Abstract
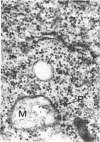
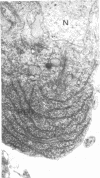
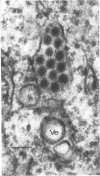
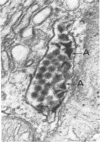
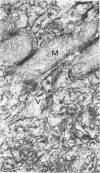
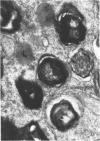
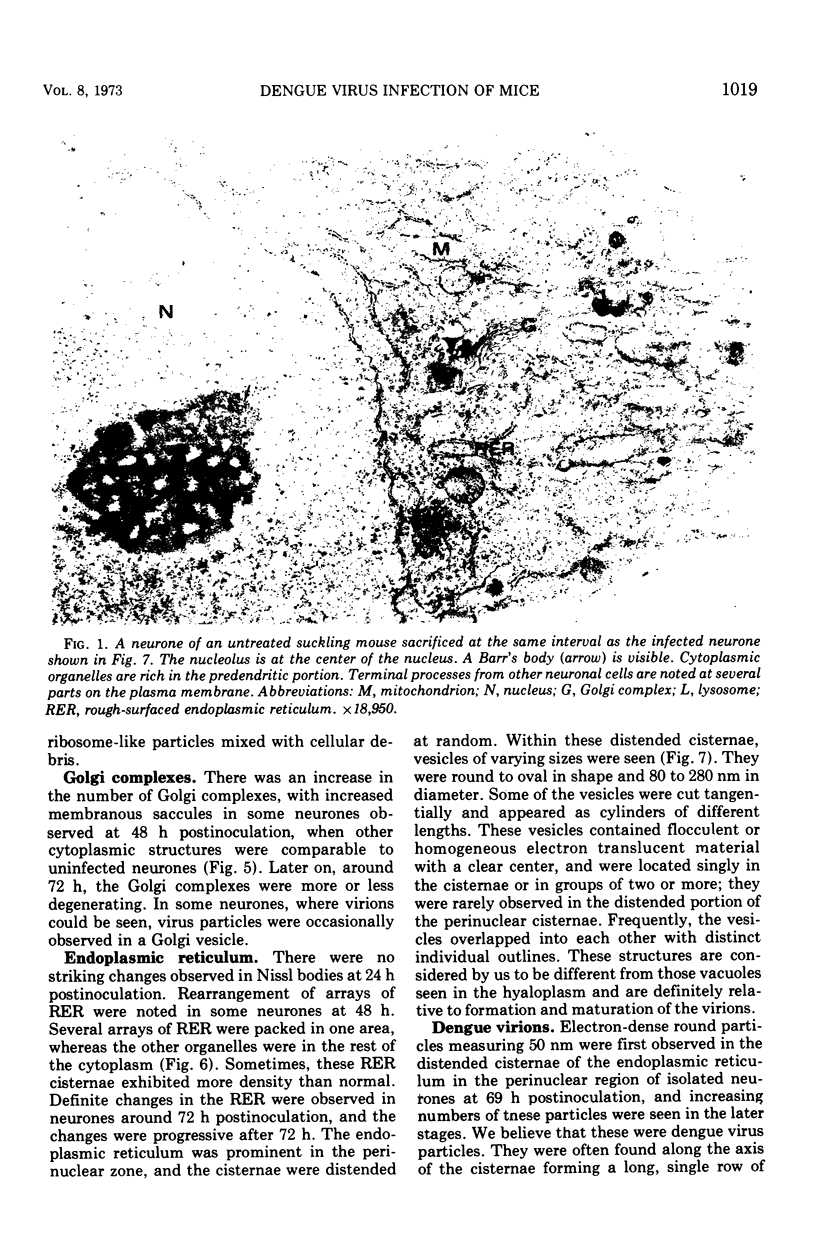
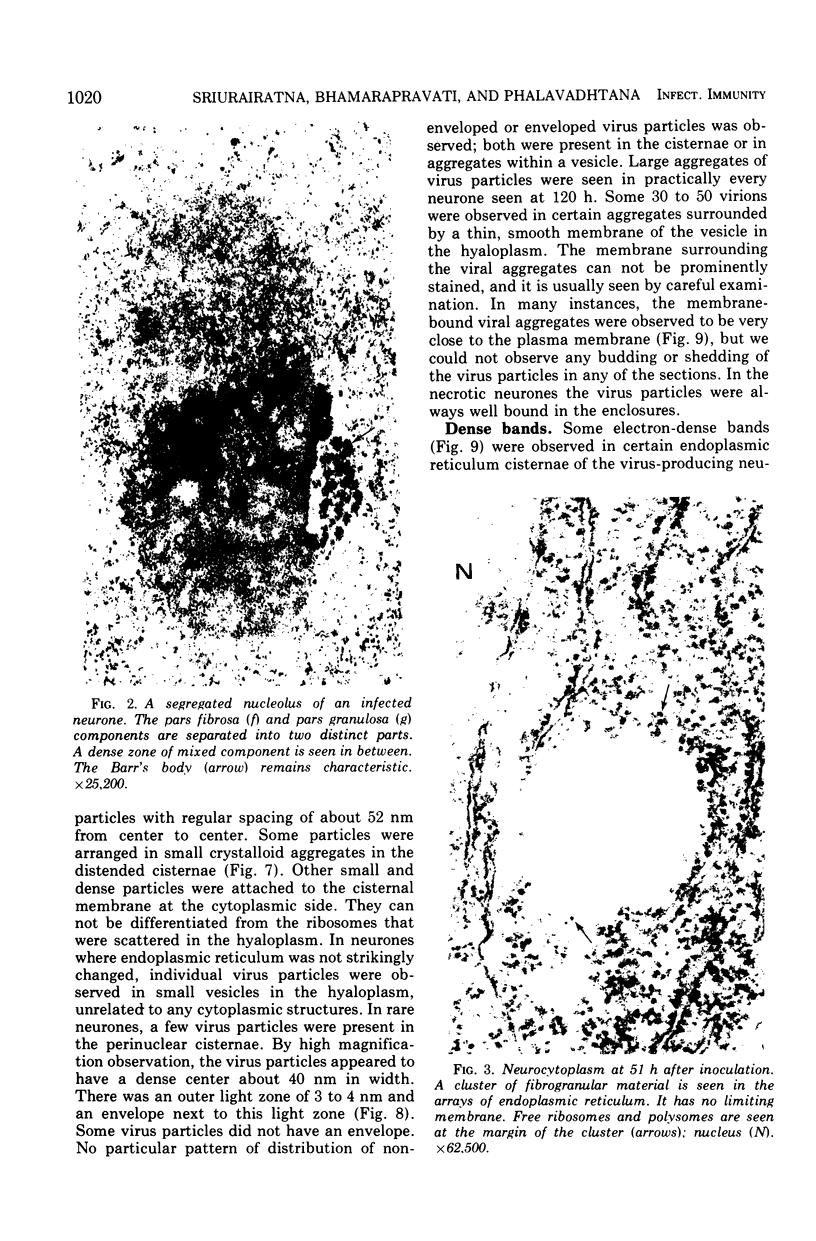
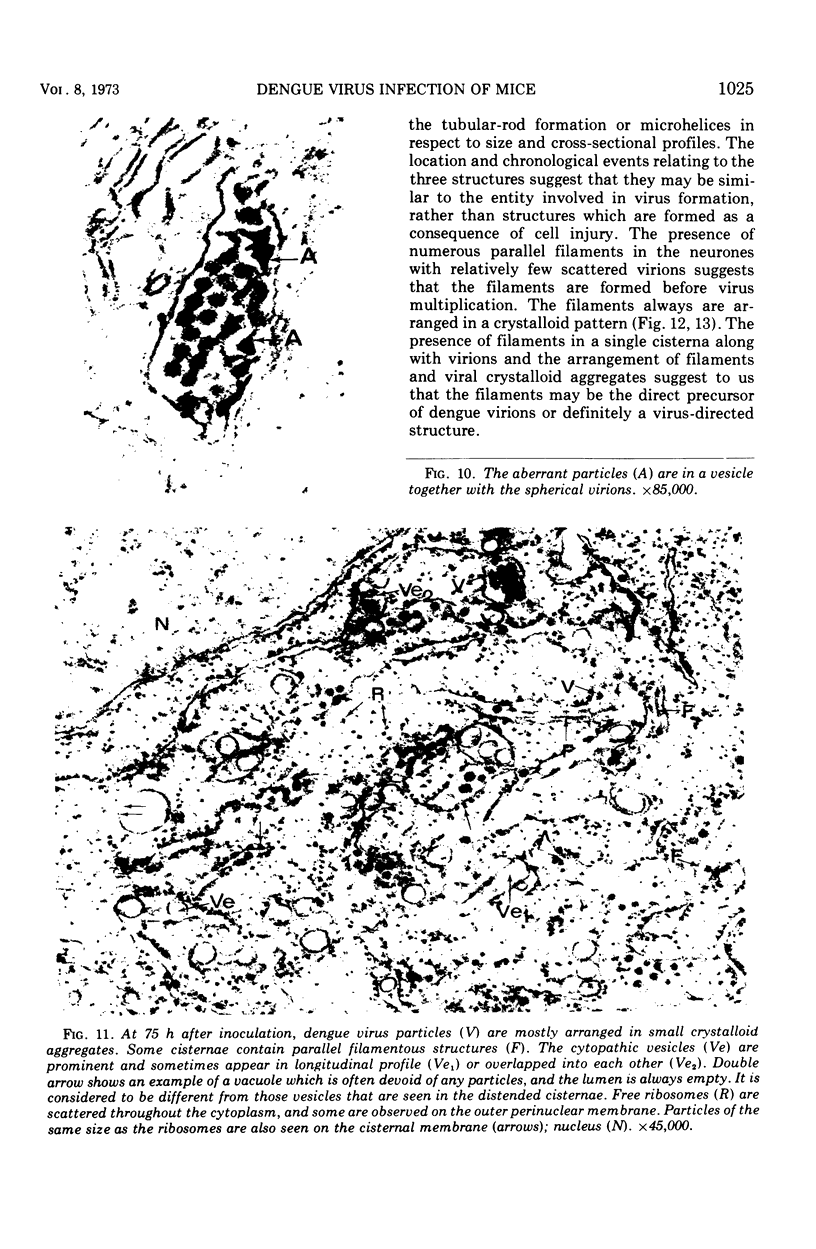
In dengue type-2 virus-infected neurones of suckling mice, formation of single-membrane vesicles is observed in the distended cisternae of the endoplasmic reticulum mostly of the perinuclear zone around 72 h after inoculation. Electron-dense 50-nm virus particles are arranged in chains in these distended cisternae; some form small crystalloid aggregates. Aberrant particles of different shapes are also seen in the distended cisternae about the same time that the virus particles appear. Parallel filamentous structures are occasionally observed in the cisternae that contain very few virions, either characteristic or aberrant. Increasing cytopathic changes are present after 75 to 96 h. There is an intense vesicular formation. Large numbers of virions and aberrant particles are seen either in the endoplasmic reticulum cisternae or smooth membrane vesicles. They are spread throughout the neurocytoplasm, extending into the dendrites. Dengue virions which are enclosed in fairly intact membrane-bound vesicles are released during cytolysis of the neurones. Morphogenesis of dengue virus type 2 is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Atchison R. W., Ordonez J. V., Sather G. E., Hammon W. M. Fluorescent antibody, complement fixation method for detection of dengue viruses in mice. J Immunol. 1966 Jun;96(6):936–943. [PubMed] [Google Scholar]
- BARUCH E. ELECTRON MICROSCOPIC STUDY OF SPINAL CORD OF MICE INFECTED WITH YELLOW FEVER VIRUS. J Ultrastruct Res. 1963 Oct;59:209–224. doi: 10.1016/s0022-5320(63)80003-9. [DOI] [PubMed] [Google Scholar]
