Abstract
Background:
Immune suppression in the tumour microenvironment remains a major limitation to successful immunotherapy of cancer. In the current study, we analysed whether the natural killer T cell-activating glycolipid α-galactosylceramide could overcome immune suppression and improve vaccination against metastatic breast cancer.
Methods:
Mice with metastatic breast cancer (4T1 model) were therapeutically treated with a Listeria monocytogenes-based vaccine expressing tumour-associated antigen Mage-b followed by α-galactosylceramide as separate agents, or as a complex of α-galactosylceramide stably incorporated into Listeria-Mage-b. Effects on metastases, tumour weight, toxicity and immune responses were determined.
Results:
Sequential treatments of mice with established 4T1 breast carcinomas using Listeria-Mage-b followed by α-galactosylceramide as a separate agent was highly effective at reducing metastases, but was accompanied by severe liver toxicity. In contrast, combined therapy using Listeria-Mage-b modified by incorporation of α-galactosylceramide resulted in nearly complete elimination of metastases without toxicity. This was associated with a significant increase in the percentage of natural killer T cells in the spleen, and an increase in natural killer cell activity and in T cell responses to Mage-b.
Conclusions:
Our results suggest that direct incorporation of α-galactosylceramide into a live bacterial vaccine vector is a promising non-toxic new approach for the treatment of metastatic breast cancer.
Keywords: Listeria monocytogenes, α-galactosylceramide, metastases, breast cancer, NKT cells
Breast cancer is the most common cancer among women worldwide, and ∼30% of cases progress to metastatic disease, which is difficult or impossible to treat effectively (Youlden et al, 2012). Current treatment options for metastatic cancer include surgery followed by chemotherapy or radiation, or other adjuvant therapy (Cardoso et al, 2012). Despite aggressive treatment, elimination of metastases or residual tumour cells after initial treatment is often incomplete, and removal of residual disease by chemotherapy is prevented by chemoresistance (Gonzalez-Angulo et al, 2007). This underlines the urgent need for new effective therapies against metastatic disease.
It has been shown in mice and humans that vaccines can have a favourable effect on metastases (Niethammer et al, 2002; Marchand et al, 2003; Kruit et al, 2005; Kim et al, 2008) but that vaccine efficacy is strongly reduced by immune suppression in the tumour microenvironment (TME; Gajewski et al, 2006). In a previous study, we developed a vaccine based on a non-pathogenic strain of Listeria monocytogenes (LM) expressing the tumour-associated antigen (TAA) Mage-b (LM-Mb; Kim et al, 2008). Listeria monocytogenes is an intracellular bacterium that has the capacity to deliver antigens through infection into antigen-presenting cells (APCs) such as dendritic cells (DCs), monocytes and macrophages with high efficiency (Paterson and Maciag, 2005). We have also demonstrated that LM infects tumour cells, which can lead to cytolytic effects through a mechanism involving induction of high levels of reactive oxygen species, and sensitises the infected tumour cells for recognition by LM-specific CD8+ T cells (Kim et al, 2009). When administered prior to tumour establishment in an aggressive mouse model of metastatic breast cancer (4T1), LM-Mb treatment resulted in strong CD8+ T-cell responses to both Mage-b and LM and an almost complete elimination of metastatic disease (Kim et al, 2008). However, when administered in a therapeutic setting (i.e., after establishment of primary 4T1 tumours), LM-Mb treatment was only moderately effective against metastatic breast cancer, and induced relatively weak CD8+ T-cell responses to Mage-b, due to strong immune suppression in the TME (Kim et al, 2009).
To overcome the immune suppression that is characteristic of tumour-bearing hosts, immunologic adjuvants that can promote robust immune responses and augment the effects of therapeutic vaccines are greatly needed. Glycolipids of the α-galactosylceramide family (αGC) represent one potentially useful class of adjuvants that have shown promise in preclinical studies for immunotherapy of cancers (Metelitsa, 2011; Yamasaki et al, 2011). These glycolipids mediate their effects on the immune system by binding to an MHC class I-like molecule called CD1d, creating a complex that is recognised by a population of conserved effector lymphocytes known as natural killer T (NKT) cells (Bendelac et al, 2007). Formation of intracellular complexes of CD1d with αGC in APCs initiates rapid NKT-cell activation (Bendelac et al, 2007), resulting in the production of Th1-associated cytokines such as IFNγ and IL-12p70, maturation of CD8α+ DCs in the lymph nodes and subsequent activation of NK and conventional T cells (Hermans et al, 2003; Venkataswamy et al, 2009). This cascade of immune reactions that is initiated by NKT cells in response to αGC has been shown in mouse models to generate innate and adaptive immunity against a wide range of cancers and infections (Brigl et al, 2003; Behar and Porcelli, 2007; Bendelac et al, 2007).
Based on these observations we hypothesised that addition of αGC to LM-Mb vaccine could improve therapeutic efficacy, in part through enhancement of specific T cell responses to Mage-b. When these two agents were used as combination therapy in the 4T1 model, αGC significantly improved the therapeutic vaccine efficacy of LM-Mb as demonstrated by the almost complete elimination of the metastases. However, the administration of these two agents sequentially caused severe and in some cases fatal toxicity to the liver, leading us to explore other novel strategies. Drawing on previous experience using direct incorporation of relatively low doses of αGC into live Mycobacterium bovis BCG to improve vaccine efficacy (Venkataswamy et al, 2009), we developed a similar approach for direct incorporation of the glycolipid into live LM organisms. This generated a vaccine that was equally effective against metastatic breast cancer compared to sequential administration of LM-Mb and αGC as separate agents, but without any apparent toxicity. The powerful anti-metastatic effect of vaccination with LM-Mb modified by direct incorporation of αGC correlated with an increase in the frequency of splenic NKT cells, and improved responses of Mage-b-specific CD8+ T and NK cells. This approach may provide a basis for new strategies to safely improve vaccine efficacy against metastatic cancer through augmentation of multiple innate and adaptive immune mechanisms.
Materials and methods
Mice
Normal female BALB/c mice aged 3 months were obtained from Charles River Laboratories and maintained in the animal facility of Albert Einstein College of Medicine according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care, and according the guidelines of the Albert Einstein Institute for Animal Studies. All mice were kept under biosafety level 2 conditions, as required for Listeria monocytogenes.
