Abstract
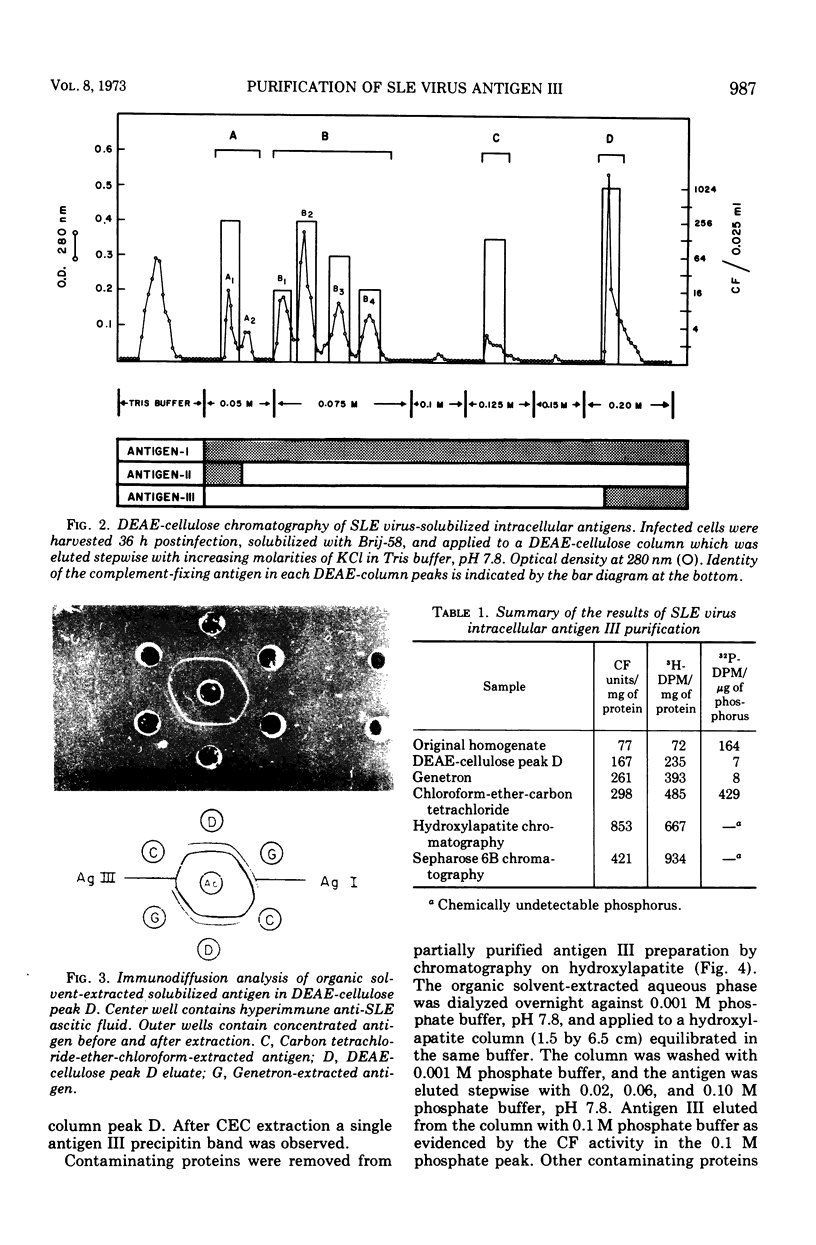
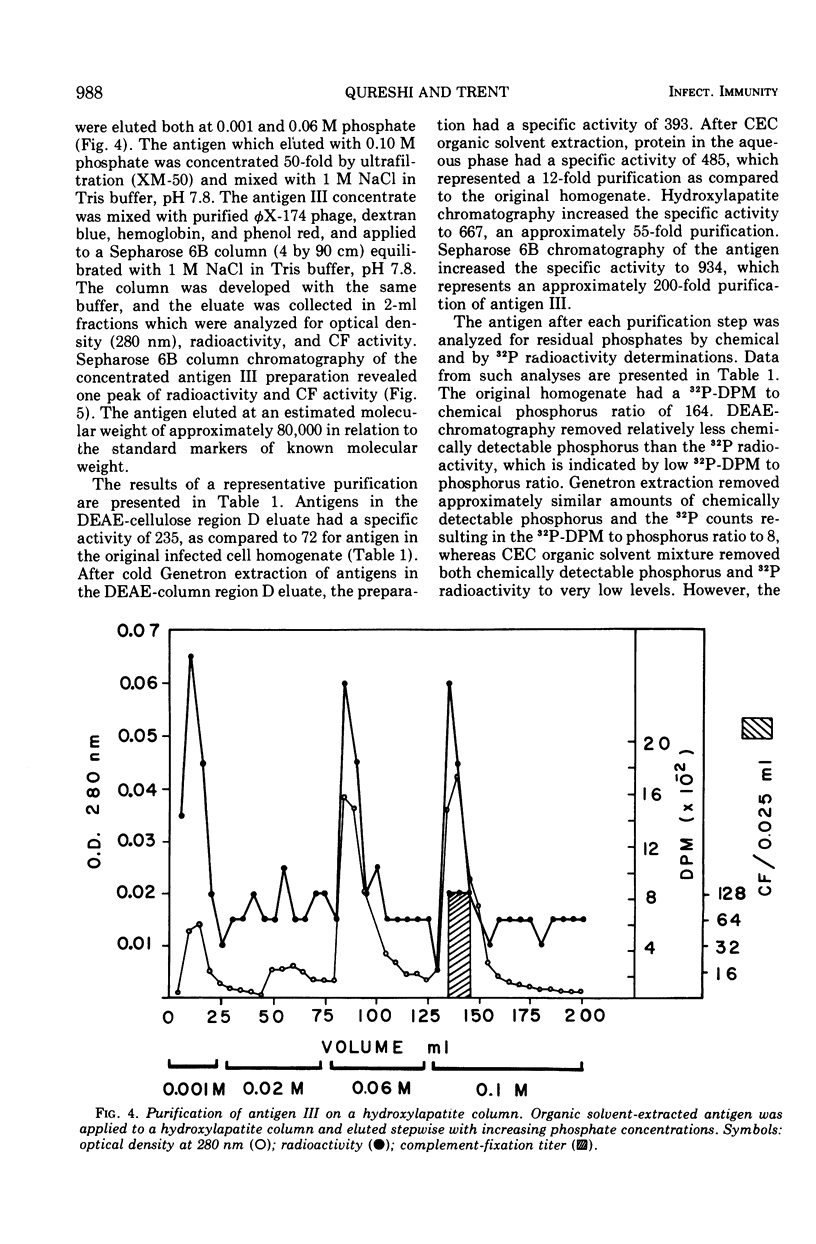
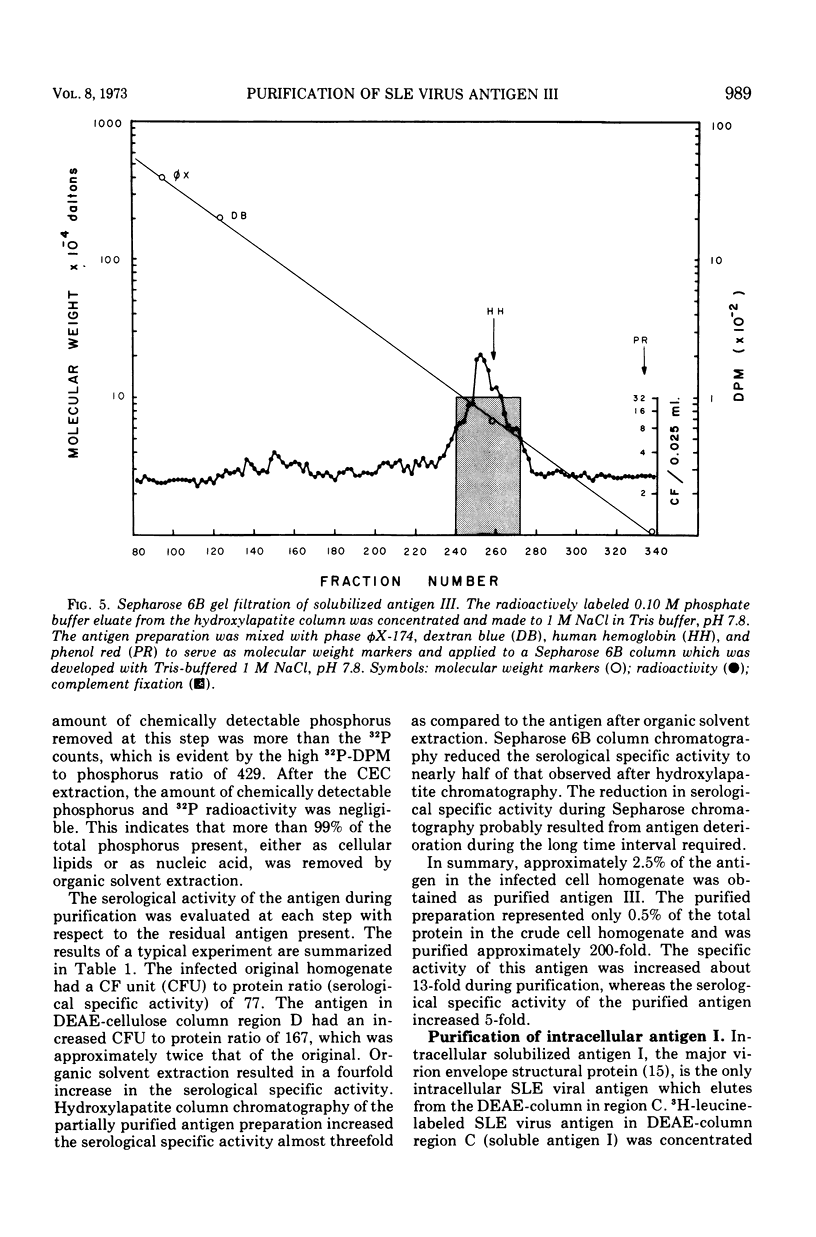
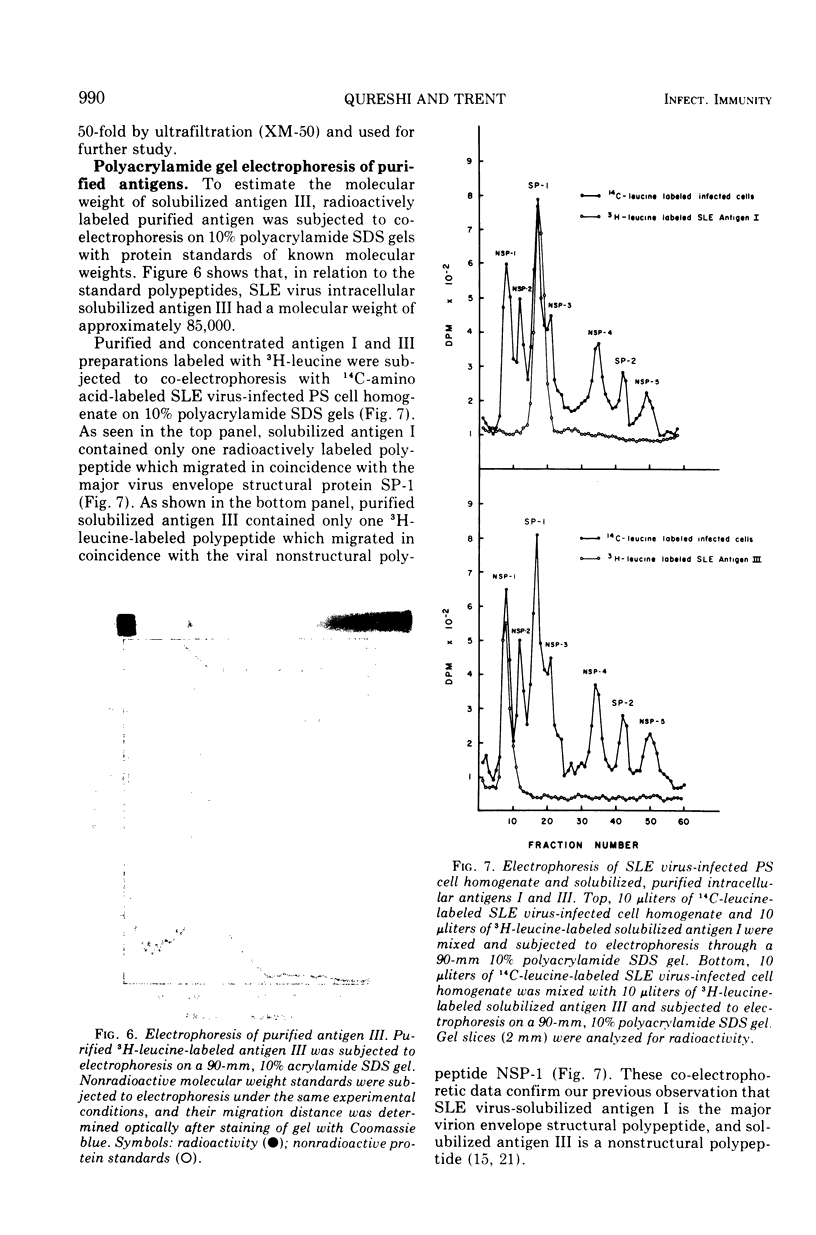
Three serologically distinct antigens were identified in homogenates of Saint Louis encephalitis (SLE) virus-infected cells after Brij-58 solubilization and diethylaminoethyl (DEAE)-cellulose chromatography. These antigens were designated as antigen I, II, and III. Immunodiffusion analyses showed that all antigen peaks which eluted from the DEAE-cellulose column contained the intracellular major envelope protein, antigen I. This protein was the only viral antigen which eluted at 0.125 M KCl in DEAE-cellulose column peak C. Optical density peak D, which eluted at 0.2 M KCl, contained both antigens I and III. Antigen III was purified from this column fraction approximately 200-fold by organic solvent extraction and chromatography on hydroxylapatite and Sepharose 6B. Purified antigen III had a molecular weight of 80,000 to 85,000 and contained only one antigenic determinant as judged by immunodiffusion analysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardoin P., Clarke D. H. The use of sonication and of calcium-phosphate chromatography for preparation of group C arbovirus hemagglutinins. Am J Trop Med Hyg. 1967 May;16(3):357–363. doi: 10.4269/ajtmh.1967.16.357. