Abstract
Background: Increasing data suggest that subclinical hypothyroidism (SCH) and thyroid autoimmunity (TAI) are associated with adverse pregnancy outcomes, but there are limited data on the association of these conditions in early pregnancy with subsequent miscarriage.
Methods: In this prospective cohort study, we screened 3315 women at low risk for thyroid dysfunction at four to eight weeks' gestation from iodine-sufficient areas of China between January 2012 and September 2012. Thyrotropin (TSH), free thyroxine (fT4), and the autoantibodies thyroid-peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) were measured. Based on these results, women were divided into four groups for comparison: euthyroidism (ET), isolated SCH, isolated TAI (positive TPOAb or/and TgAb), and SCH with TAI (SCH+TAI). The SCH group was stratified into two subgroups (SCH 1 and SCH 2) on the basis of the level of TSH (2.5≤TSH<5.22 or 5.22≤TSH<10 respectively). Accordingly, the SCH+TAI group was also stratified into two subgroups (SCH+TAI 1 and SCH+TAI 2). The outcome of interest was miscarriage, defined as spontaneous pregnancy loss prior to 20 weeks.
Results: Compared to women with ET, the risk of miscarriage was significantly higher among women with SCH 2 (7.1% vs. 2.2%, aOR 3.40 [CI 1.62–7.15]; p=0.002), isolated TAI (5.7% vs. 2.2%, aOR 2.71 [CI 1.43–5.12]; p=0.003), SCH+TAI 1 (10.0% vs. 2.2%, aOR 4.96 [CI 2.76–8.90]; p=0.000), and SCH+TAI 2 (15.2% vs. 2.2%, aOR 9.56 [CI 3.76–24.28]; p=0.000). The gestational ages of 110 women at miscarriage were lower among women with subclinical thyroid abnormalities compared to ET (11.13±3.21 weeks with subclinical thyroid abnormalities vs. 9.33±1.71 weeks with ET; p=0.024). In parallel with the higher TSH levels, there were earlier gestation ages at miscarriage between subgroups of SCH and SCH+TAI (SCH 1 vs. SCH 2: 10.79±1.77 vs. 9.70±1.47 weeks, p=0.039; SCH+TAI 1 vs. SCH+TAI 2: 9.59±1.97 vs. 8.88±1.24 weeks, p=0.031).
Conclusions: Women with SCH and TAI are at an increased risk of miscarriage between four and eight gestational weeks. Women with a combination of SCH and TAI were found to have the highest risk and earlier gestational ages of miscarriage.
Introduction
Over the past two decades, the association between subclinical hypothyroidism (SCH), thyroid autoimmunity (TAI), and adverse obstetric outcomes has been hotly debated in the domains of endocrinology, obstetrics and gynecology, and perinatology (1–6). SCH occurs in 3–7% of pregnant women, and it is often ignored due to the lack of characteristic clinical symptoms (7,8). Recent studies have reported that SCH in pregnancy was associated with miscarriage (2–5,9), and higher maternal TSH levels have been detected in women who miscarried when compared to unaffected controls (3,10). To date, several meta-analyses indicated that a clear association between TAI and miscarriage appears evident (11–14). However, the gestational ages in these above-mentioned studies were 11–13 weeks, and did not include some women who miscarried before 11 weeks. Therefore, the aim of this study was to investigate the effects of SCH and TAI during early pregnancy in women at low risk for thyroid diseases on spontaneous miscarriage.