Cells, cell culture and plasmids
The 4T1 cell line, derived from a spontaneous mammary carcinoma in a BALB/c mouse (Aslakson and Miller, 1992), was cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, 1 mM mixed non-essential amino acids, 2 mM L-glutamine, insulin (0.5 USP units ml−1), penicillin (100 units ml−1) and streptomycin (100 μg ml−1). The DNA plasmids pcDNA3.1-Mage-b (Sypniewska et al, 2005), pcDNA3.1-LacZ (Invitrogen, Grand Island, NY, USA) and pCMV-GM-CSF (Chambers and Johnston, 2003) were purified using the Endo-Free Mega kit from Qiagen (Valencia, CA, USA).
LM-based vaccine
The LM-Mb strain was developed in an earlier study (Kim et al, 2008). This was constructed in the prfA-negative XFL-7 strain (referred to as LM in this study), which lacks the positive regulatory factor A that is a central mediator of virulence (Singh et al, 2005). The vaccine strain was transformed with LM plasmid pGG-34, which encodes prfA and amino acids 311–660 of murine Mage-b fused to a non-cytolytic form of listeriolysin O (Gunn et al, 2001; Kim et al, 2008). Complementation of prfA expression by the plasmid does not fully restore virulence, but enforces retention of the plasmid during infection (Gunn et al, 2001; Singh et al, 2005).
Incorporation of αGC into live LM-Mb
The αGC used in this study ((2S, 3S, 4R)-1-O-(α-D-galactopyranosyl)-N-hexacosanoyl-2-amino-1,3,4-octadecanetriol), also known in previous studies as KRN7000 or αGalCer-C26:0, was synthesised as previously described (Yu et al, 2005), and stored as solvent-free aliquots in glass vials at −20°C. The glycolipid was reconstituted either in 100% dimethylsulphoxide (DMSO) at 100 μM for in vitro studies, or in aqueous vehicle consisting of PBS with 0.05% Tween 20 (Life Technologies, Grand Island, NY, USA) and 0.1% DMSO at 500 μM for in vivo studies. The incorporation of αGC into live LM was performed using a method similar to that described previously for M. bovis BCG (Venkataswamy et al, 2009). Briefly, αGC was solubilised at a concentration of 2.3 μM in glass vials by addition of warm (37°C) brain–heart infusion medium containing 5% tyloxapol, followed by sonication for 5 min, heating at 80°C for 2 min and vortexing for 1 min. Then, 450 μl of the 2.3 μM glycolipid solution was immediately diluted into 50 ml of warm BHI to give the required final concentrations of 20 nM glycolipid and 0.05% tyloxapol, and this was inoculated with 500 μl of a LM-Mb mid-log phase culture (OD600 0.5–0.8). Following incubation for 4–6 h at 37°C to reach mid-log phase (OD600 0.5–1.0), the bacterial culture was then immediately aliquoted in 1 ml vials and frozen at −80°C. For subsequent use, the bacteria were thawed, harvested by centrifugation, washed three times with saline and resuspended in saline for injection. In order to determine the incorporation of αGC into LM, we measured the ability of the bacteria to activate an iNKT-cell hybridoma as described in Supplementary Figure S1.
Tumour challenge and immunotherapy regimens
Mice were challenged with 104 4T1 cells injected into the mammary fat pad as described previously (Kim et al, 2008), and then treated using therapeutic regimens to compare the effects of combined LM-Mb and αGC treatment either as separate agents or as glycolipid-modified bacteria. Initial pilot studies showed that when these two agents were administered separately, anti-metastatic effects were greater when LM-Mb treatment was given prior to a series of injections of αGC, as opposed to the reverse order. Moreover, when LM-Mb and αGC were administered at the same time points, the anti-metastatic effect was less compared to LM-Mb and αGC administered sequentially, and animals developed severe liver toxicity (data not shown). Based on these observations we administered LM-Mb first followed by αGC in all subsequent experiments when used as separate agents. The first protocol consisted of three therapeutic immunisations with LM-Mb followed by three injections of αGC. Mice received 104 4T1 tumour cells in the mammary fat pad on day 0, followed by three i.p. injections of LM-Mb or LM (104 CFU), or saline on days 3, 6 and 9, and then three i.p. injections of αGC (100 ng in 200 μl vehicle) i.p. on days 14, 15 and 16. The second protocol consisted of five therapeutic immunisations with LM-Mb modified by direct incorporation of αGC (I-αGC-LM-Mb). Mice received 104 4T1 tumour cells in the mammary fat pad on day 0, followed by i.p. injections of 104 CFU of I-αGC-LM-Mb on days 3, 6, 9, 12 and 15. All mice were euthanised for the analysis of metastatic disease and immunology on day 18. The total number of metastases per mouse was determined as previously described (Kim et al, 2008). For studies carried out to determine effects on survival, mice were maintained until they succumbed spontaneously, or were terminated upon appearance of severe pre-morbid symptoms requiring euthanasia as specified by our approved animal use protocol.
Activation of iNKT cells in vivo
BALB/c mice were injected i.p. with the inert vehicle (PBS plus 0.05% Tween 20 and 0.1% DMSO), 104 CFU LM, 104 CFU I-αGC-LM or 4 nmoles of free αGC. Sera were assayed at the indicated times for IL-4, and IFNγ by capture ELISA as previously described (Yu et al, 2005). Alternatively, BALB/c mice receiving 4T1 tumour cells and therapeutic immunisations with LM-Mb or I-αGC-LM-Mb (see above) were injected with 4 nmoles of free αGC 15 days after the injection of tumour cells. After the indicated times, splenocyte single-cell suspensions were obtained and stained with anti-mouse TCR-FITC (clone H57-597, BD Biosciences, San Jose, CA, USA) and αGC-loaded mouse CD1d tetramers-APC, prepared as previously described (Venkataswamy et al, 2009). Samples were acquired using a LSR II Flow Cytometer (BD Biosciences) and analysed using FlowJo software (TreeStar Software, Ashland, OR, USA).
Flow cytometry analyses
Cells were isolated from blood as described previously (Kim et al, 2008; Castro et al, 2009). Briefly, red blood cells were lysed according standard protocols, and the remaining leukocyte population was used for analysis. Cells were first incubated with Golgi-Plug for 6 h (BD Biosciences), then with an Fc blocker (mAb 2.4G2, anti-CD16/CD32), and finally with specific fluorochrome-conjugated antibodies for the identification of different cell types. Anti-CD8α-PE (clone 53-6.7) antibodies were used to identify CD8 T cells, and anti-CD45-APC antibody (clone 30-F11) to identify the leucocyte population in tumour cell suspensions. To detect the production of intracellular cytokines, cell suspensions were processed using the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions, and stained using antibodies to IFNγ (clone XMG1.2). Appropriate isotype controls were used for each sample. All antibodies were purchased from BD Biosciences. For most samples, data of 1.5 × 105 and 3 × 105 cells were acquired using a FACSCalibur flow cytometer (BD Biosciences). However, in some cases where noted with samples derived from small tumours, limited cell numbers allowed acquisition of only104 cells. Cell debris and dead cells were excluded from the analyses based on forward and side scatter signals, and used Fixable Blue or Green Live/Dead Cell Stain kit (Invitrogen). All data analyses were done using FlowJo software (TreeStar).
