Abstract
Background:
Pazopanib, an oral angiogenesis inhibitor targeting vascular endothelial growth factor receptor (VEGFR)/platelet-derived growth factor receptor (PDGFR)/c-Kit, is approved in locally advanced/metastatic renal cell carcinoma (RCC).
Methods:
Data from trials in advanced solid tumours and advanced/metastatic RCC were used to explore the relationships between plasma pazopanib concentrations and biomarker changes, safety, and efficacy. Initially, the relationships between pharmacokinetic parameters and increased blood pressure were investigated, followed by analysis of steady-state trough concentration (Cτ) and sVEGFR2, safety, progression-free survival (PFS), response rate, and tumour shrinkage. Efficacy/safety end points were compared at Cτ decile boundaries.
Results:
Strong correlation between increased blood pressure and Cτ was observed (r2=0.91), whereas weak correlation was observed between Cτ and decline from baseline in sVEGFR2 (r2=0.27). Cτ threshold of >20.5 μg ml−1 was associated with improved efficacy (PFS, P<0.004; tumour shrinkage, P<0.001), but there was no appreciable benefit in absolute PFS or tumour shrinkage from Cτ >20.5 μg ml−1. However, the association of Cτ with certain adverse events, particularly hand–foot syndrome, was continuous over the entire Cτ range.
Conclusions:
The threshold concentration for efficacy overlaps with concentrations at which toxicity occurs, although some toxicities increase over the entire Cτ range. Monitoring Cτ may optimise systemic exposure to improve clinical benefit and decrease the risk of certain adverse events.
Keywords: pazopanib, trough concentration, renal cell carcinoma, progression-free survival, blood pressure
Pazopanib (Votrient, GlaxoSmithKline, Philadelphia, PA, USA) is an oral angiogenesis inhibitor targeting vascular endothelial growth factor receptors (VEGFR)-1, -2, and -3, platelet-derived growth factor receptors-α/-β, and c-Kit (Kumar et al, 2007; Sonpavde et al, 2008). Pazopanib is approved in the United States (U.S. Department of Health and Human Services Food & Drug Administration, 2009), European Union (European Medicines Agency, 2010), and other countries (Australian Government Department of Health and Ageing, 2010; Instituto de Salud Publica de Chile, 2010; Kidney Cancer Canada, 2010) for the treatment of renal cell carcinoma (RCC). The efficacy and safety of pazopanib in treatment-naive and cytokine-pretreated patients with advanced/metastatic RCC was demonstrated in an open-label, single-arm Phase II trial (Hutson et al, 2010) and in a randomized, double-blind, placebo-controlled Phase III trial (Sternberg et al, 2010). In the Phase II study, patients treated with single-agent pazopanib experienced an overall response rate of 35% and median progression-free survival (PFS) of 12 months (Hutson et al, 2010). In the Phase III study, pazopanib significantly improved PFS compared with placebo in the overall study population (9.2 vs 4.2 months; hazard ratio 0.46; 95% CI, 0.34–0.62; P<0.0001; Sternberg et al, 2010).
A number of factors affect the efficacy of a drug or treatment regimen in patients with cancer; these include patient and tumour characteristics, tumour biology, and systemic exposure to the drug. Among these, parameters of drug exposure are most amenable to regular monitoring, and may potentially provide insight towards dose optimisation. Emerging evidence corroborates the association of clinical and biologic effects of small-molecule tyrosine kinase inhibitors with the level of systemic exposure. For example, tumour response rates, time to progression, and overall survival were significantly better in patients with RCC who achieved a target threshold exposure to sunitinib plus its primary active metabolites (Houk et al, 2010). Similarly, clinical benefit is reduced when plasma concentrations of imatinib decrease to below ∼1000 ng ml−1 in patients with gastrointestinal stromal tumours or patients with chronic myeloid leukaemia (Eechoute et al, 2011).
In addition to the correlation with efficacy, systemic exposure of a drug may also correlate with biomarkers indicative of target inhibition. Administration of sunitinib results in changes in levels of circulating proteins associated with angiogenesis, including soluble VEGFR2 (sVEGFR2; Faivre et al, 2006; Motzer et al, 2006; Deprimo et al, 2007). The decrease in sVEGFR2 observed after administration of sunitinib in patients with RCC demonstrated a weak relationship with the steady-state trough plasma sunitinib concentrations (Deprimo et al, 2007). VEGFR2 is the predominant receptor that mediates production of nitric oxide and prostacyclin by endothelial cells, leading to vasodilation. Administration of VEGF in animals and humans causes vasodilation and decreases in blood pressure. In contrast, inhibition of VEGFR signalling increases blood pressure (Li et al, 2002; Janvier et al, 2005; Roodhart et al, 2008). Hypertension has been noted as a possible pharmacodynamic marker of anti-VEGF therapy, although its prognostic and predictive roles in the treatment of cancer are controversial (Scartozzi et al, 2009; Hurwitz et al, 2010; Rini, 2010). Thus, biomarkers of target inhibition such as sVEGFR2 and blood pressure may confirm inhibition of the targeted receptor and provide insight into the optimal dose of a therapeutic agent.
Preclinical studies have demonstrated that the inhibitory activity of pazopanib towards VEGFR2 phosphorylation, angiogenesis, and tumour growth is concentration dependent (Kumar et al, 2007; Harris et al, 2008). In these models, the in vivo activity of pazopanib required steady-state concentrations of at least 40 μM (17.5 μg ml−1) during the entire dosing interval rather than transient maximal concentrations (Kumar et al, 2007). These results suggest that constant target inhibition during treatment is required to maximise the therapeutic effect of pazopanib in preclinical models of in vivo activity.