Method
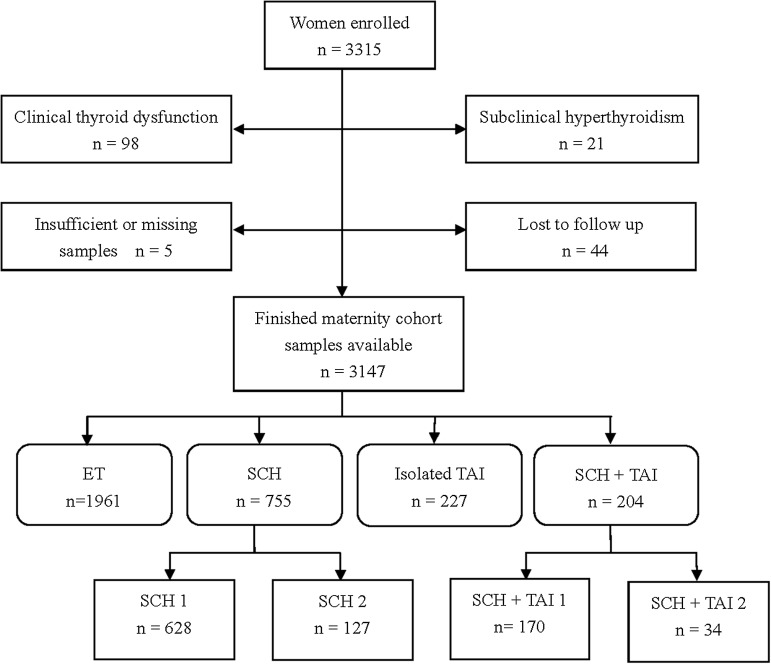
This was a prospective cohort study. Unselected women who attended the gynecology and obstetrics clinics in 13 hospitals and six prenatal clinics in three cities (Shenyang, Dalian, and Dandong) of Liaoning Province in China were recruited between January 2012 and September 2012. These clinics are all located in regions of China classified as iodine sufficient. Inclusion criteria were: (a) four to eight weeks intrauterine gestation; (b) resident in the designated areas for more than five years; and (c) spontaneously conceived singleton pregnancy. Women with the following high risk factors (1,15) for thyroid disorders during pregnancy were excluded: (a) hereditary diseases and chronic disorders, such as hypertension, diabetes, and anemia (Hb<110 g/L); (b) history of thyroid diseases (thyroid ultrasound was performed for the presence of goiter and/or thyroid nodules) or family history of thyroid disease; (c) extra TAI diseases; (d) medication that may impact thyroid function (except for estrogen); (e) pregnancy conceived through assisted reproductive technology; (f) uterine malformations (septate or bicornuate uterus) and uterine fibroids; (g) an intrauterine demise; (h) history of therapeutic head or neck irradiation; (i) body mass index (BMI) of >30 kg/m2; or (j) prior preterm birth. In accordance with the two guidelines published by the American Thyroid Association and the Endocrine Society (1,15), women with high risk factors for thyroid dysfunction during pregnancy were excluded at enrollment. Thus, the study population consisted of patients with a low risk for thyroid diseases and miscarriage. Of the 3315 women enrolled, 3147 pregnant women were analyzed after considering the exclusion criteria (Fig. 1).
FIG. 1.
Flowchart of study population.
All participants answered a questionnaire about demographic and obstetric characteristics (maternal age, gestational age, parity, and previous miscarriage), educational status, income, smoking, alcohol intake, personal and family history of thyroid disorders (including first- and second-degree relatives), personal history of type 1 diabetes or other autoimmune diseases, and history of therapeutic head or neck irradiation.
All women enrolled underwent an ultrasound on the day of enrolment to confirm there was an ongoing intrauterine gestation, and the duration of gestation was calculated based on the dates of their last menstrual period and confirmed by ultrasonography. The outcome of interest was miscarriage, defined as spontaneous pregnancy loss occurring before 20 weeks' gestation. All participants were followed with thyroid function tests and an ultrasound examination at the endocrine clinics each month. Miscarriage was defined as pregnancy loss after ultrasound detection of a gestational sac or histological evidence of pregnancy on curettage. Miscarriage was confirmed and reported by the physicians at the gynecology and obstetrics clinics in 13 hospitals and six prenatal clinics.
Study samples, laboratory determinations, and categorization
Samples of spot urine and regular serum blood were obtained from each participant in the morning after an overnight fast. All specimens were frozen until shipment and assayed as soon as possible. Thyrotropin (TSH), free thyroxine (fT4), thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TgAb), and urinary iodine (UI) were determined by a centralized laboratory. TSH, fT4, TPOAb, and TgAb concentrations were measured by an electrochemiluminescence immunoassay with Cobas Elesys 601 (Roche Diagnostics, Switzerland). The functional sensitivity of the TSH assay was 0.002 mIU/L. The intra-assay coefficients of variation (CV) of serum TSH, fT4, TPOAb, and TgAb were 1.57–4.12%, 2.24–6.33%, 2.42–5.63%, and 1.3–4.9% respectively. The interassay CV values were 1.26–5.76%, 4.53–8.23%, 5.23–8.16%, and 2.1%–6.9% respectively. UI excretion was measured by the colorimetric ceric ion arsenious acid ash method, based on the Sandell–Kolthoff reaction. The intra- and interassay CV values were <7%. Iodine nutrition status in the cohort was expressed by the median of UI concentration (μg/L) (16).