ELISPOT
Spleen cells were isolated from vaccinated and control mice with 4T1 tumours and analysed for T cell responses to Mage-b- and NK-cell responses to LM by ELISPOT as described previously (Kim et al, 2008; Chandra et al, 2013). To detect Mage-b-specific immune responses, 4 × 105 spleen cells of vaccinated or control mice were transfected with plasmids pcDNA3.1-Mage-b and pCMV-GM-CSF (1 μg of each plasmid per 5 × 106 spleen cells) using Lipofectamine 2000 (Life Technologies), as described previously (Kim et al, 2008). Expression of Mage-b by the transfected spleen cells was verified by RT–PCR followed by Southern blotting (Supplementary Figure S2). To detect LM-induced NK cell responses, 2 × 105 spleen cells were infected with 2 × 105 CFU of LM for 1 h, and subsequently treated with gentamicin (50 μg ml−1) until the end of restimulation (72 h). After 72 h, the frequency of IFNγ-producing cells was determined by ELISPOT according to standard protocols (BD Biosciences), using an ELISPOT reader (CTL Immunospot S4 analyzer, Cellular Technology Ltd., Cleveland, OH, USA). To determine the CD8+ T-cell or NK-cell component of the responses, spleen cells were depleted of CD8 T cells using anti-CD8α (Clone 53-6.7)-conjugated magnetic beads, or depleted of NK cells using anti-CD49b (Clone DX5)-conjugated magnetic beads according to the manufacturer's instructions (Miltenyi, Auburn, CA, USA).
Assessment of toxicity
Liver toxicity was assessed by visual inspection after killing the mice, and a numerical grade was assigned corresponding to the size and number of visible necrotic plaques. The toxicity was graded as follows: T0=no lesions (normal appearance); T1=uniform light discolouration and firmness; T2=white plaques visible covering ∼5% of the liver surface; T3=white plaques covering ∼10% of liver surface; T4=white plaques covering up to ∼30% of liver surface; and T5=white plaques covering ⩾30% of liver surface. Haematoxylin and eosin (H&E) staining of thin sections of livers was also done to confirm the presence and extent of hepatic inflammation and necrosis. Briefly, liver tissues were fixed in 10% formaldehyde for 48 h, and then kept in 70% ethanol until use. Sections of 1 mm thickness were stained with H&E, and analysed for pathological damage by light microscopy. All pathological analyses were performed by a trained veterinary pathologist. Survival was followed for up to 18 days, and survival curves plotted for the various treatment groups.
Statistical analysis
Effects of treatments on tumour growth, metastasis and immune responses were analysed using the Mann–Whitney test, Unpaired t-test or ANOVA with Sidak post-test for multiple comparisons as indicated. Survival studies were analysed with the Mantel–Cox test. Values of P<0.05 were considered statistically significant.
Results
Efficacy of combination therapy with LM-Mb and αGC in metastatic breast cancer
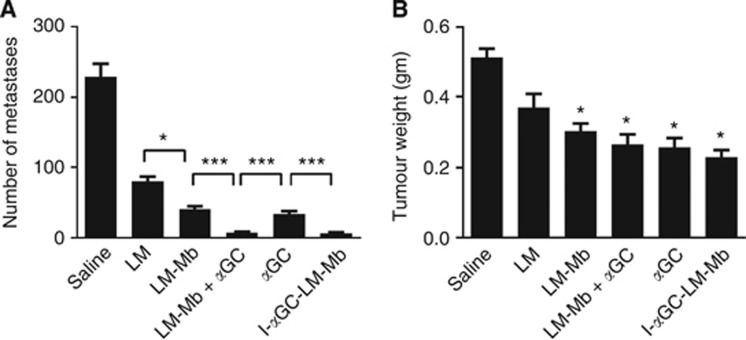
The therapeutic effect of combining the LM-Mb vaccine with αGC treatment or αGC physically incorporated into live LM-Mb vaccine on metastatic breast cancer was assessed in the 4T1 mouse tumour model. Mice treated with unmodified LM showed marked reduction in metastases when killed at day 18 after initial tumour implantation (Figure 1A), consistent with previous studies showing that this treatment induces a variety of tumoricidal mechanisms (Kim et al, 2009). An improved anti-metastatic effect was observed with LM-Mb compared to LM, suggesting an effect of Mage-b-specific immunity, and a similar level of anti-metastatic activity was observed with administration of αGC as a single agent. Strikingly, the combination of αGC with LM-Mb, either as a separately administered agent (LM-Mb+αGC) or by direct incorporation of the glycolipid into the live bacteria (I-αGC-LM-Mb), gave a significantly better anti-metastatic effect compared to LM-Mb alone or αGC alone (Figure 1A). Both of the regimens using combined LM-Mb and αGC treatment reduced the number of macroscopically visible metastases nearly to zero, with only a rare nodule being detected in these mice. The number of metastases were reduced by I-αGC-LM-Mb in all tissues/organs compared to the saline group (MLN, spleen, kidney, liver and diaphragm), but most dramatically in the MLN (Supplementary Table S1). Significant reductions in the weights of primary tumours were also observed in all treatment groups compared to the saline group (with an exception for LM), although combination treatment did not show significant improvement over single-agent treatment in this parameter (Figure 1B).
Figure 1.
Therapeutic effects of Listeria-based vaccines and αGC in the 4T1 breast cancer model. BALB/c mice were injected with 104 4T1 tumour cells in the mammary fat pad and subsequently treated with various single agents or combination regimens. Single agents were given therapeutically as i.p. injections on days 3, 6 and 9, and consisted of saline (sham treatment), attenuated Listeria monocytogenes (LM), LM expressing Mage-b (LM-Mb) or αGC. Combination regimens consisted of three therapeutic injections of LM-Mb (days 3, 6 and 9) followed by three injections of αGC (days 14, 15 and 16; LM-Mb+αGC), or five therapeutic injections of LM-Mb modified by direct incorporation of αGC (I-αGC-LM-Mb; see Materials and Methods for details). Mice were euthanised on day 18 and analysed for the frequency of metastases (A) and primary tumour weight (B). This experiment was performed three times with consistent results using N=5 mice per group, and the results shown average values for means and s.e. for data from all three experiments. For both metastases and tumour weight, all treated groups were significantly different from the saline-treated control groups, with an exception for the tumour weight of the groups treated with LM only. *P<0.05, ***P<0.001 (Mann–Whitney test) for comparisons between individual groups as indicated in A, and for comparisons of individual treated groups to saline control group in B.