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bond J. O., Hammon W. M. Epidemiologic studies of possible cross protection between dengue and St. Louis encephalitis arboviruses in Florida. Am J Epidemiol. 1970 Nov;92(5):321–329. doi: 10.1093/oxfordjournals.aje.a121213. [DOI] [PubMed] [Google Scholar]
- Bond J. O. St. Louis encephalitis and dengue fever in the Caribbean area: evidence of possible cross-protection. Bull World Health Organ. 1969;40(1):160–163. [PMC free article] [PubMed] [Google Scholar]
- Brandt W. E., Chiewslip D., Harris D. L., Russell P. K. Partial purification and characterization of a dengue virus soluble complement-fixing antigen. J Immunol. 1970 Dec;105(6):1565–1568. [PubMed] [Google Scholar]
- CLARKE D. H. Antigenic analysis of certain group B arthropodborne viruses by antibody absorption. J Exp Med. 1960 Jan 1;111:21–32. doi: 10.1084/jem.111.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Brandt W. E., McCloud T. G., Shapiro D., Russell P. K. Immunological and biophysical separation of dengue-2 antigens. J Virol. 1971 Jan;7(1):15–23. doi: 10.1128/jvi.7.1.15-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Nichols C. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J Virol. 1972 Nov;10(5):1021–1030. doi: 10.1128/jvi.10.5.1021-1030.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESSLER A. E., BENDER C. E., PARKINSON M. C. A new and rapid method for isolating viruses by selective fluorocarbon deproteinization. Trans N Y Acad Sci. 1956 Jun;18(8):701–703. doi: 10.1111/j.2164-0947.1956.