Because the reference values given by the manufacturer of the analyzer (Roche) were collected through experimentation on populations that did not include pregnant women, the data were categorized using percentiles of our laboratory values. To determine the first eight-week gestational reference intervals for TSH and fT4, based on the recommendation of the National Academy of Clinical Biochemistry (NACB) of the United States (17), 1961 healthy women with singleton pregnancies in our study were chosen. The pregnancy-specific reference ranges (2.5–97.5 percentiles) for TSH and fT4 levels were 0.29–5.22 mIU/L and 12.27–20.72 pmol/L respectively. Positive thyroid autoantibodies were defined as a TPOAb concentration of >34 IU/mL, and/or TgAb of >115 IU/mL.
The subjects were divided into four groups with respect to thyroid hormone levels and thyroid autoantibody concentrations (Fig. 1). Group 1: euthyroidism (ET, n=1961), normal TSH (0.29≤TSH<2.5 mIU/L) and fT4, and TPOAb and/or TgAb negative. Group 2: isolated SCH (n=755), TSH levels between 2.5 and 10 mIU/L, normal fT4, and TPOAb and/or TgAb negative. On the basis of the level of TSH, the SCH group was stratified into two subgroups: SCH 1 with 2.5≤TSH<5.22 mIU/L and SCH 2 with 5.22≤TSH<10 mIU/L. Group 3: Isolated TAI (n=227), positive TPOAb and/or TgAb with normal TSH and fT4. Group 4: SCH+TAI (n=204), SCH with TAI. Based on the level of TSH, this group was also stratified into two subgroups: TAI+SCH 1 and TAI+SCH 2.
The study was approved by the ethical committee of the First Affiliated Hospital of China Medical University and by the ethical committees of the hospitals involved in the study, and all participating women gave informed written consent at initial presentation.
Statistical analysis
Statistical analysis was performed using the SPSS v13.0 software package (SPSS, Inc., Chicago, IL). The odds ratios and confidence intervals of women with subclinical thyroid abnormalities and women with ET were analyzed relative to the risk of miscarriage using Review Manager v5.1 software. We used descriptive statistics to characterize the study cohort. Continuous data are reported as mean and standard deviation (SD), and skewed data were log-transformed before analysis. Categorical data are reported as percentages. Characteristics between the four or six groups were compared using the Mann–Whitney U-test or analysis of variance (ANOVA) for continuous variables and Fisher's exact test or the chi-square test for categorical variables as appropriate, with post hoc Bonferroni correction.
In addition to the univariate analysis, a multivariate logistic regression model was used to assess the association between maternal thyroid status and miscarriage, controlling for the following potential confounders: mother's age, parity, BMI, previous miscarriage, smoking, drinking, and UI. Results are reported as adjusted odds ratios (aOR) with 95% CI. A p-value of<0.05 was considered to be statistically significant.
Results
Clinical characteristics of subjects
Of all 3315 enrolled women, there were 959 (28.9%) women with a TSH value >2.5 mIU/L, and 161 (4.9%) women with a TSH concentration >5.22 mU/L. Positive TgAb and/or TPOAb were present in 431 women (13.0%). The percentage of women with both positive TPOAbs and TgAbs was significantly higher than that of women with isolated positive TPOAbs or TgAbs (6.1% vs. 3.2% and 3.7%; p<0.05).
The average age of the 3147 study subjects was 29.59±3.71 years, and the mean duration of pregnancy at enrollment was 7.10±1.12 weeks. Of these, 3015 (95.8%) women were nulliparous, 810 women (25.7%) had a history of miscarriage, 16 (0.5%) smoked, and 26 (0.9%) consumed alcohol during pregnancy. Median UI concentrations in women from the cities of Shenyang, Dalian, and Dandong were 166.3 μg/L, 154.4 μg/L, and 176.2 μg/L respectively.
With the exception of TSH levels and gestational age at miscarriage, no significant differences were found among the four groups for the demographic and baseline characteristics analyzed (Table 1).
Table 1.
