (KODAK) for 2 days, developed in D-19 developer (20 °C) for 4 min, rinsed rapidly in deionized water, and fixed in GBX (KODAK) for 10 min.
Data Analysis of Receptor Audioradiography
Autoradiograms from receptor binding were analyzed by using the MERCATOR software (Explora Nova, Paris, France). All optical density values were converted to nCi/mg tissue equivalent by means of standard curves generated using the autoradiographic [125I] microscale standards. Specific DRD3 binding was obtained by substracting nonspecific binding from total [125I]7-0H-PIPAT binding. An average of two or three coronal sections/animal (n=3 animals) was measured (in the two sides of the brain) for each region to generate mean binding values. Only values above 2 × SEM from the nonspecific binding values were considered detectable. Autoradiograms were scanned using a CoolscanV-ED (Nikon) for illustration purposes. Images in figure 1 were generated with the Photoshop software (Adobe Systems, CA)
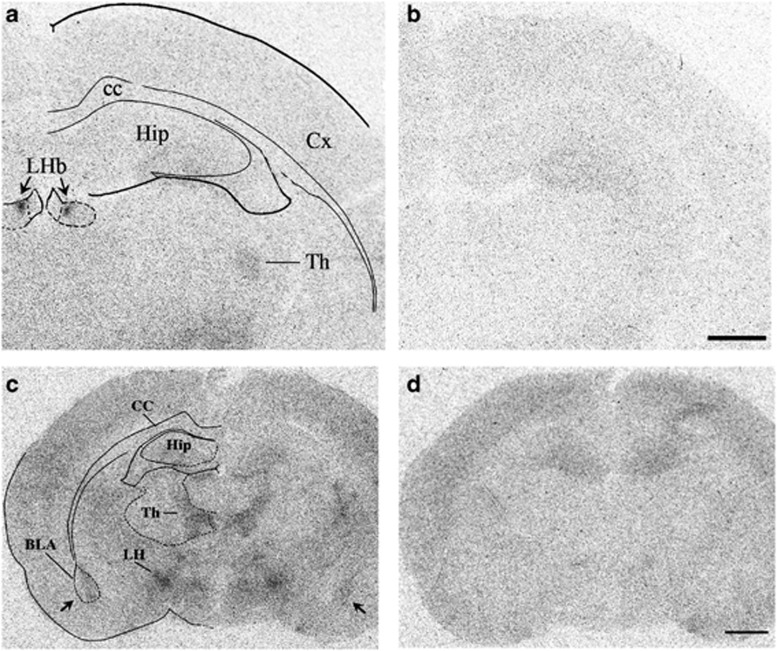
Figure 1.
Binding of [125I]7-OH-PIPAT, a selective D3 ligand, to various brain regions. Binding to the lateral habenula (LHb; a) and basolateral amygdala (BLA; c). Other areas of binding were the thalamus (Th in a) and lateral hypothalamus (LH in c). [125I]7-OH-PIPAT binding in these areas, but not in the hippocampus (Hip) and cortex (Cx), was completely inhibited in the presence of 1 μM of dopamine, leaving a negligible and uniform nonspecific labeling (b and d). Bars=1 mm. cc, corpus callosum.
RESULTS
Autoradiographic Localization of [125I]7-OH-PIPAT-Binding Sites in the Lateral Habenula and the Basolateral Amygdala of the Rat Brain
We used [125I]7-OH-PIPAT, a selective and highly sensitive D3 radioligand, on coronal rat brain sections to detect the presence of D3 receptors in the lateral habenula and in the basolateral subdivision of the amygdala complex. Autoradiograms of brain sections at several levels of these regions demonstrated that [125I]7-OH-PIPAT-specific binding was prominent and restricted to the medial portion of the lateral habenula (Figure 1a; Table 1). Detailed analysis of autoradiograms at the level of the habenular complex indicated that D3 receptor expression was restricted to the parvocellular and central subnuclei of the medial division of the lateral habenula, which concentrates the dopamine mesohabenular TH-immunoreactive nerve terminals (Geisler et al, 2003). A distinct, although lower, [125I]7-OH-PIPAT-specific binding was also detected in the medial part of the basolateral amygdala (Figure 1c; Table 1), thalamus, and lateral hypothalamus (Figure 1a and c; Table 1). [125I]7-OH-PIPAT binding in these areas was completely inhibited in the presence of 1 μM of dopamine, leaving a negligible and uniform nonspecific labeling (Figure 1b and d). In contrast, [125I]7-OH-PIPAT binding in the hippocampus complex was not inhibited in the presence of dopamine (Figure 1a–d).