Accordingly, the present analyses were conducted to determine the pharmacokinetic and pharmacodynamic relationship between pazopanib and markers of clinical efficacy and safety. Markers of target engagement, blood pressure and sVEGFR2, and end points of safety and efficacy were examined to investigate the range of plasma pazopanib concentrations that could optimise benefit to patients with RCC.
Materials and Methods
The initial analysis of the relationship between plasma pazopanib concentrations and increased blood pressure was conducted with data from patients with advanced solid tumours enroled in a first-in-human Phase I study (Hurwitz et al, 2009). That dose-escalation study provided individual hypertension data over a wide range of pazopanib concentrations; patients received pazopanib doses of 50 and 100 mg three times weekly, 50–2000 mg once daily, and 300 and 400 mg twice daily. Blood pressure and pharmacokinetic data were available for 54 of the 63 enroled patients.
Serial blood samples (4 ml) were collected for determination of plasma pazopanib concentrations on days 1 and 22 in the Phase I study as described previously (Hurwitz et al, 2009). Plasma pazopanib area under the curve (AUC), maximum concentration (Cmax), and trough concentrations (Cτ) observed on study day 22 were included to investigate the pharmacokinetic and pharmacodynamic relationships between systemic exposure and increased blood pressure. Cτ was defined as the plasma pazopanib concentration at 24 h after the first dose on day 22 in the once-daily and twice-daily cohorts and as the predose concentration on day 22 in the three-times-weekly cohorts. AUC from 0 to 24 h after dosing was measured in the three-times-weekly and once-daily cohorts. AUC from 0 to 12 h after dosing was measured in the twice-daily dosing cohorts.
Blood pressure was measured in the Phase I study during clinic visits at baseline, on study days 8, 15, and 22, and every 3 weeks thereafter until the patient was withdrawn from the study. Patients also recorded blood pressure with a digital monitor at least twice daily at home between clinic visits, and all blood pressure data were included in the analysis. A significant increase in blood pressure was defined as a 15 mm Hg or greater increase from baseline in the mean arterial blood pressure (MAP; calculated as (2 × diastolic pressure+systolic pressure)/3) on at least three separate occasions, initiation or escalation of antihypertensive medications, or both. The occurrence of a significant increase in blood pressure in a patient was described as a binomial response (yes or no). Only elevations in MAP or escalation of antihypertensive regimens that occurred while the patients remained on their initial starting dose of pazopanib were included in this analysis.
Patients were grouped according to the quintiles of each pharmacokinetic parameter (AUC, Cmax, Cτ). Quintiles of pharmacokinetic parameters were chosen to examine trends that may be obscured by the variability in the data. The proportion of patients who experienced a significant increase in blood pressure was determined within each quintile. A simple linear model was fit to the proportion of patients with increased blood pressure vs the midpoint of the parameter quintile to investigate the correlation between each pharmacokinetic parameter and an increase in blood pressure. The correlation between AUC and Cτ was also investigated.
Pharmacokinetics/pharmacodynamics in patients with RCC
The analysis of the relationship between plasma pazopanib concentrations and sVEGFR2, clinical efficacy, and safety in patients with RCC used data from 225 patients with locally advanced or metastatic RCC treated with pazopanib monotherapy in a single-arm Phase II trial (Hutson et al, 2010). The primary efficacy end point was overall response rate, and PFS was a secondary end point. Response was assessed by RECIST version 1.0 (Therasse et al, 2000) at week 12 and every 8 weeks thereafter. Serial blood samples (2 ml) were collected at week 4 at the following times: predose (within 60 min before study drug administration; Cτ) and 1–2 h, 3–4 h, and 6–8 h post dose.
sVEGFR2
Plasma concentrations of sVEGFR2 were measured by enzyme-linked immunosorbent assay before initiation of treatment with pazopanib (baseline) and at 4, 8, and 12 weeks of pazopanib treatment. The post-baseline nadir (lowest concentration measured during the first 12 weeks) in plasma sVEGFR2 was identified for each patient, and the percent change from baseline in the sVEGFR2 concentration (%ΔsVEGFR2) was calculated as: %ΔsVEGFR2=(baseline−nadir)/baseline × 100.
Linear and nonlinear (Emax) models were used to describe the relationship between %ΔsVEGFR2 and plasma pazopanib concentrations:
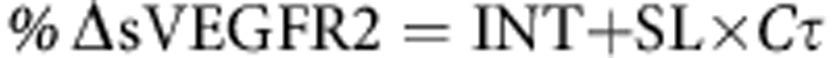 |
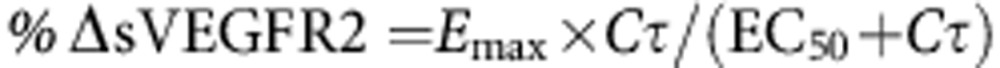 |
where Cτ is the predose plasma pazopanib concentration at the week 4 visit, SL is the slope of the linear relationship between Cτ and the decrease in sVEGFR2, INT is the intercept for the relationship, Emax is the maximum percent decrease from baseline for the sVEGFR2 nadir, and EC50 is the Cτ at which the percent change from baseline for the sVEGFR2 nadir is 50% of the maximum decrease. Models were fit to the data with R version 2.14.0 using the linear models or nonlinear least squares' packages. Model fits were compared by the Akaike information criteria.