Characteristics of Different Groups of Thyroid Status
| Characteristic | ET 0.29≤TSH<2.5 mIU/L (n=1961) | SCH 2.5≤TSH<10.0 mIU/L (n=755) | Isolated TAI TSH<2.5 mIU/L (n=227) | SCH+TAI 2.5≤TSH<10.0 mIU/L (n=204) | p |
|---|---|---|---|---|---|
| Maternal age (years), M±SD | 29.86±3.51 | 29.53±3.38 | 29.88±3.69 | 29.90±3.54 | 0.14 |
| GA (weeks) | 7.20±1.11 | 7.12±1.06 | 7.06±0.97 | 7.16±1.13 | 0.06 |
| GA at abortion (weeks) | 11.13±3.21 | 10.47±1.74 | 9.91±1.74 | 9.40±1.81 | 0.02 |
| BMI (kg/m2) | 21.88±3.21 | 22.00±3.37 | 21.64±3.31 | 21.94±3.51 | 0.51 |
| WC (cm) | 77.19±8.72 | 77.22±8.99 | 76.79±9.21 | 77.17±8.83 | 0.92 |
| SBP (mmHg) | 112.78±10.81 | 112.47±10.82 | 113.12±10.93 | 113.62±11.96 | 0.06 |
| DBP (mmHg) | 71.67±8.14 | 71.61±8.43 | 72.91±8.40 | 72.83±8.45 | 0.07 |
| Heart rate (per/min) | 80.52±9.36 | 79.93±8.24 | 80.81±8.79 | 81.33±10.37 | 0.74 |
| Parity, n (%) | 0.45 | ||||
| 0 | 1876 (95.7) | 720 (95.4) | 221 (97.4) | 198 (97.1) | |
| 1+ | 85 (4.3) | 35 (4.6) | 6 (2.6) | 6 (2.9) | |
| Previous miscarriage, n (%) | 0.14 | ||||
| 0 | 1476 (75.3) | 557 (73.8) | 156 (68.7) | 148 (72.5) | |
| 1 | 420 (21.4) | 167 (22.1) | 59 (26.0) | 46 (22.5) | |
| 2+ | 65 (3.3) | 31 (4.1) | 12 (5.3) | 10 (4.9) | |
| Educational status, n (%) | 0.60 | ||||
| Junior middle school | 581 (29.6) | 200 (26.5) | 69 (30.4) | 61 (29.9) | |
| Senior middle school | 204 (10.4) | 89 (11.8) | 23 (10.1) | 26 (12.7) | |
| College | 1091 (55.6) | 435 (57.6) | 123 (54.2) | 111 (54.4) | |
| Postgraduate | 85 (4.3) | 31 (4.1) | 12 (5.3) | 6 (2.9) | |
| Monthly per capita income (RMB)(RMB yuan) n (%) | 0.70 | ||||
| <1000 | 490 (25.0) | 174 (23.0) | 54 (23.8) | 52 (25.5) | |
| 1000–3000 | 616 (31.4) | 232 (30.7) | 67 (29.5) | 66 (32.4) | |
| 3000–5000 | 564 (28.8) | 245 (32.5) | 74 (32.6) | 59 (28.9) | |
| >5000 | 291 (14.9) | 104 (13.8) | 32 (14.1) | 27 (13.2) | |
| Smoking, n (%) | 7 (0.4) | 6 (0.8) | 0 (0) | 3 (1.5) | 0.97 |
| Drinking, n (%) | 15 (0.8) | 7 (0.9) | 2 (0.9) | 2 (1.0) | 0.78 |
| UI (μg/L), median (IQR) | 155.2 (106.98) | 161.3 (109.08) | 157.3 (118.72) | 165.9 (108.33) | 0.16 |
| fT4 (pmol/L), median (IQR) | 16.16 (1.90) | 15.60 (1.91) | 16.13 (1.92) | 15.67 (2.12) | 0.06 |
| TSH (mIU/L), median (IQR) | 1.40 (0.99) | 3.21 (1.33) | 1. 60 (0.53) | 3.79 (1.03) | 0.00 |
ET, euthyroid; SCH, subclinical hypothyroidism; isolated TAI, isolated thyroid autoimmunity; SCH+TAI, SCH with the positive TPO-Ab/Tg-Ab; GA, gestational age; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; UI, urinary iodine fT4, free thyroxine; TSH, thyrotropin; IQR, interquartile range.
Table 2 shows that TSH levels, TAI, and previous miscarriage were associated with miscarriage in univariate analysis.
Table 2.