Direct incorporation of αGC into LM-Mb avoids severe toxicity
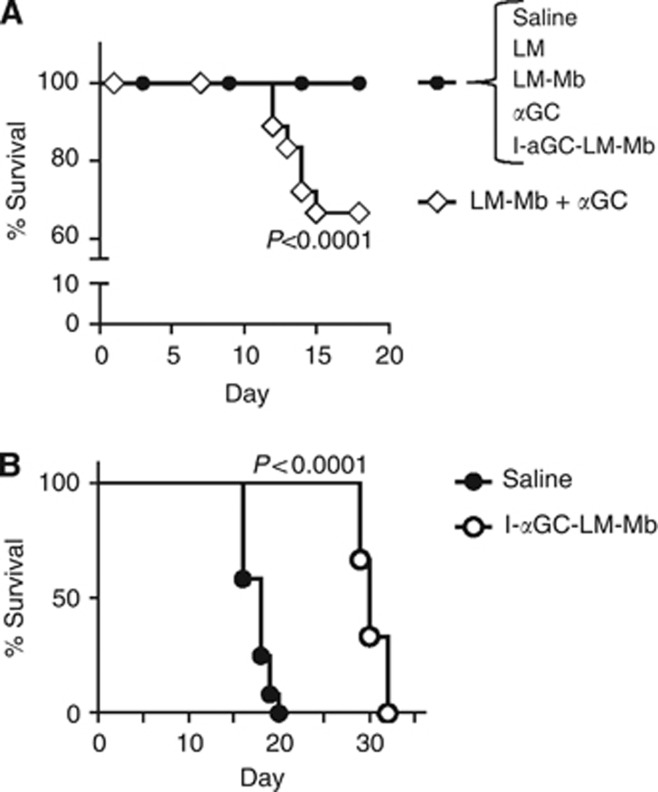
Although αGC was equally efficacious in reducing metastases when administered as a free glycolipid following LM-Mb treatment or simultaneously using the direct incorporation approach, analysis of survival of treated mice revealed a striking difference between these two approaches. This was first examined in a short-term survival study in which mice receiving various therapies were observed for 18 days following implantation of 4T1 cells in the mammary fat pad. Whereas all sham-treated 4T1 bearing mice (i.e., saline injections only) survived at least 18 days from the time of tumour initiation, we observed a significant fatality rate starting around day 11 in animals treated with the sequential administration of LM-Mb and αGC as separate agents. In contrast, no deaths were observed over this time period in any of the other treatment groups, including those which received LM-Mb with directly incorporated αGC (I-αGC-LM-Mb; Figure 2A). We then carried out a second more extended survival study to compare time to death in 4T1 tumour-bearing mice receiving therapeutic vaccinations with I-αGC-LM-Mb vs mice receiving only saline injections. Although mice that received only sham immunisations with saline all succumbed by day 20, mice that received I-αGC-LM-Mb all survived past day 20 and showed a significant extension (30%) of overall survival (Figure 2B). This extension of survival was consistent with the marked anti-metastatic effect and low toxicity of the I-αGC-LM-Mb treatment.
Figure 2.
Survival of 4T1 tumour-bearing mice treated with various regimens. BALB/c mice were challenged with 4T1 tumour cells and immunised therapeutically i.p. with saline or the single or combination regimens as described in Figure 1. The percentage of live animals was determined daily for all groups until all mice were killed on day 18 after tumour challenge (A). This experiment was repeated three times with N=5 mice per group, and the results were averaged. Extended survival study for BALB/c mice (N=8 per group) challenged on day 0 with 4T1 tumour cells, and treated therapeutically with i.p. injections of saline alone or 104 CFU of I-αGC-LM-Mb administered every 3 days (B). P values for differences between survival curves are indicated (Mantel–Cox test).
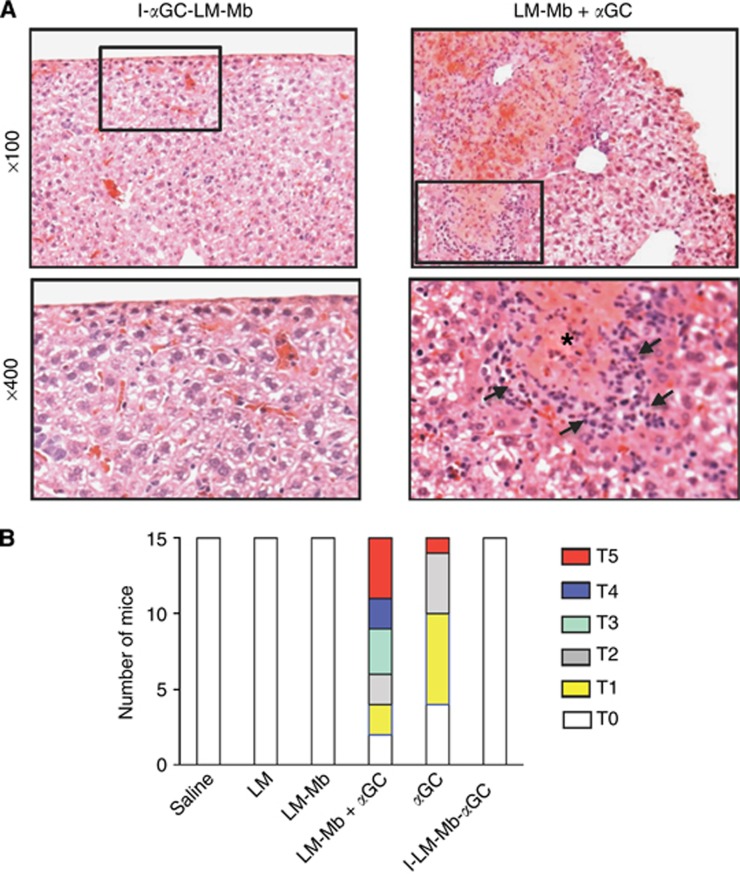
The apparent toxicity leading to accelerate death in animals receiving the separately injected LM-Mb and αGC regimen was investigated by necropsy performed on animals killed at day 18. Focal areas of hepatic necrosis were found and verified by histologic studies of thin sections of the liver (Figure 3A). Such foci were also observed with lower frequency and smaller size in mice that received αGC alone, but not at all in animals that were treated with I-αGC-LM-Mb or LM-Mb alone (Figure 3B).