Table 1. [125I]7-0H-PIPAT-Specific Binding in Several Regions of the Rat Brain (Specific Binding=Total Binding−Nonspecific Binding).
| LHb | BLA | Th | LH | |
|---|---|---|---|---|
| Specific binding | 13.45±1.27 | 3.31±0.33 | 4.40±1.30 | 8.22±0.99 |
Each value represents the mean (±SEM nCi/mg tissue equivalent) of determinations from three animals.
Effect of Intra-Amygdala, Lateral Habenula, or Nucleus Accumbens Infusion of SB-277011-A on Cue-Induced Reinstatement of Nicotine Seeking
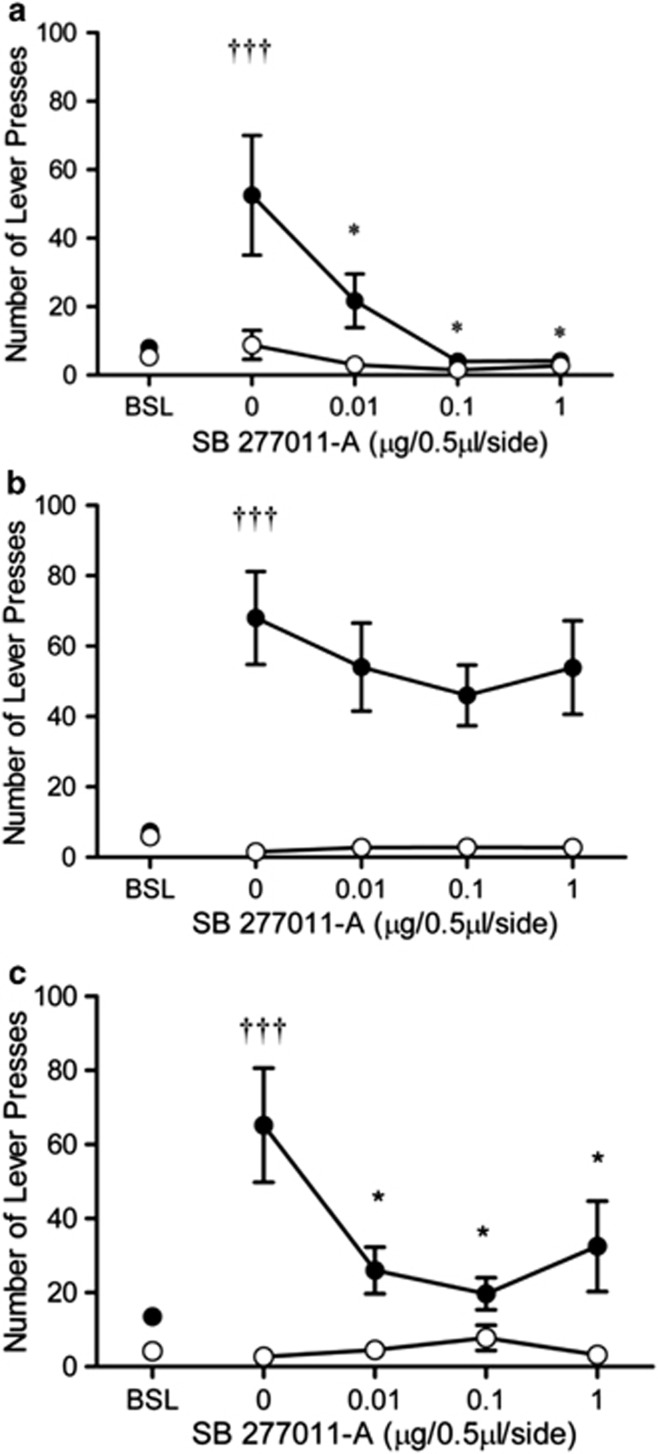
Following infusion of SB-277011-A into the basolateral amygdala or lateral habenula, a dose-dependent decrease in cue-induced reinstatement was observed, whereas no effects of infusion in the nucleus accumbens were seen (Figure 2). For all brain regions, responding during reinstatement was greater than that during extinction. A two-way ANOVA revealed a significant dose × lever interaction for the basolateral amygdala (F4,24=8.61, p<0.001; n=7), lateral habenula (F4,24=6.943, p<0.01; n=8), and nucleus accumbens (F4,28=13.403, p<0.01; n=7). A one-way repeated-measures ANOVA on the effect of dose revealed a significant effect for the basolateral amygdala (F4,24=8.01, p<0.01), nucleus accumbens (F4,28=11.74, p<0.01), and lateral habenula (F4,24=6.20, p<0.05) for the active lever. Tukey's post hoc test revealed a significant difference between extinction and vehicle treatment (p<0.01) for all brain areas for the active lever. For the basolateral amygdala and lateral habenula, significant differences between vehicle and each dose of SB-277011-A (p<0.01) were found for the active lever. There were no significant effects on inactive lever pressing.