- BEARCROFT W. G. Electron-microscope studies on the liver cells of yellow-fever-infected rhesus monkeys. J Pathol Bacteriol. 1960 Oct;80:421–426. [PubMed] [Google Scholar]
- BHAMARAPRAVATI N., HALSTEAD S. B., SOOKAVACHANA P., BOONYAPAKNAVIK V. STUDIES ON DENGUE VIRUS INFECTION. 1. IMMUNOFLUORESCENT LOCALIZATION OF VIRUS IN MOUSE TISSUE. Arch Pathol. 1964 May;77:538–543. [PubMed] [Google Scholar]
- Blinzinger K., Müller W., Anzil A. P. Microhelices in the endoplasmic reticulum of murine neurons infected with a group B arbovirus. Arch Gesamte Virusforsch. 1971;35(2):194–202. doi: 10.1007/BF01249710. [DOI] [PubMed] [Google Scholar]
- Brandt W. E., Cardiff R. D., Russell P. K. Dengue virions and antigens in brain and serum of infected mice. J Virol. 1970 Oct;6(4):500–506. doi: 10.1128/jvi.6.4.500-506.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Russ S. B., Brandt W. E., Russell P. K. Cytological localization of Dengue-2 antigens: an immunological study with ultrastructural correlation. Infect Immun. 1973 May;7(5):809–816. doi: 10.1128/iai.7.5.809-816.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain M. M., Doane F. W., McLean D. M. Morphological development of Chikungunya virus. Can J Microbiol. 1966 Oct;12(5):895–900. doi: 10.1139/m66-122. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., Babcock V. I., Southam C. M., Bailey R. B., Shipkey F. H. Semliki Forest virus in HEp-2 cell cultures. J Virol. 1967 Oct;1(5):996–1009. doi: 10.1128/jvi.1.5.996-1009.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filshie B. K., Rehacek J. Studies of the morphology of Murray Valley encephalitis and Japanese encephalitis viruses growing in cultured mosquito cells. Virology. 1968 Mar;34(3):435–443. doi: 10.1016/0042-6822(68)90063-9. [DOI] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Friedman R. M. Development of Semliki forest virus in mouse brain: an electron microscopic study. Exp Mol Pathol. 1970 Feb;12(1):1–13. doi: 10.1016/0014-4800(70)90070-5. [DOI] [PubMed] [Google Scholar]