Safety
Adverse events (AEs) from the Phase II study that occurred during the first 12 weeks of pazopanib treatment were used to explore concentration–effect relationships to allow sufficient time for the AEs to emerge and to minimise the confounding effects of factors that tend to increase over time on study, such as dose modifications, addition of concomitant medications, and disease progression. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. The incidence of all AEs was summarised according to the week 4 pazopanib Cτ quartiles to allow for a sufficient number of events within each group to reveal concentration-related trends in the frequency. Statistical analyses of clinical efficacy data were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Efficacy
Disease assessments were performed at week 12 of pazopanib treatment and every 8 weeks thereafter. The response rate was defined as the percentage of patients who achieved either a confirmed complete or partial response. A best response of stable disease required stable disease for at least 12 weeks after the first dose of pazopanib. Progression-free survival was defined as the interval between the first day of treatment and the earliest date of disease progression or death due to any cause, whichever occurred first.
The Phase II study originally was designed using randomized discontinuation design. All patients initially received pazopanib 800 mg once daily for 12 weeks. After the week 12 disease assessment, patients with stable disease were randomized to receive pazopanib or placebo. After a planned interim analysis of safety and efficacy data, the design was amended to an open-label treatment where all patients received pazopanib 800 mg once daily based on the recommendations of an independent data monitoring committee. Patients who were randomized to receive placebo (n=28) were excluded from the pharmacokinetic/pharmacodynamic analysis; therefore, a total of 177 patients were included in the analysis of efficacy end points. Statistical analyses of clinical efficacy data were conducted with R version 2.14.0.
A PFS result was available for all patients included in the analysis, and sufficient data were available to group the results by pazopanib Cτ at week 4. After identifying the deciles of pazopanib Cτ at week 4, patients were split into two groups at each decile: those with a Cτ concentration less than or equal to the decile boundary and those with a Cτ concentration greater than the decile boundary. In the first analysis, the groups defined by the Cτ deciles were used to determine a threshold value for Cτ such that patients with Cτ below this threshold had statistically significant worse PFS compared with patients with Cτ above the threshold. In all, nine different values of Cτ were examined. At each Cτ decile tested, the PFS of patients in the two groups were compared using a log-rank test. The results were displayed graphically by plotting the log-rank P-value on the y axis vs each Cτ decile border on the x axis. The Cτ that produced the lowest P-value for the comparison of PFS between groups was considered to be a threshold value for further analysis. Responses (complete response, partial response, stable disease, progressive disease, and not evaluable or unknown response) in the two groups defined by the Cτ threshold were tested for independence using Fisher's exact test.
A similar analysis was conducted to identify a threshold value for Cτ such that mean tumour reduction in patients with Cτ below the threshold was significantly less than mean tumour shrinkage in patients with Cτ above the threshold. The maximum tumour shrinkage based on the sum of the longest diameters of target lesions after the start of treatment was identified for each patient. The mean maximum post-baseline tumour shrinkage in patients with Cτ above and below the deciles tested was compared using a t-test. No corrections for multiple testing were done in any analysis. All P-values reported are two sided with a significance level of 0.05.
Once the Cτ that yielded the most significant difference in PFS was identified, a Kaplan–Meier plot of the PFS for patients with Cτ above and below the threshold was generated. An empirical cumulative distribution function of the maximum tumour shrinkage relative to baseline for patients with Cτ values above and below the selected threshold value was constructed. For each group, these maximum tumour shrinkage values were ordered from largest to smallest tumour shrinkage post baseline, then plotted with the percentage of tumour shrinkage on the x axis and the percentage of patients with at least that amount of tumour shrinkage on the y axis.
Analyses were conducted to determine whether additional clinical benefit was observed at pazopanib Cτ values greater than the week 4 Cτ decile threshold that resulted in the most significant difference in PFS and mean maximum post-dose tumour shrinkage. Data from patients with week 4 Cτ values below the threshold selected based on differences in PFS or mean maximum tumour reduction were removed. The analyses of PFS and tumour reduction described above were repeated at each remaining Cτ decile boundary.
Results
Blood pressure in patients with solid tumours
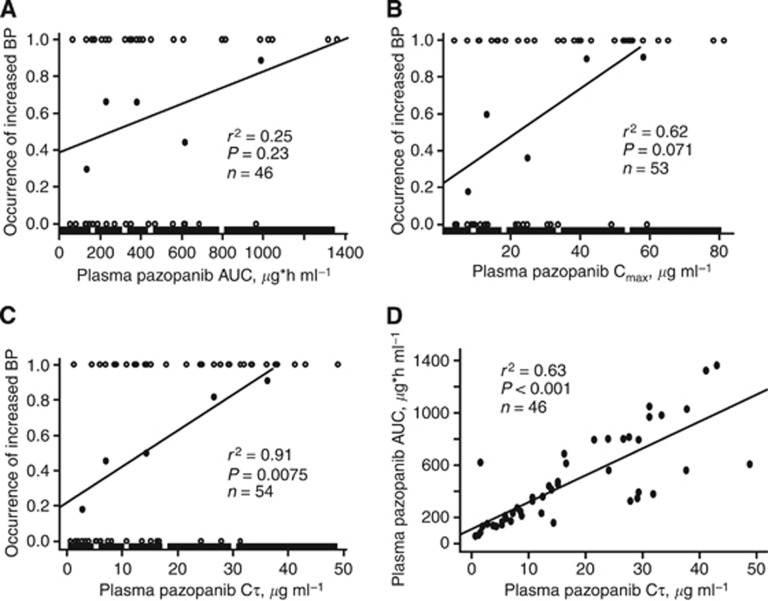
Plots of the occurrences of a significant increase in blood pressure vs AUC, Cmax, and Cτ are displayed in Figure 1A–C, respectively, for patients with solid tumours enroled in the Phase I study. The relationship between the occurrence of a significant increase in blood pressure and Cτ yielded the largest r2 value (0.91) and the lowest P-value (0.0075) of the pharmacokinetic parameters. A significant relationship between AUC and Cτ was observed (r2=0.63, P<0.001; Figure 1D).