Figure 2.
The effect of SB-277011-A (0.01–1 μg/μl/side), or vehicle (0), locally infused into the basolateral amygdala (BLA; n=7; a), nucleus accumbens (NAcc; n=8; b), or lateral habenula (LHb; n=7; c) on the mean (±SEM) number of active (filled symbols) or inactive (open symbols) lever presses during cue-induced reinstatement of nicotine seeking. †††Significant reinstatement was obtained following infusion of vehicle as compared with extinction (p<0.05). *Intra-basolateral amygdala and intra-lateral habenula infusions of SB-277011-A significantly reduced cue-induced reinstatement at all the doses tested compared with vehicle (p<0.01). Intra-nucleus accumbens infusions of SB-27701-A had no effect on active lever responding. No effect of vehicle or drug was seen on inactive lever presses.
Effect of Intra-Basolateral Amygdala and Intra-Lateral Habenula Infusions of SB-277011-A on Food Self-Administration
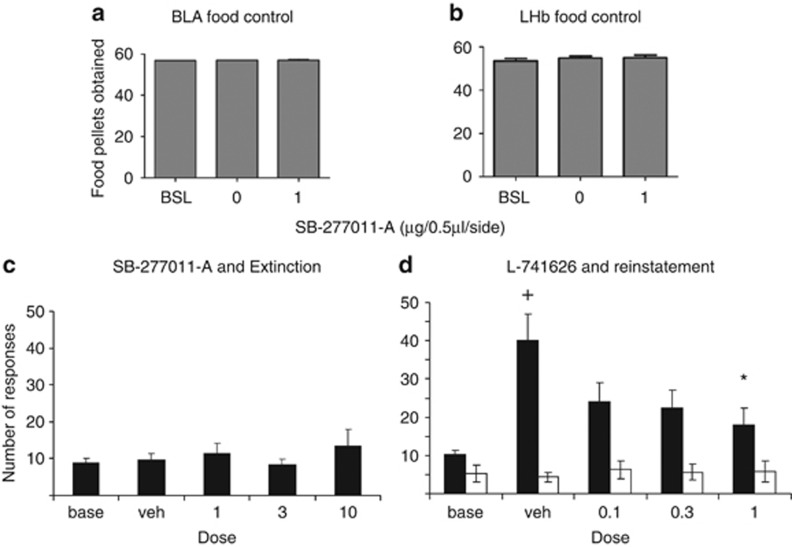
Infusion of SB-277011-A into the basolateral amygdala (Figure 3a) or lateral habenula (Figure 3b) had no effect on intake of food under a FR-5 schedule. A one-way repeated-measures ANOVA revealed no effect of dose for either the basolateral amygdala (p>0.05; n=5) or lateral habenula (p>0.05; n=6).
Figure 3.
Effect of intra-basolateral amygdala (BLA; n=5; a) or intra-lateral habenula (LHb; n=6; b) infusions of SB-277011-A (1 μg/0.5 μl/side), or vehicle, on food-taking behavior. No effect is observed as a result of D3 antagonism on the number of reinforcements obtained compared with baseline responding under FR-5 schedule. Effect of systemic SB-277011-A on extinction responding (n=6; c) or the selective D2 antagonist L741626 (d) on cue-induced reinstatement of nicotine seeking (n=7). A significant effect of L741626 on cue-induced reinstatement was found (p<0.05). Data are expressed as the mean (±SEM).