- Higashi N., Matsumoto A., Tabata K., Nagatomo Y. Electron microscope study of development of Chikungunya virus in green monkey kidney stable (VERO) cells. Virology. 1967 Sep;33(1):55–69. doi: 10.1016/0042-6822(67)90093-1. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. T. VIRUS INVASION OF THE CENTRAL NERVOUS SYSTEM: A STUDY OF SINDBIS VIRUS INFECTION IN THE MOUSE USING FLUORESCENT ANTIBODY. Am J Pathol. 1965 Jun;46:929–943. [PMC free article] [PubMed] [Google Scholar]
- Janzen H. G., Rhodes A. J., Doane F. W. Chikungunya virus in salivary glands of Aedes aegypti (L.): an electron microscope study. Can J Microbiol. 1970 Jul;16(7):581–586. doi: 10.1139/m70-097. [DOI] [PubMed] [Google Scholar]
- Kitaoka M., Shimizu A., Tuchinda P., Kim-Anake C. Electron microscopic observations on dengue type 2 virus. Biken J. 1971 Sep;14(3):361–364. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic and biological studies on the growth of Venezuelan equine encephalitis virus in KB cells. Virology. 1962 Jan;16:52–62. doi: 10.1016/0042-6822(62)90201-5. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Stollar V., Schlesinger R. W. Studies on the nature of dengue viruses. V. Structure and development of dengue virus in Vero cells. Virology. 1971 Nov;46(2):344–355. doi: 10.1016/0042-6822(71)90036-5. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Gary G. W., Jr, Whitfield S. G., Forrester F. T. St. Louis encephalitis virus infection in mice. Electron microscopic studies of central nervous system. Lab Invest. 1968 Dec;19(6):652–662. [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Tzianabos T. Electron microscopic observations of mouse brain infected with Bunyamwera group arboviruses. J Virol. 