Figure 1.
Pazopanib exposure and blood pressure. Relationship, in patients with solid tumours, between the occurrence of a significant increase in blood pressure (BP) and steady-state plasma pazopanib AUC (A), Cmax (B), and Cτ (C); relationship between steady-state AUC and Cτ (D). Open circles represent individual observations (0=no significant increase; 1=significant increase) and closed circles represent the proportion of patients with a significant increase in blood pressure within each plasma pazopanib Cτ quintile range. The thick line represents the plasma pazopanib Cτ quintile range.
Pharmacokinetics/pharmacodynamics in patients with advanced/metastatic RCC
Predose plasma pazopanib concentrations at 4 weeks and AE data were available for 205 of the 225 patients (91%) in the Phase II study. Longitudinal tumour size measurements sufficient to assess tumour shrinkage, and response rates were available from 197 patients (88%). Plasma concentrations of sVEGFR2 and PFS data were available from 178 and 177 patients (79%), respectively.
Efficacy
A summary of the PFS comparison at each week 4 pazopanib Cτ decile is presented in Table 1 (see also Supplementary Figure S1). The week 4 plasma pazopanib Cτ that yielded the most significant separation in median PFS was 17.4 μg ml−1. However, the greatest week 4 Cτ that yielded a significant separation in the median PFS was 20.5 μg ml−1. The week 4 Cτ boundaries of the 40th percentile (25.1 μg ml−1) and greater did not result in a significant separation of the median PFS. In the comparison of the median PFS of patients grouped by week 4 Cτ values, excluding patients with Cτ ⩽20.5 μg ml−1 resulted in no statistically significant difference in median PFS at any Cτ boundary.
Table 1. Comparison of progression-free survival at each week 4 plasma pazopanib Cτ decile in the overall study population and in the subset of patients with Cτ above 20.5 μg ml−1.
| |
Median PFS, weeks (n) |
|
|
|---|---|---|---|
| Week 4 threshold, μg ml−1 | Above threshold | Below threshold | P-value for difference |
|
Overall study population (N=177) | |||
| 12.5 | 43.7 (159) | 20.3 (18) | 0.035 |
| 17.4 | 49.4 (141) | 13.9 (36) | 0.00235 |
| 20.5 | 52.0 (124) | 19.6 (53) | 0.00378 |
| 25.1 | 52.0 (106) | 29.6 (71) | 0.0529 |
| 28.1 | 52.1 (88) | 29.6 (89) | 0.146 |
| 31.15 | 53.0 (71) | 35.3 (106) | 0.252 |
| 34.2 | 59.9 (53) | 31.7 (124) | 0.115 |
| 38.5 | 53.0 (36) | 38.4 (141) | 0.391 |
| 47.6 |
49.4 (18) |
39.3 (159) |
0.609 |
|
Excluding patients with Cτ ⩽20.5 μg ml−1 (n=124) | |||
| 25.1 | 52.0 (106) | 51.7 (18) | 0.760 |
| 28.1 | 52.1 (88) | 43.7 (36) | 0.799 |
| 31.1 | 53.0 (71) | 43.7 (53) | 0.779 |
| 34.2 | 59.9 (53) | 39.3 (71) | 0.519 |
| 38.5 | 53.0 (36) | 52.0 (88) | 0.962 |
| 47.6 | 49.4 (18) | 52.0 (106) | 0.940 |
Abbreviations: PFS=progression-free survival.
Cτ represents predose plasma pazopanib concentration at week 4.
The comparisons of mean maximum tumour reduction in patients with Cτ above and below each decile concentration at week 4 are presented in Table 2. The greatest difference in the mean maximum tumour reduction was observed at the week 4 plasma pazopanib Cτ of 20.5 μg ml−1. Unlike PFS results, the difference in mean maximum tumour reduction was statistically significant for all Cτ decile boundaries, except the 80th and 90th percentiles (38.5 and 47.6 μg ml−1, respectively). This was the result of including patients with Cτ ⩽20.5 μg ml−1 because there were no statistically significant differences in the maximum tumour reduction between patients grouped by Cτ decile values after exclusion of patients with week 4 Cτ ⩽20.5 μg ml−1 (Table 2).
Table 2. Mean tumour shrinkage in patients at each week 4 plasma pazopanib Cτ decile in the overall study population and in the subset of patients with Cτ above 20.5 μg ml−1.
| |
Mean tumour shrinkage, % |
|
||
|---|---|---|---|---|
| Threshold concentration, μg ml−1 | Above threshold | Below threshold | Difference | P-value for difference |
|
Overall study population (N=177) | ||||
| 12.5 | −31.7 | −2.96 | 28.7 | <0.001 |
| 17.4 | −34.5 | −4.85 | 29.7 | <0.001 |
| 20.5 | −37.9 | −6.86 | 31.0 | <0.001 |
| 25.1 | −37.5 | −16.0 | 21.5 | <0.001 |
| 28.1 | −39.0 | −18.6 | 14.9 | <0.001 |
| 31.15 | −39.8 | −21.3 | 18.5 | <0.001 |
| 34.2 | −40.2 | −24.0 | 16.2 | 0.0020 |
| 38.5 | −36.0 | −27.1 | 8.9 | 0.136 |
| 47.6 |
−31.0 |
−28.7 |
2.3 |
0.693 |
|
Excluding patients with Cτ ⩽20.5 μg ml−1 (n=124) | ||||
| 25.4 | −37.5 | −40.5 | −3 | 0.581 |
| 28.1 | −39.0 | −35.2 | 3.8 | 0.460 |
| 30.5 | −39.7 | −35.4 | 4.3 | 0.381 |
| 33.7 | −40.2 | −36.2 | 4.0 | 0.457 |
| 37.9 | −36.0 | −38.7 | −2.7 | 0.654 |
| 47.3 | −31.2 | −39.2 | −8.0 | 0.181 |
Cτ represents predose plasma pazopanib concentration at week 4.