Effect of Systemic SB-277011-A on Extinction
Administration of four counterbalanced doses of SB-277011-A to rats resulted in a stable pattern of responding (Figure 3c). A one-way repeated-measures ANOVA revealed no effect of dose (p>0.05; n=6).
Effect of L741626 on Cue-Induced Reinstatement of Nicotine Seeking
Administration of four counterbalanced doses of the selective D2 antagonist resulted in decreases in cue-induced reinstatement (Figure 3d). A two-way dose × lever ANOVA revealed a significant interaction (F4,24=4.970, p<0.05). A one-way ANOVA revealed a significant effect of dose (F4,24=4.389, p<0.01; n=7) for the active lever. Post hoc tests with Tukey's revealed a significant difference between extinction baseline and vehicle treatment, and a significant difference between vehicle and the high dose (1 mg/kg) of L741626. No effects on the inactive lever were revealed.
Histological Examination
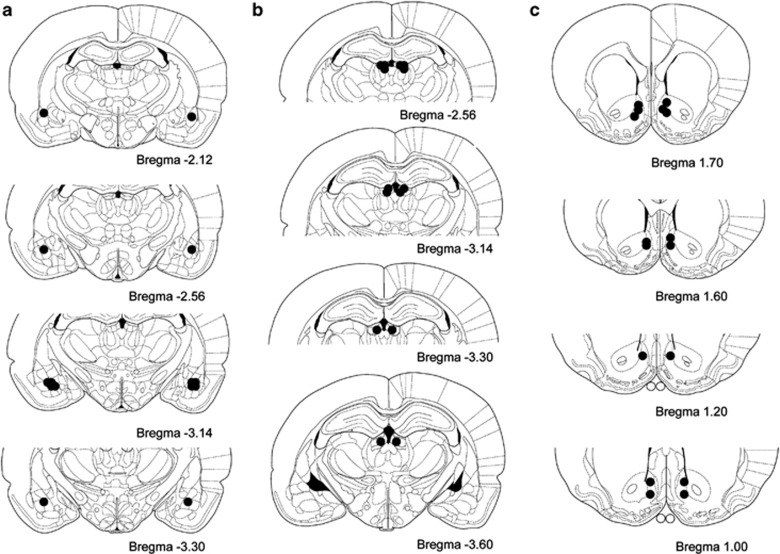
Figure 4 shows the location of microinjectors tips within the brain areas investigated in the current study. Rats were only included in the study when the histological analysis showed that microinjectors tips were bilaterally placed within the target site.
Figure 4.
Location of microinjector tips in the basolateral amygdala (a), lateral habenula (b), or nucleus accumbens (c) of the rats included in the nicotine cue-induced reinstatement experiment plotted on coronal sections of the rat brain taken from the atlas of Paxinos and Watson (1997). Numbers refer to anterior–posterior coordinates relative to bregma.
DISCUSSION
The purpose of the present study was to determine the effect of intracerebral infusion of the D3 antagonist SB-277011-A into the basolateral amygdala, lateral habenula, or nucleus accumbens on cue-induced reinstatement of nicotine seeking. It was found that SB-277011-A attenuated cue-induced nicotine seeking when infused into the basolateral amygdala or lateral habenula but not the nucleus accumbens. Control experiments demonstrated that SB-27011-A did not have any effect on food seeking when infused into the basolateral amygdala or lateral habenula and that systemic SB-277011-A did not attenuate responding in extinction. The selective D2 antagonist L-741,626 decreased cue-induced reinstatement suggesting that this receptor subtype is also involved in nicotine seeking.
The present study found that D3 receptors are localized to the lateral habenula, basolateral amygdala, thalamus, and hypothalamus. In particular, the binding in the lateral habenula was dense, as compared with that in the basolateral amygdala. The distribution and densities of binding sites were consistent with that displayed by the D3 immunoreactivity in the lateral habenula in previous reports (data not shown; Diaz et al, 2000). The present findings thus confirm and extend previous reports (Bouthenet et al, 1991; Diaz et al, 2000) as to the distribution of D3 receptors in brain areas that are important in addictions, including nicotine dependence (Heidbreder, 2005).