Figure 3.
Hepatotoxicity of αGC administered as a separate agent but not when incorporated into LM-Mb. Representative micrographs of H&E stained sections from the livers of mice 4T1 tumour-bearing mice that received I-αGC-LM-Mb (left) or LM-Mb+αGC (right) as described in Figure 1, and killed on day 18 (A). Low power views (top) show normal appearance of liver tissue typical for I-αGC-LM-Mb-treated mice, and foci of necrosis and inflammatory cells that were common in mice receiving LM-Mb+αGC. Higher magnification views of the boxed areas are shown in the lower panels, with an area of necrosis marked by the asterisk and arrows indicating leukocyte infiltrates for the LM-Mb+αGC sample. Summary of semiquantitative grading of toxicity based on gross liver pathology (B). BALB/c mice were challenged with 4T1 tumour cells and immunised therapeutically i.p. with saline or the single or combination regimens as described in Figure 1. Toxicity was graded in each mouse by visual inspection of livers at necropsy using a scale from T0 to T5 as described in Materials and Methods. This experiment was repeated three times with N=5 mice per group and the results of all experiments are combined in the graph shown.
Activation of NKT cells in spleens of 4T1 tumour-bearing mice that received I-αGC-LM-Mb
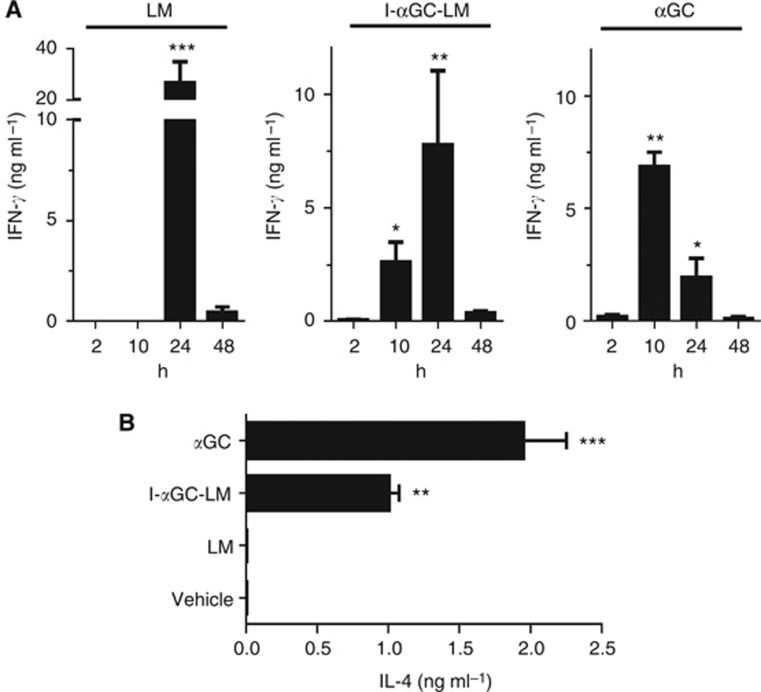
To determine whether LM bacteria modified by direct incorporation of αGC could activate NKT cells in vivo, we assessed the rapid production of IFNγ and IL-4, two cytokines that are characteristically for NKT-cell activation. For this purpose we injected naive mice once with LM, αGC or LM modified by direct incorporation of αGC (I-αGC-LM) and obtained serum samples at various time points after injection to determine cytokine levels. (The slower adaptive responses to Mb is irrelevant in this analysis of rapid innate-like NKT-cell responses.) Previous studies have shown that αGC administered as a free glycolipid induces the production of IFNγ and IL-4 that peak in the serum at ∼10–12 and 2 h, respectively (Venkataswamy et al, 2009). Infection with LM also induces the rapid production of IFNγ in naive mice by multiple cell types, including NK, NKT and CD8+ T cells (North and Conlan, 1998; Brigl et al, 2003; Soudja et al, 2012). We found that I-αGC-LM stimulated a serum IFNγ response that was apparent at 10 h, and peaked at 24 h. In contrast, LM infection generated a large transient serum IFNγ response, which was first detected at 24 h (Figure 4A). The accelerated IFNγ production seen with I-αGC-LM was consistent with direct NKT-cell activation by the αGC incorporated into the bacteria. Also supporting the conclusion that NKT cells were directly activated, we observed a significant IL-4 response at 2 h after injection of I-αGC-LM, whereas LM alone did not induce detectable IL-4 (Figure 4B).
Figure 4.
Rapid induction of IFNγ and IL-4 by immunisation with I-αGC-LM. BALB/c mice were injected i.p. with inert vehicle, LM, I-αGC-LM or free αGC. Sera were assayed at the indicated times for IFNγ by capture ELISA (A). Values of IFNγ for vehicle-treated mice were below the limit of detection (<0.1 ng ml−1). Sera obtained from mice (N=3 per group) at 2 h after treatment as in A were assayed for IL-4 by capture ELISA (B). Graphs are a representative of three experiments with N=3 mice per group. Mean and s.d. are shown, and significant elevations of IFNγ above background levels are indicated by asterisks. *P<0.05, **P<0.01, ***P<0.001; ANOVA with Sidak post-test for multiple comparisons.