Univariate Analysis of Variable Related to Miscarriage
| Characteristic | Miscarried (n=110) | Unaffected (n=3037) | p |
|---|---|---|---|
| Maternal age (years), M±SD | 29.49±3.81 | 29.8±3.48 | 0.22 |
| GA (weeks) | 6.92±1.12 | 7.10±1.11 | 0.41 |
| BMI (kg/m2) | 22.20±3.66 | 21.89±3.26 | 0.15 |
| WC (cm) | 76.49±8.72 | 77.22±8.83 | 0.76 |
| SBP (mmHg) | 112.24±11.54 | 112.98±10.88 | 0.24 |
| DBP (mmHg) | 71.99±9.19 | 71.82±8.22 | 0.19 |
| Heart rate (per/min) | 80.63±10.15 | 80.45±9.10 | 0.31 |
| Parity, n (%) | 0.23 | ||
| 0 | 103 (93.6) | 2912 (95.9) | |
| 1+ | 7 (6.4) | 125 (4.1) | |
| Previous miscarriage, n (%) | 0.004 | ||
| 0 | 69 (62.7) | 2268 (74.7) | |
| 1 | 34 (30.9) | 658 (21.7) | |
| 2+ | 7 (6.4) | 111 (3.7) | |
| Educational status, n (%) | 0.21 | ||
| Junior middle school | 22 (20.0) | 887 (29.2) | |
| Senior middle school | 33 (30.0) | 309 (10.2) | |
| College | 50 (45.5) | 1710 (56.3) | |
| Postgraduate | 3 (2.7) | 131 (4.3) | |
| Monthly per capita income (RMB)(RMB yuan) n (%) | 0.47 | ||
| <1000 | 20 (18.2) | 748 (24.6) | |
| 1000–3000 | 44 (40.0) | 937 (30.9) | |
| 3000–5000 | 37 (33.6) | 905 (29.8) | |
| >5000 | 7 (6.4) | 447 (14.7) | |
| Smoking, n (%) | 1 (0.9) | 15 (0.5) | 0.44 |
| Drinking, n (%) | 2 (1.8) | 24 (0.8) | 0.24 |
| Ab+, n (%) | 34 (30.9) | 397 (13.1) | 0.018 |
| TPOAb+, n (%) | 14 (12.7) | 252 (8.3) | 0.101 |
| TgAb+, n (%) | 23 (20.9) | 220 (7.2) | 0.000 |
| UI (μg/L), median (IQR) | 171.53 (105.17) | 168.52 (117.08) | 0.80 |
| fT4 (pmol/L), median (IQR) | 15.76 (1.78) | 15.89 (1.82) | 0.38 |
| TSH (mIU/L), median (IQR) | 2.45 (0.99) | 1.81 (1.01) | 0.000 |
Ab+, positive TPOAb and/or TgAb.
TSH levels
As shown in Table 3, women with thyroid abnormalities have higher TSH levels compared with ET subjects. To analyze the impact of SCH and TAI on the risk of miscarriage, women in the SCH and SCH+TAI groups were stratified according to the TSH levels and autoantibody status. The TSH levels, miscarriage rates, and gestational ages at abortion are reported in Table 3. There was no significant difference in TSH levels between the ET and isolated TAI groups (1.40 vs. 1.60 mIU/L, p=0.09). Moreover, there was no correlation between TPOAb/TgAb levels and serum TSH levels (data not shown). The SCH+TAI group showed higher TSH levels than the SCH group (3.79 vs. 3.21 mIU/L, p=0.04). Interestingly, there was no remarkable difference in the TSH level between the TAI+SCH 1 and SCH 1 groups (3.06 vs. 3.33 mIU/L, p=0.06). However, the median TSH level was significantly higher in the TAI+SCH 2 group than in the SCH 2 group (4.96 vs. 5.84 mIU/L, p=0.03; Table 3).
Table 3.