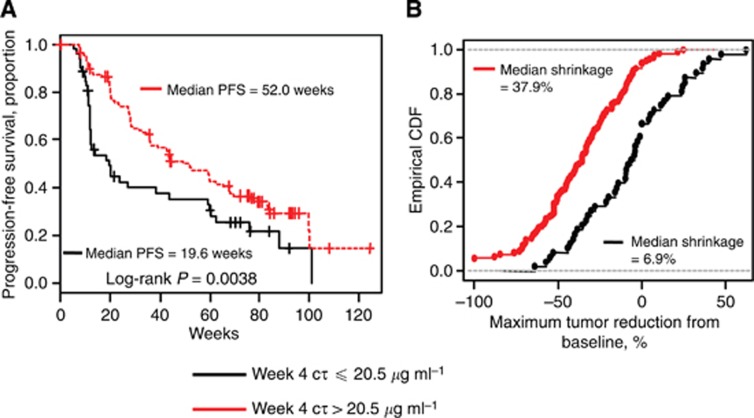
The week 4 Cτ of 20.5 μg ml−1 was the greatest value that significantly discriminated median PFS and mean maximum tumour reduction. Therefore, the week 4 Cτ of 20.5 μg ml−1 was used as the threshold for further analyses. The Kaplan–Meier curves of PFS and the empirical distribution function of the change in tumour size for patients with a week 4 Cτ ⩽20.5 μg ml−1 and Cτ above 20.5 μg ml−1 are displayed in Figure 2. The median PFS for patients with a week 4 Cτ ⩽20.5 μg ml−1 was 19.6 weeks. In contrast, the median PFS for patients with a week 4 Cτ above 20.5 μg ml−1 was 52.0 weeks. Median observed tumour shrinkage in patients with a week 4 Cτ above 20.5 μg ml−1 (37.9%) was more than five-fold greater than the median observed tumour shrinkage in patients with a week 4 Cτ ⩽20.5 μg ml−1 (6.9%).
Figure 2.
Steady-state plasma pazopanib concentration and clinical response. The Kaplan–Meier curves showing PFS (A) and empirical cumulative distribution function (CDF) of maximum decrease from baseline in tumour size (B) for patients with week 4 predose steady-state plasma pazopanib concentration (Cτ) ⩽20.5 μg ml−1 (black) and >20.5 μg ml−1 (red).
The percentage of patients who experienced stable disease as their best response was 40% for those with a week 4 Cτ ⩽20.5 μg ml−1 and those with a week 4 Cτ above 20.5 μg ml−1. However, the percentage of patients with a week 4 Cτ above 20.5 μg ml−1 who had a best response of partial or complete response was more than double that of patients with a week 4 Cτ ⩽20.5 μg ml−1 (45 and 19%, respectively). Likewise, the percentage of patients with progressive disease was more than double when week 4 Cτ was ⩽20.5 μg ml−1 (38%) compared with patients with a week 4 Cτ above 20.5 μg ml−1 (11%).
sVEGFR2
Plasma sVEGFR2 concentrations decreased at least 10% from the baseline value to the nadir in 167 of 178 patients (94%) from whom data were available. The fit of the linear and Emax models to the percent change from baseline in sVEGFR2 nadir vs plasma pazopanib concentration is displayed in Supplementary Figure S2. The correlation between plasma pazopanib concentrations and the maximum decrease in sVEGFR2 was weak using the linear model (r2=0.27). However, the linear model produced a slightly lower Akaike information criterion relative to the fit of the Emax model to the data (1415 vs 1417) suggesting a slightly better fit of the data.
Adverse events
A summary of the most common (total incidence ⩾5%) treatment-emergent AEs by plasma pazopanib concentration quartile is presented in Table 3. The incidence of increased MAP, diarrhoea, hair colour change, alanine aminotransferase increase, stomatitis, and hand–foot syndrome increased as the plasma pazopanib concentrations increased, with the highest incidence occurring in the fourth Cτ quartile. The highest incidence of CTCAE grade 3/4 hypertension occurred in the third and fourth quartiles of Cτ. The increases in the incidences of hand–foot syndrome and MAP in the fourth quartile of Cτ were the most marked. In contrast, the incidence of nausea, fatigue, vomiting, dysgeusia, and rash displayed no obvious relationship with pazopanib Cτ.
Table 3. Frequency of adverse events by week 4 pazopanib Cτ quartile (n=205).
| |
Pazopanib concentration range,
μg ml−1 |
|||
|---|---|---|---|---|
| Adverse events, all grades % (grade 3/4)a | 0.8 to ⩽19 | >19 to ⩽28 | 28 to ⩽36 | 36 to ⩽85 |
| Hypertensiona | 27 (2) | 33 (8) | 38 (10) | 35 (10) |
| Increased MAPb | 38 | 65 | 67 | 78 |
| Diarrhoea | 24 (0) | 49 (0) | 65 (0) | 67 (2) |
| Hair colour change | 18 (0) | 37 (0) | 37 (0) | 51 (0) |
| Alanine aminotransferase increase | 8 (4) | 14 (6) | 15 (12) | 16 (4) |
| Hand–foot syndrome | 0 | 4 (2) | 6 (0) | 24 (6) |
| Stomatitis | 0 | 4 (0) | 2 (0) | 10 (0) |
| Nausea | 35 (2) | 37 (0) | 31 (2) | 41 (0) |
| Fatigue | 25 (2) | 41 (4) | 35 (4) | 31 (2) |
| Vomiting | 14 (0) | 14 (0) | 13 (2) | 18 (0) |
| Dysgeusia | 10 (0) | 27 (0) | 17 (0) | 25 (0) |
| Rash | 10 (2) | 10 (0) | 15 (0) | 8 (2) |
Abbreviation: MAP=mean arterial blood pressure.