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Characterization of the tumorlike (T) antigen induced by type 12 adenovirus. I. Purification of the antigen from infected KB cells and a hamster tumor cell line. J Virol. 1968 Jan;2(1):7–14. doi: 10.1128/jvi.2.1.7-14.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCloud T. G., Brandt W. E., Russell P. K. Molecular size and charge relationships of the soluble complement-fixing antigens of dengue viruses. Virology. 1970 Jul;41(3):569–572. doi: 10.1016/0042-6822(70)90180-7. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Tanigaki N., Yagi Y., Pressman D. An efficient method for isolation of HL-A antigens from hematopoietic cell lines. J Immunol. 1971 Aug;107(2):394–401. [PubMed] [Google Scholar]
- Price W. H., Thind I. S. Protection against West Nile virus induced by a previous injection with dengue virus. Am J Epidemiol. 1971 Dec;94(6):596–607. doi: 10.1093/oxfordjournals.aje.a121358. [DOI] [PubMed] [Google Scholar]
- Qureshi A. A., Trent D. W. Group B arbovirus structural and nonstructural antigens. I. Serological identification of Saint Louis encephalitis virus soluble antigens. Infect Immun. 1973 Feb;7(2):242–248. doi: 10.1128/iai.7.2.242-248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. K., Chiewsilp D., Brandt W. E. Immunoprecipitation analysis of soluble complement-fixing antigens of dengue viruses. J Immunol. 1970 Oct;105(4):838–845. [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Isolation of H-2 alloantigens solubilized by the detergent NP-40. J Immunol. 1971 Nov;107(5):1363–1367. [PubMed] [Google Scholar]
- Smith T. J., Brandt W. E., Swanson J. L., McCown J. M., Buescher E. L. Physical and biological properties of dengue-2 virus and associated antigens. J Virol. 1970 Apr;5(4):524–532. doi: 10.1128/jvi.5.4.524-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira G., Popescu M., Cymbalista S., Biezunski N., Goldblum N. Isolation of the SV40 induced tumor ("T") antigen from transformed hamster kidney cells. Arch Gesamte Virusforsch. 1972;37(2):236–242. doi: 10.1007/BF01268006. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Qureshi A. A. Structural and nonstructural proteins of Saint Louis encephalitis virus. J Virol. 1971 Mar;7(3):379–388. doi: 10.1128/jvi.7.3.379-388.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, SWEET B. H., KITAOKA M., TAMIYA T. Immunological studies with group B arthropod-borne viruses. I. Broadened neutralizing antibody spectrum induced by strain 17D yellow fever vaccine in human subjects previously infected with Japanese encephalitis virus. Am J Trop Med Hyg. 1962 Jul;11:550–561. [PubMed] [Google Scholar]