1968 Nov;2(11):1315–1325. doi: 10.1128/jvi.2.11.1315-1325.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTA Z. ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS VIRUS IN PORCINE KIDNEY STABLE (PS) CELLS. Virology. 1965 Mar;25:372–378. doi: 10.1016/0042-6822(65)90057-7. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTHAM C. M., SHIPKEY F. H., BABCOCK V. I., BAILEY R., ERLANDSON R. A. VIRUS BIOGRAPHIES. I. GROWTH OF WEST NILE AND GUAROA VIRUSES IN TISSUE CULTURE. J Bacteriol. 1964 Jul;88:187–199. doi: 10.1128/jb.88.1.187-199.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalova N. M., Reingold V. N., Gagarina A. V., Kornilova E. A., Popov G. V., Chumakov M. P. Electron microscopic study of the central nervous system in mice infected by Omsk hemorrhagic fever (OHF) virus. Virus reproduction in cerebellum neurons. J Ultrastruct Res. 1972 Sep;40(5):458–469. doi: 10.1016/s0022-5320(72)80035-2. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Brandt W. E., Swanson J. L., McCown J. M., Buescher E. L. Physical and biological properties of dengue-2 virus and associated antigens. J Virol. 1970 Apr;5(4):524–532. doi: 10.1128/jvi.5.4.524-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Tan K. B. Electron microscopy of cells infected with Semliki forest virus temperature-sensitive mutants: correlation of ultrastructural and physiological observations. J Virol. 1970 May;5(5):632–638. doi: 10.1128/jvi.5.5.632-638.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASUZUMI G., TSUBO I. ANALYSIS OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS (JBE) VIRUS. II. ELECTRON MICROSCOPE STUDIES OF NEURONS INFECTED WITH JBE VIRUS. J Ultrastruct Res. 1965 Apr;12:304–316. doi: 10.1016/s0022-5320(65)80101-0. [DOI] [PubMed] [Google Scholar]