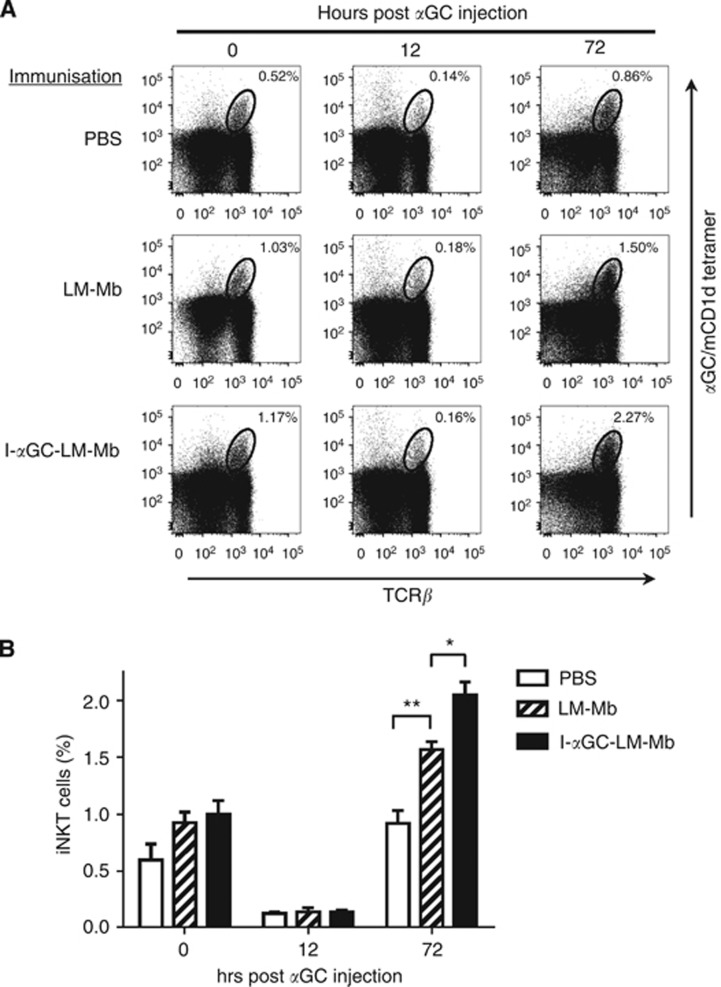
To confirm that NKT cells were rapidly activated by treatment with I-αGC-LM-Mb in tumour-bearing mice in vivo, we analysed the percentages of NKT cells in the spleens of mice with 4T1 tumours and metastases that were therapeutically immunised with I-αGC-LM-Mb using CD1d tetramers loaded with αGC. The percentage of NKT cells stained with the tetramer in the spleens of tumour-bearing mice that had received five therapeutic treatments with I-αGC-LM-Mb was either unchanged or slightly increased compared to saline or LM-treated controls at 0 h post αGC injection (Figure 5A and B), while tetramer binding of NKT cells showed a transient decrease in the spleen at 12 h, followed by a rebound to greater than baseline levels at 72 h (Figure 5A and B). This was consistent with the normal activation pattern observed for intact NKT-cell populations in healthy naive mice, which is characterised by rapid TCR downmodulation leading to loss of tetramer staining at earlier time points, followed by TCR re-expression and proliferative expansion of the tetramer staining population by 72 h (Wilson et al, 2003). In fact, animals that had received prior treatment with either LM-Mb or I-αGC-LM-Mb showed significantly increased expansion of NKT cells at 72 h after subsequent injection of free αGC, with I-αGC-LM-Mb showing the greater effect (Figure 5A and B). In summary, NKT-cell activation by repeated I-αGC-LM-Mb treatments did not lead to either depletion or anergy of NKT cells in vivo, and may actually have primed NKT cells to respond more vigourously to subsequent stimulation.
Figure 5.
Absence of NKT-cell anergy following repeated therapeutic immunisations with I-αGC-LM. BALB/c mice were challenged with 4T1 tumour cells and immunised therapeutically with saline, LM-Mb and I-αGC-LM-Mb as described in Figure 1. Fifteen days after the first immunisation, splenocytes were obtained and stained for NKT cells using αGC-loaded CD1d tetramers (0 h time point). In parallel, a group of mice receiving the same treatment was injected i.p. with free αGC and splenocytes were stained for NKT cells after 12 and 72 h. (A and B) Representative dot plots depicting staining with anti-TCRβ and αGC-loaded CD1d tetramers are shown in A, and the results using N=3 mice per group are summarised in B. Mean values and s.e. are shown. *P<0.05, **P<0.01 (ANOVA with Sidak post-test).
Improved T cell and NK-cell responses in mice vaccinated with I-αGC-LM-Mb
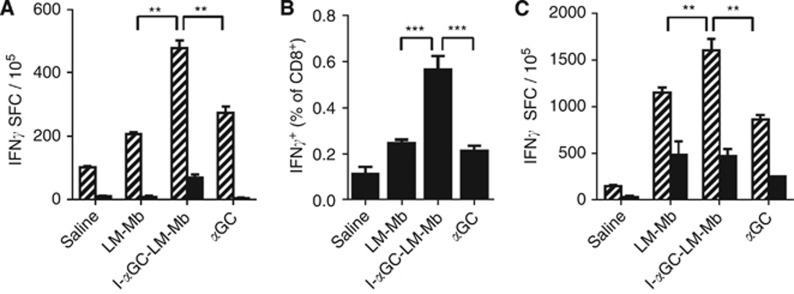
The activation of NKT cells has been frequently shown to lead secondarily to increased CD8+ T-cell cross-priming and to NK-cell activation (Hermans et al, 2003; Venkataswamy et al, 2009). Given that NKT-cell activation was augmented by treatment of tumour-bearing mice with I-αGC-LM-Mb, we analysed whether this treatment could also enhance CD8 T cell (CD8α+) and NK-cell (CD49b/DX5+) responses using the production of IFNγ by these cells as an activation marker. First, we analysed antigen-dependent recall responses to Mage-b in the spleens of I-αGC-LM-Mb-treated and control mice upon restimulation with Mage-b in vitro using IFNγ ELISPOT assays. This showed significantly higher numbers of IFNγ secreting cells in response to stimulation ex vivo by expression of Mage-b by plasmid transfection in the spleens of mice treated with I-αGC-LM-Mb compared to the control mice treated with LM-Mb, αGC or saline (Figure 6A). These responses were substantially diminished following depletion using anti-CD8α-conjugated magnetic beads. Very low numbers of IFNγ-producing cells were observed after restimulation by transfecting a plasmid encoding only a control protein, lacZ (Supplementary Figure S3), demonstrating the antigen specificity of the restimulation assay. Although NK cells and a subset of DCs express low levels of CD8α and can potentially contribute to IFNγ responses (Vremec et al, 2007; Shortman and Heath, 2010), the antigen dependence of the observed responses, both at the level of in vivo vaccination and in vitro restimulation, indicated that the responding cells were most likely CD8α+ T cells. Similarly, enumeration of CD8α+ cells spontaneously producing intracellular IFNγ (i.e., as a result of in vivo treatment but without in vitro restimulation) in peripheral blood samples by flow cytometry showed a significantly higher response in mice treated with I-αGC-LM-Mb compared to mice treated with LM-Mb or αGC alone (Figure 6B). In primary tumours, the percentage of CD8α+ cells producing intracellular IFNγ+ was significantly higher for mice that received I-αGC-LM-Mb compared to mice that received saline, but not compared to mice that received LM-Mb or αGC alone (Supplementary Figure S4). In summary, these results were consistent with a superior effect of I-αGC-LM-Mb on stimulating the cross-priming of CD8α+ T cells specific for TAAs.