In the present study, intra-basolateral amygdala and intra-lateral habenula infusion of SB-277011-A reduced the level of cue-induced reinstatement of nicotine seeking, suggesting a role for the basolateral amygdala and lateral habenula D3 receptors in the modulation of cue-induced reinstatement of nicotine seeking. No effects were observed on responding for food following infusion of the same doses of SB-277011-A into these brain areas, suggesting that the attenuation of reinstatement observed was not due to nonspecific effects of the drug. Further, no effects were observed on the inactive lever. Responding on the inactive lever can increase when the ability of the lever to maintain discriminative responding is altered and thus, no effects in this type of control were observed. Also supporting a selectivity of the effect is the finding that systemic SB-277011-A had no effect on extinction responding. The effect is thus likely due to an impact on the properties of the conditioned stimulus that reinstated behavior as opposed to an activational effect of the operant chamber or residual responding on the lever. In addition, it is unlikely that the effects of the D3 blockade observed in the current study are due to a disruption of other factors, such as an effect on cue light perception, memory, or learning, or due to a decrease in attention. Recent studies showed that D3 blockade has an important role in enhancing memory, attention, and learning (Nakajima et al, 2013). Moreover, D3 antagonists were shown to improve learning deficits in memory-impaired rats (Laszy et al, 2005). The current findings are, therefore, most likely to be attributed to a specific effect on the ability of conditioned stimuli, previously paired with the administration of nicotine, to reinstate extinguished nicotine-seeking behavior in the absence of nicotine (primary reinforcer).
In contrast to the findings in the basolateral amygdala and lateral habenula, the present study showed that intra-nucleus accumbens infusions of SB-277011-A had no effect on cue-induced reinstatement of nicotine-seeking behavior. It should be noted that the tips of the cannulae in the present study were located preferentially in the nucleus accumbens shell, and not the nucleus accumbens core, posing the question as to differences in function between the nucleus accumbens core and nucleus accumbens shell. Indeed, the lack of effect in the nucleus accumbens shell in the present study is in keeping with the consensus of literature that the nucleus accumbens core, but not the nucleus accumbens shell, mediates cue-induced reinstatement (Bossert et al, 2007; Fuchs et al, 2004; Rogers et al, 2008). It may be that D3 receptors function in a different capacity with respect to cue-maintained seeking of drugs. Indeed, in a recent study, it was found that infusion of SB-277011-A into the nucleus accumbens shell or core decreased the incubation of drug seeking over time, whereas infusion into the basolateral amygdala was without an effect (Xi et al, 2013). These findings are complementary to the present ones and highlight parallel circuits involving D3 receptors in cue-maintained drug seeking.
The present findings are in keeping with a study showing that the infusion of SB-277011-A (4 μg/0.3 μl/side) into the basolateral amygdala reduced cocaine seeking maintained by the presentation of cocaine-associated cues, under a second-order schedule of reinforcement (Di Ciano, 2008), whereas infusion into the nucleus accumbens shell had no effect. This is consistent with the established body of evidence linking the basolateral amygdala, and its dopaminergic innervations, to conditioned reinforcement processes, which may be relevant to nicotine dependence (Everitt et al, 1999). Specifically, dopaminergic mechanisms may interact with glutamatergic receptors in the nucleus accumbens core (Di Ciano and Everitt, 2004) to control behaviors mediated by conditioned reinforcers such as cue-induced reinstatement. For example, it was demonstrated that infusion of an NMDA antagonist into the nucleus accumbens core increased cue-induced reinstatement (D'Souza and Markou 2014). The authors interpret this as an evidence that glutamate in the nucleus accumbens may negatively modulate nicotine seeking. It remains to be determined whether the nucleus accumbens core functions in cue-induced reinstatement of nicotine seeking and whether these mechanisms may be dopaminergic or glutamatergic.