Graded according to Common Terminology Criteria for Adverse Events (CTCAE; MAP was not graded by CTCAE).
MAP increase ⩾15 mm Hg on ⩾3 separate occasions, initiation or escalation of antihypertensive medications, or both.
Pharmacokinetics
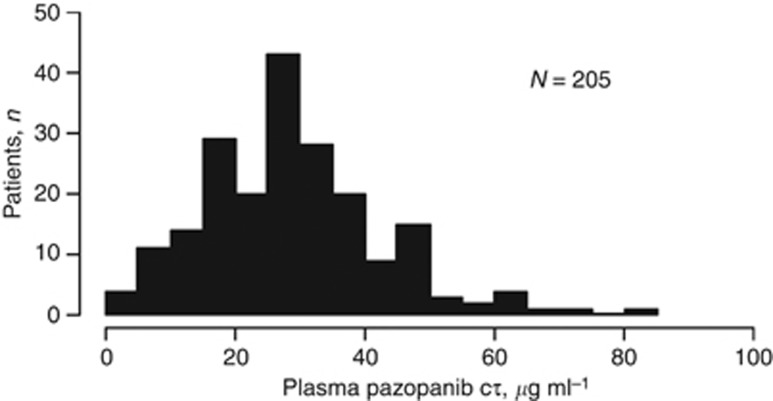
The mean and median Cτ values on week 4 in the Phase II study were 28.8 and 28.1 μg ml−1, respectively (Figure 3). Dose reductions were allowed in this study. In all, 184 of the 205 patients with week 4 Cτ data received pazopanib 800 mg once daily for at least 2 weeks; the mean week 4 Cτ value in those patients was 29.3 μg ml−1. For patients who experienced dose reductions or dose interruptions within 2 weeks of collection of the week 4 plasma sample, the mean week 4 Cτ value was 24.8 μg ml−1.
Figure 3.
Distribution of predose steady-state plasma pazopanib concentrations (Cτ).
Discussion
Results of the current analysis identified a threshold for pazopanib Cτ, which must be achieved for optimal efficacy in patients with RCC. This target pazopanib Cτ is similar to the threshold steady-state concentration of at least 40 μM (17.5 μg ml−1) that was required for the inhibition of VEGF-induced VEGFR2 phosphorylation in mouse lungs. Cτ measured immediately before pazopanib administration is assumed to be the lowest plasma concentration over the dosing interval. Therefore, these results suggest that maintenance of plasma pazopanib concentrations above 20.5 μg ml−1 for the entire dosing interval was associated with the most significant increases in PFS and tumour shrinkage. This hypothesis is supported by the observation that pazopanib Cτ was better than Cmax as a predictor of the occurrence of an increase in blood pressure in patients with solid tumours. Furthermore, Mancuso et al (2006) observed rapid vascular regrowth in a tumour cell line on interruption of VEGF inhibition, and Cacheux et al (2008) observed rapid regrowth of tumours in patients with metastatic colorectal cancer after interruption of bevacizumab therapy. In a study reported by Burstein (Burstein et al, 2008), levels of biomarkers of VEGFR inhibition (VEGF, sVEGFR2, sVEGFR3) were cyclical and coincident with the schedule of sunitinib used in the study (4 weeks on, 2 weeks off). Moreover, tumour growth of superficial cutaneous or nodal lesions was observed in several patients during the 2-week period when no sunitinib was administered. Together, these results suggest that maintaining persistent suppression of the targeted signalling pathway may lead to better efficacy of these agents.
However, administration of sunitinib at the standard intermittent regimen of 50 mg daily for 4 weeks followed by 2 weeks without treatment produced a numerically greater median PFS vs 37.5 mg daily administered on a continuous basis (8.5 vs 7.0 months; hazard ratio 0.77; P=0.070) in a randomized study in patients with advanced RCC (Motzer et al, 2012). The incidence and severity of AEs were similar between the continuous and intermittent regimens. These results suggest that the level of suppression of the target receptor achieved by administration of lower daily doses of sunitinib did not provide an advantage in clinical outcomes vs intermittent administration of higher doses. Although alternative dosing regimens of pazopanib were not investigated in the present study, results suggest that the clinical benefit of pazopanib in RCC could be improved by increasing the proportion of patients with trough plasma pazopanib concentrations above a target threshold.
A concentration-dependent increase in the incidence of several AEs was also observed during the Phase II trial (Table 3; Supplementary Figure S3). Some toxicities such as hypertension and alanine aminotransferase elevations appeared to reach a plateau at higher concentrations. In contrast to hypertension, alanine aminotransferase elevations, and the results for measures of clinical efficacy, the incidence of diarrhoea, hand–foot syndrome, hair colour change, and stomatitis continued to increase as the trough plasma pazopanib concentration increased. There was no evidence of additional increases in PFS or tumour shrinkage if trough plasma pazopanib concentrations were maintained above threshold values >20.5 μg ml−1. In addition to suggesting that escalation to a maximally tolerated dose of targeted agents such as pazopanib may not be necessary, these results reinforce the previous inference that increasing the dose of a targeted agent to a maximally tolerated level may only serve to affect the risk-to-benefit ratio negatively.