Figure 6.
Enhancement of CD8+ and NK cell responses following therapeutic immunisation with I-αGC-LM-Mb. BALB/c mice were challenged with 4T1 tumour cells and immunised therapeutically i.p. with saline, LM-Mb, I-αGC-LM-Mb or αGC as described in Figure 1. Spleens were collected on day 18 and analysed for IFNγ-producing cells by ELISPOT. Measurement of IFNγ spot-forming cells (SFC) determined by ELISPOT following restimulation in vitro with Mage-b, either without (hatched bars) or with (solid bars) depletion of CD8α+ cells (A). White blood cells were analysed directly ex vivo by flow cytometry for intracellular IFNγ+ cells, gating on CD8α+ lymphocytes (B). Measurement of IFNγ SFC in the spleen was determined by ELISPOT following restimulation in vitro with LM, either without (hatched bars) or with (solid bars) depletion of DX5+ NK cells (C). These experiments were repeated three times with N=5 mice per group. Means and s.e. are shown. **P<0.01, ***P<0.001 (Mann–Whitney test).
We also measured NK-cell (DX5+) responses in the spleens of I-αGC-LM-Mb-treated and control mice. For this purpose, we infected the spleen cells with LM in vitro and determined the percentage of IFNγ-producing cells by ELISPOT with and without anti-DX5 magnetic bead depletion. We found that the number of cells secreting IFNγ in response to restimulation by LM infection was significantly increased in I-αGC-LM-Mb-treated mice compared to mice treated with saline, or with LM-Mb or αGC alone. These responses were substantially reduced by depletion of DX5+ cells, consistent with a large NK-cell component (Figure 6C). Overall, our results indicated that treatment of tumour-bearing mice with combined therapy in the form of I-αGC-LM-Mb gave superior CD8α+ T-cell priming against specific TAAs and also enhanced NK cell activation.
Accumulation of I-αGC-LM-Mb in metastases and primary tumours
Previous studies showed enhanced survival and replication of LM in primary and metastatic tumours compared to normal tissues, most likely as a consequence of local immune suppression in the TME (Chandra et al, 2013; Quispe-Tintaya et al, 2013). To assess the impact of αGC incorporation on survival and replication of I-αGC-LM-Mb in tissues, we analysed bacterial loads in the metastases, tumours, spleens and livers at days 1, 3 and 6 after a single administration of either LM-Mb or I-αGC-LM-Mb (0.5 × 107 CFU; Supplementary Table S2). This showed that both LM-Mb and I-αGC-LM-Mb accumulated and persisted at high levels in primary tumour and metastatic tissues, with higher CFU per gram of tissue than in spleen and liver. Incorporation of αGC showed modest reductions in CFU in some cases at day 1 (spleen and liver) and day 3 (spleen), but by day 6 these differences were no longer apparent. We thus conclude that incorporation of αGC into I-αGC-LM-Mb does not significantly diminish its ability to persist and replicate within tumours and metastases.
Discussion
Although treatment with αGC as a free glycolipid injection has shown remarkable anti-tumour activity in a variety of mouse models of cancer, phase I clinical trials of this approach in human cancer patients have not shown clear evidence of therapeutic benefit (Schneiders et al, 2011). In the study presented here, we have explored various therapeutic approaches using αGC and a LM-based tumour vaccine expressing TAA Mage-b to achieve strong synergistic effects, particularly in the suppression of metastatic disease. In one approach, the LM-Mb vaccine and αGC were administered as separate components, while an alternate strategy used direct incorporation of αGC into live LM-Mb prior to injection into tumour-bearing animals. This latter approach has been used successfully in previous work to improve the vaccine efficacy of live M. bovis BCG (Venkataswamy et al, 2009). The combination of LM-Mb and αGC, with the glycolipid administered either as a separate series of injections or directly incorporated into the LM-Mb bacteria, was highly effective at reducing the number of metastases, and almost completely eliminated grossly visible metastatic nodules. Most significantly, while the administration of LM-Mb and αGC as separate injections in sequential manner was associated with marked toxicity due to hepatic necrosis, we found that the direct incorporation of the glycolipid into LM-Mb completely eliminated the toxicity while still preserving the marked clinical benefit.
The apparent synergy that we observed between LM and αGC of inducing anti-tumour immunity without toxicity is likely to be related to the incorporation of αGC into the LM-Mb. It is likely that the high efficiency of infection of CD8α+ DC by LM resulted in enhanced intracellular concentration of αGC compared to the uptake of free αGC, and may have improved the activation of NKT cells compared to free αGC in vivo. Indeed, we found that I-αGC-LM-Mb significantly increased the population of NKT cells in the spleen compared to free αGC in 4T1 tumour-bearing mice in vivo. Activated NKT cells are known for producing multiple cytokines, such as IL-2, IFNγ and IL-4 that can lead to an immune-stimulating environment (Bendelac et al, 2007). We found that I-αGC-LM-Mb stimulated iNKT-cell hybridoma cells in vitro, and when injected into mice, I-αGC-LM-Mb rapidly induced elevated levels of IFNγ and IL-4 in the serum consistent with direct activation of iNKT cells in vivo.
In addition to directly activating iNKT cells, we also found that repeated immunisations of 4T1 tumour-bearing mice with I-αGC-LM-Mb significantly increased the percentage of CD8α+ cells producing IFNγ, compared to LM-Mb or αGC alone in vivo. Restimulation in vitro with Mage-b also demonstrated that antigen-specific CD8+ T-cell responses were significantly enhanced in spleens of mice that received I-αGC-LM-Mb compared to mice that received LM-Mb or free αGC. The 4T1 metastases and primary tumour highly express Mage-b (Kim et al, 2008) and are therefore a target for Mage-b-specific CD8+ T cells.