The lateral habenula is emerging as a neural site potentially involved in neuropsychiatric disorders including depression, schizophrenia, and drug addiction (Lecourtier et al, 2004). Recently, this region has been found to be involved in several processes including learning (Lecourtier and Kelly, 2007) and reward (Morissette and Boye, 2008). The relationship between the lateral habenula and the dopaminergic neurons in the ventral tegmental area is of special interest, given the projection of the ventral tegmental area to the basolateral amygdala. In primates, the response of the lateral habenula was found to be opposing and preceding that of the ventral tegmental area dopamine neurons following the presentation of reward-predictive or non-predictive stimuli, and weak stimulation of the lateral habenula resulted in the inhibition of the dopamine neurons (Matsumoto and Hikosaka, 2007). A recent study has found that deep brain stimulation of the lateral habenula decreased cocaine self-administration, promoted extinction behavior, and attenuated cocaine-induced reinstatement of cocaine seeking (Friedman et al, 2010), suggesting a role for the lateral habenula in drug-conditioning and reinstatement processes that may be relevant to nicotine dependence. Given the inhibitory relationship of the lateral habenula with the dopamine system, the results of the study by Friedman et al (2010) are consistent with the present results in that inhibition of the dopamine system produced by stimulation of the lateral habenua resulted in decreased drug seeking, similar to the present findings that inhibition of the dopamine system (through antagonism of dopamine receptors) attenuated nicotine seeking. Further evidence for a role of the lateral habenula in addictions in general was provided by the findings of Zhang et al (2005), who demonstrated that the reintroduction of heroin-associated cues, which reinstated extinguished heroin-seeking behavior, also increased c-Fos immunoreactivity in the lateral habenula, a sign of neuronal activation (Zhang et al, 2005). Taken together, the findings of the current study add to a growing interest in a role for the habenular complex in nicotine-seeking behavior, and support a growing body of evidence implicating the lateral habenula–dopaminergic interaction in reward signalling, reward prediction, and drug conditioning.
In the present study, an effect of the selective antagonist, L741626, was found on cue-induced reinstatement of nicotine seeking. This is consistent with the previous finding that eticlopride decreased cue-induced reinstatement of nicotine seeking (Liu et al, 2010). Eticlopride has traditionally been classed as a D2 antagonist, but it targets all types of D2 receptors, including the D3 and D4 subtypes, the latter has recently been shown to control nicotine seeking (Yan et al, 2012). Thus, it has been difficult to determine which receptor subtype was mediating the effects of D2 ligands. In the present study, the selective D2 antagonist, L741626, was used and was able to block cue-induced reinstatement of nicotine seeking suggesting that D2 is involved in mediating nicotine-seeking behavior. Together with our earlier reports that systemic administration of selective D3 (Khaled et al, 2010) and D4 antagonist (Yan et al, 2012) blocked cue-induced reinstatement of nicotine seeking, this suggests that all D2 subtypes mediate nicotine seeking. Further demonstration of the specific involvements of the selective receptor subtypes could be provided by using transgenic mice lacking the D3 receptor, as already reported by some investigators (Song et al, 2012). These receptor subtypes have been shown to have different behavioral correlates (Watson et al, 2012) and also to be differentially regulated in the addiction process (Boileau et al, 2012; Staley and Mash, 1996; Volkow et al, 2001). Thus, it is likely that their contributions to cue-induced reinstatement may be complementary, perhaps being mediated by different brain systems. For example, it was found that administration of eticlopride into the caudate reduced cue-induced reinstatement of morphine seeking (Gao et al, 2013), whereas administration of SB-277011-A into the caudate had no effect on stimulus-controlled behavior as measured using second-order schedules of cocaine reinforcement (Di Ciano, 2008). Thus, the caudate may represent an area of further investigation with respect to dissociable mechanisms of D2 vs D3 receptor involvement.
In summary, the current study demonstrates a role for the basolateral amygdala and lateral habenula D3 receptors in cue-induced reinstatement of nicotine-seeking behavior, whereas the nucleus accumbens D3 receptors appear to have no such role. Given the presence of D3 receptors in the nucleus accumbens shell, future studies may investigate the exact contribution of this brain region to drug seeking. The present findings emphasize the regional selectivity of D3 receptors in controlling cue-induced reinstatement of nicotine seeking. Interactions between these brain areas in the control of nicotine seeking remain to be determined. Given the finding that D2 receptors are also involved in reinstatement, future studies may uncover the relative contribution of D3 and D2 receptors to nicotine seeking and the different brain areas that may subserve these roles. The current findings expand the basic understanding of the mechanisms and areas through which D3 receptors influence stimulus-reward associations leading to relapse. Extrapolation of the current data to humans suggests a strong potential for D3-selective antagonists as therapeutic agents for the prevention of relapse to smoking.
FUNDING AND DISCLOSURE
Dr Le Foll's research is supported by CAMH, the Campbell Family Mental Health Research Institute, CIHR, and the National Institute on Drug Abuse at the National Institutes of Health (1R21DA033515-01). Dr Le Foll has received grant and salary support from Pfizer and is a consultant for Richter Pharmaceuticals, Lundbeck, Mylan, Ethypharm, and Pfizer. The current study was supported by an Early Researcher Award Grant awarded to Dr Le Foll from the Ontario Ministry of Research and Innovation and by the Scholars' Program of Interdisciplinary Capacity Enhancement (SPICE) awarded to MATM Khaled. The remaining authors declare no conflict of interest.