Characteristics of Different Thyroid Status Groups
| Characteristic | ET 0.29≤TSH<2.5 (n=1961) | SCH 2.5≤TSH<10.0 (n=755) | SCH 1 2.5≤TSH<5.22 (n=628) | SCH 2 5.22≤TSH<10.0 (n=127) | Isolated TAI TSH<2.5 (n=227) | SCH+TAI 2.5≤TSH<10.0 (n=204) | TAI+SCH 1 2.5≤TSH<5.22 (n=170) | TAI+SCH 2 5.22≤TSH<10.0 (n=34) | All (n=1186) |
|---|---|---|---|---|---|---|---|---|---|
| TSH (mIU/L), median (IQR) | 1.40 (0.89) | 3.21 (1.46) | 3.06 (1.03) | 4.96 (1.33) | 1. 60 (0.93) | 3.79 (1.21)a | 3.33 (1.23) | 5.84 (1.17)a | 3.47 (1.56)* |
| Miscarriage, n (%) | 43 (2.2%) | 31 (4.1%)* | 22 (3.5%) | 9 (7.1%)* | 13 (5.7%)* | 23 (11.3%)* | 17 (10.0%)* | 6 (15.2%)* | 67 (5.6%)* |
| GA at abortion (weeks) | 11.13±3.21 | 10.47±1.74* | 10.79±1.77* | 9.70±1.47* | 9.91±1.74* | 9.40±1.81* | 9.59±1.97* | 8.88±1.24* | 9.33±1.71* |
p<0.05 between the SCH and SCH+TAI groups and between the SCH 2 and TAI+SCH 2 groups; *p<0.05 relative to ET women.
Miscarriage risk
In all, 110 of the 3147 women had a miscarriage (3.5%). Miscarriage rates, OR, and CI of different groups according to the risk of miscarriage are shown in Figure 2.
FIG. 2.
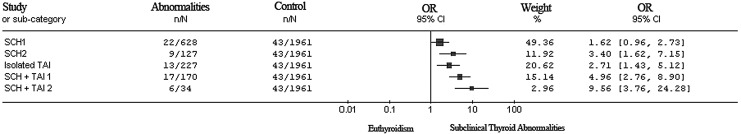
Forest plots of odds ratios and confidence intervals of women comparing thyroid abnormalities according to the risk of miscarriage.
Compared to women with ET (0.29≤TSH<2.5 mIU/L), SCH 1 (2.5≤TSH<5.22 mIU/L) was associated with a nonstatistically significant increase in miscarriage risk (3.5 vs. 2.2%, aOR 1.62 [CI 0.96–2.73]; p=0.083). However, the risk of miscarriage was significantly higher in the SCH 2 group (TSH between 5.22 and 10 mIU/L). The risk of miscarriage in the isolated TAI group increased, especially with coexistent SCH. TAI enhanced the risk of miscarriage in the SCH 1 and SCH 2 groups (Fig. 2; 7.1% vs. 2.2%, aOR 3.40 [CI 1.62–7.15]; p=0.002), isolated TAI (5.7% vs. 2.2%, aOR 2.71 [CI 1.43–5.12]; p=0.003), SCH+TAI 1 (10.0% vs. 2.2%, aOR 4.96 [CI 2.76–8.90]; p=0.000), and SCH+TAI 2 (15.2% vs. 2.2%, aOR 9.56 [CI 3.76–24.28]; p=0.000; Table 3). Moreover, there is a significant increased miscarriage rate in the SCH+TAI group compared to the SCH group (4.1% vs. 11.3%, p=0.000), whereas the comparison of the subgroups of SCH and SCH+TAI did not show statistically significant increases for miscarriage rates (3.5% vs. 7.1%; 10.0% vs. 15.2%, p>0.05).
Gestational ages at miscarriage
Among the110 women with a miscarriage, the mean gestational age at miscarriage was 11.13±3.21 weeks. The gestational age at miscarriage was earlier in all groups with any abnormal TSH and TAI. In parallel with the higher TSH levels, earlier gestational ages at miscarriage were observed in the SCH subgroups and the SCH+TAI subgroups (SCH 1 vs. SCH 2: 10.79±1.77 vs. 9.70±1.47; SCH+TAI 1 vs. SCH+TAI 2: 9.59±1.97 vs. 8.88±1.24 weeks).
Discussion
The present study was performed to evaluate the association between subclinical thyroid abnormalities and miscarriage during the early weeks of pregnancy. The results revealed that, through the eighth week of pregnancy, women whose TSH values were above the upper limit of the pregnancy-specific interval (SCH 2 with 5.22≤TSH<10 mIU/L) and women with isolated TAI had an elevated risk of miscarriage, but women with TSH values within the reference range for the pregnant population (SCH 1 with 2.5≤TSH<5.22 mIU/L) did not have an increased risk of miscarriage. The high risk of miscarriage in both SCH and TAI-positive subjects is highly associated with an early gestational age of miscarriage.