Dose reductions to 400 mg pazopanib occurred in ∼30% of patients with RCC in the Phase II study. The most common AEs that led to dose interruptions or dose reductions were diarrhoea, hypertension, and increased ALT/AST (Hutson et al, 2010). All of those AEs demonstrated a concentration-dependent increase in frequency in the current analysis. Results suggest that the frequency of these common AEs could be reduced by decreasing systemic exposure to pazopanib through dose reductions or interruptions while maintaining plasma pazopanib concentrations above the threshold for optimal clinical effect in many patients.
The pazopanib dose of 800 mg once daily was selected for Phase II and Phase III clinical trials based on target trough plasma concentrations that were associated with clinical and biologic effects in preclinical models and in patients with solid tumours (Kumar et al, 2007; Hurwitz et al, 2009). However, inter-patient heterogeneity in the metabolism and/or absorption of pazopanib can lead to differences in systemic exposure. Pazopanib is a Biopharmaceutics Classification System Class II drug, with high permeability and low solubility. The majority of an oral pazopanib dose is eliminated in the faeces, primarily as an unabsorbed drug, which is consistent with solubility-limited oral absorption. Pazopanib is a substrate of human p-glycoprotein (Pgp) and breast cancer resistance protein (BCRP; Deng et al, 2013). Differences in the expression of Pgp and BCRP may contribute to the intra-patient variability in systemic exposure to pazopanib after oral administration. Administration of esomeprazole in the evening and pazopanib in the morning resulted in 42 and 40% decreases in Cmax and AUC, respectively (Tan et al, 2013). Administration of pazopanib with food increased Cmax and AUC by approximately two-fold (Heath et al, 2010). Use of medications that alter gastric pH or administration in a partially fasted state also may contribute to the inter- and intra-patient variability of pazopanib pharmacokinetics.
In light of that variability and the relationship between systemic exposure and clinical benefit and toxicities, dose adjustments based on trough plasma concentrations may provide a method to optimise therapy with pazopanib by maintaining therapeutically effective blood levels, while minimising AEs whenever possible. Dose adjustment concurrent with therapeutic drug monitoring is widely used for compounds with a narrow therapeutic index, such as digoxin, antiepileptic agents, aminoglycosides, and immunosuppressants; this approach also has been recommended in certain situations for antiretroviral medications, where consistent target suppression is necessary (Pretorius et al, 2011). Use of a trough concentration may provide a convenient method of monitoring systemic exposure to pazopanib in a clinical setting, thereby allowing optimisation of the dosing regimen to gain maximum efficacy. Algorithms for therapeutic drug monitoring of tyrosine kinase inhibitors have been proposed (Haouala et al, 2009; Wang et al, 2009; Klumpen et al, 2011). Results from the present analysis indicate that plasma pazopanib concentrations obtained early (week 4) in the treatment period were predictors of effects consistent with VEGFR inhibition (increased blood pressure and decreases in sVEGFR2), clinical benefit, and the incidence of AEs. Recent improvements in bioanalytical detection have allowed the analysis of drug concentrations from small volumes (<20 μl) of dried blood, making therapeutic monitoring of pazopanib concentrations in blood more feasible (Emmons and Rowland, 2010).
Further clinical studies are necessary to confirm the target threshold for pazopanib Cτ and assess the effects of pharmacokinetics-guided dosing vs standard pazopanib treatment. Strategies to optimise systemic exposure of pazopanib, such as dose escalation above 800 mg once daily or administration of pazopanib with food in patients with concentrations below the target threshold, must be investigated in a prospective fashion in order for pharmacokinetics-guided dosing to be feasible. Finally, the safety and efficacy of pharmacokinetics-guided dosing relative to the standard dosing regimen for pazopanib should be determined in a randomized clinical trial.
Acknowledgments
We thank Jerome F Sah, PhD, ProEd Communications, Inc., for his medical editorial assistance with this manuscript. Financial support for this study (Study VEG105192; clinicaltrials.gov identifier NCT00334282) and medical editorial assistance was provided by GlaxoSmithKline Pharmaceuticals, Philadelphia, Pennsylvania.
CC, SS, RA, and LP are employees and stockholders of GlaxoSmithKline. ABS, HAB, YL, and DR are former employees of GlaxoSmithKline and hold company stock. MM has been a consultant or advisory board consultant for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Mundipharma, Novartis, Stallergenes, and Pfizer. TEH has been a consultant, paid researcher, and participant in advisory boards or speaker's bureaus for Pfizer, GlaxoSmithKline, Novartis, AVEO, Johnson & Johnson, and Dendreon.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Previous Publication: Portions of the data were presented at the 2010 ASCO Annual Meeting (J Clin Oncol 28:15s, 2010, abstract 3048).
Supplementary Material
References
- Australian Government Department of Health and Ageing 2010. Drugs designated as orphan drugs: Pazopanib (PATORMA) http://www.tga.gov.au/industry/pm-orphan-drugs.htm .
- Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26 (11:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- Cacheux W, Boisserie T, Staudacher L, Vignaux O, Dousset B, Soubrane O, Terris B, Mateus C, Chaussade S, Goldwasser F. Reversible tumor growth acceleration following bevacizumab interruption in metastatic colorectal cancer patients scheduled for surgery. Ann Oncol. 2008;19 (9:1659–1661. doi: 10.1093/annonc/mdn540. [DOI] [PubMed] [Google Scholar]
- Deng Y, Sychterz C, Suttle AB, Dar MM, Bershas D, Negash K, Qian Y, Chen EP, Gorycki PD, Ho MY. Bioavailability, metabolism and disposition of oral pazopanib in patients with advanced cancer. Xenobiotica. 2013;43 (5:443–453. doi: 10.3109/00498254.2012.734642. [DOI] [PubMed] [Google Scholar]
- Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, Loos WJ, Wiemer EA, Mathijssen RH. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res. 2011;17 (3:406–415. doi: 10.1158/1078-0432.CCR-10-2250. [DOI] [PubMed] [Google Scholar]
- Emmons G, Rowland M. Pharmacokinetic considerations as to when to use dried blood spot sampling. Bioanalysis. 2010;2 (11:1791–1796. doi: 10.4155/bio.10.159. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency 2010. Votrient (pazopanib) authorisation details. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001141/human_med_001337.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124&jsenabled=true .
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24 (1:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, Ris HB, Leyvraz S, Widmer N, Decosterd LA. Therapeutic Drug Monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877 (22:1982–1996. doi: 10.1016/j.jchromb.2009.04.045. [DOI] [PubMed] [Google Scholar]
- Harris PA, Boloor A, Cheung M, Kumar R, Crosby RM, Davis-Ward RG, Epperly AH, Hinkle KW, Hunter RN, 3rd, Johnson JH, Knick VB, Laudeman CP, Luttrell DK, Mook RA, Nolte RT, Rudolph SK, Szewczyk JR, Truesdale AT, Veal JM, Wang L, Stafford JA. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-m ethyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. 2008;51 (15:4632–4640. doi: 10.1021/jm800566m. [DOI] [PubMed] [Google Scholar]
- Heath EI, Chiorean EG, Sweeney CJ, Hodge JP, Lager JJ, Forman K, Malburg L, Arumugham T, Dar MM, Suttle AB, Gainer SD, LoRusso P. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88 (6:818–823. doi: 10.1038/clpt.2010.199. [DOI] [PubMed] [Google Scholar]
- Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66 (2:357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Douglas PS, Middleton JP, Sledge GW, Johnson DH, Reardon DA, Chen D, Rosen O. Analysis of early hypertension (HTN) and clinical outcome with bevacizumab (BV) J Clin Oncol. 2010;28 (15s:abstr 3039. doi: 10.1634/theoncologist.2012-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, Hodge JP, Merkle EM, Pandite L. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15 (12:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, Epstein RJ, Baker KL, McCann L, Crofts T, Pandite L, Figlin RA. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28 (3:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- Instituto de Salud Publica de Chile 2010. Votrient coated tablets [product registration]. http://200.68.11.21/RegistrosISP/fiFichaProducto.asp?RegistroISP=F-18018/10 .
- Janvier A, Nadeau S, Baribeau J, Perreault T. Role of vascular endothelial growth factor receptor 1 and vascular endothelial growth factor receptor 2 in the vasodilator response to vascular endothelial growth factor in the neonatal piglet lung. Crit Care Med. 2005;33 (4:860–866. doi: 10.1097/01.ccm.0000159563.97092.a7. [DOI] [PubMed] [Google Scholar]
- Kidney Cancer Canada 2010. Votrient (pazopanib) approved by Health Canada for the treatment of renal cell carcinoma. http://www.kidneycancercanada.org/main.php?p=600&lan=1 .
- Klumpen HJ, Samer CF, Mathijssen RH, Schellens JH, Gurney H. Moving towards dose individualization of tyrosine kinase inhibitors. Cancer Treat Rev. 2011;37 (4:251–260. doi: 10.1016/j.ctrv.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, Gilmer TM, Truesdale AT, Epperly AH, Boloor A, Stafford JA, Luttrell DK, Cheung M. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6 (7:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- Li B, Ogasawara AK, Yang R, Wei W, He GW, Zioncheck TF, Bunting S, de Vos AM, Jin H. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension. 2002;39 (6:1095–1100. doi: 10.1161/01.hyp.0000018588.56950.7a. [DOI] [PubMed] [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116 (10:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, Wilding G, Agarwal N, Thompson JA, Cella D, Bello A, Korytowsky B, Yuan J, Valota O, Martell B, Hariharan S, Figlin RA. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30 (12:1371–1377. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24 (1:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- Pretorius E, Klinker H, Rosenkranz B. The role of therapeutic drug monitoring in the management of patients with human immunodeficiency virus infection. Ther Drug Monit. 2011;33 (3:265–274. doi: 10.1097/FTD.0b013e31821b42d1. [DOI] [PubMed] [Google Scholar]
- Rini BI. Biomarkers: hypertension following anti-angiogenesis therapy. Clin Adv Hematol Oncol. 2010;8 (6:415–416. [PubMed] [Google Scholar]
- Roodhart JM, Langenberg MH, Witteveen E, Voest EE. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3 (2:132–143. doi: 10.2174/157488408784293705. [DOI] [PubMed] [Google Scholar]
- Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20 (2:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Hutson TE, Sternberg CN. Pazopanib, a potent orally administered small-molecule multitargeted tyrosine kinase inhibitor for renal cell carcinoma. Expert Opin Investig Drugs. 2008;17 (2:253–261. doi: 10.1517/13543784.17.2.253. [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28 (6:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- Tan AR, Gibbon DG, Stein MN, Lindquist D, Edenfield JW, Martin JC, Gregory C, Suttle AB, Tada H, Botbyl J, Stephenson JJ. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71 (6:1635–1643. doi: 10.1007/s00280-013-2164-3. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92 (3:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Food & Drug Administration 2009. Pazopanib [approval notice] ( ( http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm187509.htm .
- Wang Y, Chia YL, Nedelman J, Schran H, Mahon FX, Molimard M. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31 (5:579–584. doi: 10.1097/FTD.0b013e3181b2c8cf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.