Acknowledgments
We would like to acknowledge the technical assistance of Munmun Chatterjee, Zane Qureshi, and Peter W Marinelli.
References
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Noguchi K, Ellison G. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res. 2001;906:127–134. doi: 10.1016/s0006-8993(01)02570-7. [DOI] [PubMed] [Google Scholar]
- Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology (Berl) 2012;219:123–135. doi: 10.1007/s00213-011-2382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Differential role of N-methyl-D-aspartate receptor-mediated glutamate transmission in the nucleus accumbens shell and core in nicotine seeking in rats. Eur J Neurosci. 2014;39:1314–1322. doi: 10.1111/ejn.12491. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attentuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropscyhopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol. 2005;526:186–198. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala- ventral striatal subsystems. Ann NY Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, et al. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking behaviour, but does not affect nicotine intake. Br J Pharmacol. 2011;164:1652–1660. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Li Y, Zhu N, Brimijoin S, Sui N. Roles of dopaminergic innervation of nucleus accumbens shell and dorsolateral caudate-putamen in cue-induced morphine seeking after prolonged abstinence and the underlying D1- and D2-like receptor mechanisms in rats. J Psychopharmacol. 2013;27:181–191. doi: 10.1177/0269881112466181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Andres KH, Veh RW. Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. J Comp Neurol. 2003;458:78–97. doi: 10.1002/cne.10566. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Kelleher RT, Morse WH. Second-order schedules of drug injection. Fed Proc. 1975;34:1771–1776. [PubMed] [Google Scholar]
- Hamamura T, Ichimaru Y. Amphetamine sensitization augments amphetamine-induced Fos expression in the lateral habenula. Brain Res. 1997;767:140–143. doi: 10.1016/s0006-8993(97)00697-5. [DOI] [PubMed] [Google Scholar]
- Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005;526:101–112. doi: 10.1016/j.ejphar.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, et al. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- Laszy J, Laszlovszky I, Gyertyan I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl) 2005;179:567–575. doi: 10.1007/s00213-004-2096-z. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Guranda M, Wilson AA, Houle S, Rusjan PM, Wing VC, et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-(+)-PHNO PET study in humans. Neuropsychopharmacology. 2014;39:415–424. doi: 10.1038/npp.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Schwartz JC, Sokoloff P. Disruption of nicotine conditioning by dopamine D(3) receptor ligands. Mol Psychiatry. 2003;8:225–230. doi: 10.1038/sj.mp.4001202. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- Levant B. Differential distribution of D3 dopamine receptors in the brains of several mammalian species. Brain Res. 1998;800:269–274. doi: 10.1016/s0006-8993(98)00529-0. [DOI] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF. Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol. 2010;21:153–160. doi: 10.1097/FBP.0b013e328337be95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Morissette MC, Boye SM. Electrolytic lesions of the habenula attenuate brain stimulation reward. Behav Brain Res. 2008;187:17–26. doi: 10.1016/j.bbr.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, et al. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur Neuropsychopharmacol. 2013;23:799–813. doi: 10.1016/j.euroneuro.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Compact, 3rd edn: The Rat Brain in Stereotaxic Coordinates. Academic Press: Sydney, Australia; 1997. [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Marsden CA, Millan MJ, Fone KC. Blockade of dopamine D3 but not D2 receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropsychopharmacol. 2012;15:471–484. doi: 10.1017/S1461145711000435. [DOI] [PubMed] [Google Scholar]
- WHO 2006Tobacco: Deadly in Any Form or Disguise WHO Press: Geneva, Switzerland; p48. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, et al. Blockade of mesolimbic dopamine D(3) receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Li J, Peng XQ, Song R, Gaal J, et al. Blockade of dopamine D3 receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addict Biol. 2013;18:665–677. doi: 10.1111/j.1369-1600.2012.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, et al. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhou W, Liu H, Zhu H, Tang S, Lai M, et al. Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neurosci Lett. 2005;386:133–137. doi: 10.1016/j.neulet.2005.06.008. [DOI] [PubMed] [Google Scholar]