It has been shown that LM also infects a variety of tumour cells in vitro and in vivo (Kim et al, 2009). This converts poorly immunogenic tumour cells into sensitised targets for LM-activated CD8 T and NK cells, and partly explains why unmodified LM also has a considerable anti-metastatic effect. Moreover, we found that LM could be used for the selective delivery of anti-cancer agents such as rapidly decaying radioisotopes to the metastases (Quispe-Tintaya et al, 2013). We demonstrated that LM was rapidly cleared in normal tissues by the immune system but prevented from elimination in the TME by the strong immune suppression. Therefore, it is likely that in the current study I-αGC-LM-Mb has delivered αGC to metastases as well, and may have attracted NKT cells selectively to these sites. Consistent with this, we found that the number of CFU of I-αGC-LM-Mb per gram of tissue was significantly higher (14- to 34-fold) in the metastases than in the spleens or livers of tumour-bearing mice. In preliminary experiments, we observed that this was correlated with an approximately two- to three-fold enrichment of the frequency of NKT cells in metastases compared to spleen in mice that received I-αGC-LM-Mb (unpublished results).
Our results raise the question of why I-αGC-LM-Mb was more effective against the metastases than primary tumours. In a previous study, we found that the percentage of MDSC was much higher in blood (up to ∼80% of circulating leukocytes) than in the primary tumour (∼6% of tumour infiltrating leukocytes) in the 4T1 model and in human cancer patients (Diaz-Montero et al, 2009; Chandra et al, 2013). Since metastases of 4T1 spread via the blood stream and Listeria infects the MDSC, delivery of I-αGC-LM-Mb to the metastases through MDSC may have been more efficient than to the primary tumour. Indeed, the number of CFU of I-αGC-LM-Mb was significantly higher in metastases than primary tumour on day 1 after injection of the bacteria that may have led to a greater accumulation of NKT cells in metastases compared to primary tumours.
In summary, we have demonstrated that a novel combination of a recombinant LM expressing Mage-b modified by direct incorporation of the NKT-cell-activating glycolipid αGC almost completely eliminated metastases in the 4T1 model without apparent toxicity. This correlated with the activation of NKT cells, NK cells and Mage-b-specific CD8α+ T cells in tumour-bearing mice. The results described here provide a foundation for future studies that could combine a method targeting primary tumours, such as surgery or local radiation therapy, with anti-metastatic treatment using I-αGC-LM-Mb as an approach to achieve prolonged remission or cure of aggressive breast cancer, and perhaps of other metastatic cancers.
Acknowledgments
This study was supported by a pilot grant from the Albert Einstein Cancer Center, NCI grant 1R21 CA129470-01, and by RO1 AI45889, and by the Paul F Glenn Center for the Biology of Human Aging Research. Flow cytometry studies were carried out using core facilities supported by the AECC (NIH/NCI CA013330) and the Einstein Center for AIDS Research (NIH AI-51519). LJC is a recipient of the Pew Foundation International Scholars Fellowship Award. We thank Dr Rani Sellers for the useful discussions and pathological examinations.
SAP is a consultant for Vaccinex, Inc. (Rochester, NY), which has licensed technologies for development of NKT-cell-related therapies for cancer and other diseases.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52 (6:1399–1405. [PubMed] [Google Scholar]
- Behar SM, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–250. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4 (12:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, Francis P, Gligorov J, Kyriakides S, Lin N, Pagani O, Senkus E, Thomssen C, Aapro M, Bergh J, Di Leo A, El Saghir N, Ganz PA, Gelmon K, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Leadbeater M, Mayer M, Rodger A, Rugo H, Sacchini V, Sledge G, van't Veer L, Viale G, Krop I, Winer E. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21 (3:242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, Lu S, Gravekamp C. Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br J Cancer. 2009;101 (8:1329–1337. doi: 10.1038/sj.bjc.6605329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RS, Johnston SA. High-level generation of polyclonal antibodies by genetic immunization. Nat Biotechnol. 2003;21 (9:1088–1092. doi: 10.1038/nbt858. [DOI] [PubMed] [Google Scholar]
- Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer. 2013;108 (11:2281–2290. doi: 10.1038/bjc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58 (1:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29 (3:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167 (11:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171 (10:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br J Cancer. 2008;99 (5:741–749. doi: 10.1038/sj.bjc.6604526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69 (14:5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruit WH, van Ojik HH, Brichard VG, Escudier B, Dorval T, Dreno B, Patel P, van Baren N, Avril MF, Piperno S, Khammari A, Stas M, Ritter G, Lethé B, Godelaine D, Brasseur F, Zhang Y, van der Bruggen P, Boon T, Eggermont AM, Marchand M. Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer. 2005;117 (4:596–604. doi: 10.1002/ijc.21264. [DOI] [PubMed] [Google Scholar]
- Marchand M, Punt CJ, Aamdal S, Escudier B, Kruit WH, Keilholz U, Håkansson L, van Baren N, Humblet Y, Mulders P, Avril MF, Eggermont AM, Scheibenbogen C, Uiters J, Wanders J, Delire M, Boon T, Stoter G. Immunisation of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer. 2003;39 (1:70–77. doi: 10.1016/s0959-8049(02)00479-3. [DOI] [PubMed] [Google Scholar]
- Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140 (2:119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, Eliceiri BP, Reisfeld RA. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8 (12:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- North RJ, Conlan JW. Immunity to Listeria monocytogenes. Chem Immunol. 1998;70:1–20. [PubMed] [Google Scholar]
- Paterson Y, Maciag PC. Listeria-based vaccines for cancer treatment. Curr Opin Mol Ther. 2005;7 (5:454–460. [PubMed] [Google Scholar]
- Quispe-Tintaya W, Chandra D, Jahangir A, Harris M, Casadevall A, Dadachova E, Gravekamp C. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci USA. 2013;110 (21:8668–8673. doi: 10.1073/pnas.1211287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiders FL, Scheper RJ, von Blomberg BM, Woltman AM, Janssen HL, van den Eertwegh AJ, Verheul HM, de Gruijl TD, van der Vliet HJ. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140 (2:130–141. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234 (1:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175 (6:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37 (3:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypniewska RK, Hoflack L, Tarango M, Gauntt S, Leal BZ, Reddick RL, Gravekamp C. Prevention of metastases with a Mage-b DNA vaccine in a mouse breast tumor model: potential for breast cancer therapy. Breast Cancer Res Treat. 2005;91 (1:19–28. doi: 10.1007/s10549-004-6454-7. [DOI] [PubMed] [Google Scholar]
- Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, Reddington F, Besra GS, Jacobs WR, Jr, Porcelli SA. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183 (3:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D, O'Keeffe M, Hochrein H, Fuchsberger M, Caminschi I, Lahoud M, Shortman K. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109 (3:1165–1173. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]
- Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100 (19:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, Shimizu N, Ueno N, Yamamoto S, Taniguchi M, Motohashi S, Nakayama T, Okamoto Y. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138 (3:255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36 (3:237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci USA. 2005;102 (9:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.