Although we excluded most participants with high risk factors for thyroid disorders, as listed in the guidelines of the ATA and the Endocrine Society (1,15), SCH, TAI, and previous miscarriage during gestational weeks 4–8 were still associated with pregnancy loss in the low-risk population. After adjusting for confounding factors, SCH and TAI remained independently associated with miscarriage. Similar results were also reported in the studies performed during 11–13 gestational weeks in the United States and Europe (3,4,18,19). Higher TSH levels and TAI were here taken as indicative of decreased thyroid function, which is essential for fetal growth and development (8,20). No correlation between fT4 and miscarriage was observed in our study, which we speculate may be attributable to the pronounced sensitivity of TSH to imbalances in the hypothalamus-pituitary-thyroid axis (3,21).
In our study, an interesting finding showed that isolated TAI with normal TSH level was associated with a higher risk of miscarriage, but when both TAI and SCH coexisted in an individual, the risk of miscarriage increased significantly. This situation was more remarkable with increasing serum TSH levels. The ORs for miscarriage rose from 1.62 to 4.96 and from 3.40 to 9.56 in the SCH 1 and SCH 2 groups with TAI, which indicates that TAI itself is associated with an increased risk of miscarriage.
The increased rate of pregnancy loss in TAI-positive women with TSH levels <2.5 mIU/L (isolated TAI) indicates that the presence of TAI without subclinical thyroid dysfunction is significantly associated with an increase in the miscarriage rate, which is consistent with the results of previous studies (12,20,22–24), although this remains controversial. A minor increase in the TSH level (1.48 mIU/L vs. 1.11 mIU/L) might be associated with an increased miscarriage rate, and there is no significant difference in the positive incidence of TPOAb (3). Furthermore, some studies have reported that TAI can lead to higher TSH levels and consequently be associated with loss of pregnancy (3,19,25). For this purpose, we analyzed the differences in TSH levels and in miscarriage risk among women in the SCH and SCH+TAI groups. The analyses show that antibody-positive women with TSH levels (SCH+TAI) between 5.22 and 10 mIU/L had a significantly increased risk of miscarriage compared to antibody-negative women (SCH). Our data indicate that this difference was much more pronounced when SCH was accompanied by the presence of TAI. TAI was found to affect the rate of miscarriage in both a TSH-dependent and TSH-independent manner (26). An additive or synergistic phenomenon may exist between the two conditions, contributing to the occurrence of thyroid dysfunction during pregnancy and ultimately resulting in pregnancy loss, but the precise mechanism underlying this process is still unclear. Two hypotheses may help to explain the association of TAI with pregnancy loss. First, thyroid autoantibodies may be considered as markers for generalized autoimmune dysfunction in the body because they have been known to be associated with an increased risk for pregnancy loss. In our previous study, we found that TgAb correlates with miscarriage rate, and Unuane et al. reported that TAI is considered as one of the risk factors of infertility (23,27). Second, TAI-positive euthyroid women are more prone to develop subclinical or overt hypothyroidism during pregnancy due to hormonal imbalance, particularly in the first trimester (27), although our findings did not reveal significantly higher serum TSH levels in the women with positive TPOAb compared to the women without TPOAb.
In healthy pregnant women with no previous history of thyroid disease, miscarriage occurs earlier in women with SCH (6.5±0.9 weeks) than in women with isolated positive TPOAb (8.2±2.1 weeks), who in turn experience miscarriage earlier than ET women (8.2±1.6 weeks; p=0.02) (24). Consistent with these findings, the mean gestational age at miscarriage was earlier among SCH women than among ET women. We also observed that women with isolated TAI and SCH+TAI experienced miscarriage earlier than those with ET. Women with both SCH and higher TSH levels and TAI had the lowest gestational age at miscarriage. Subgroup analyses demonstrate that women with higher TSH levels had a lower gestational age at abortion, both with and without TAI. In women with TAI, the higher the TSH levels, the earlier the gestational age at miscarriage. In the retrospective study by De Vivo et al. (24), 216 apparently healthy pregnant women with no previous history of thyroid disease were enrolled. The authors concluded that women suffering from SCH have a lower gestational age at miscarriage, while the results of our prospective cohort study showed that either SCH or TAI are associated with earlier miscarriage. Hence, this difference may be due to the different methods used in the two studies. In addition, the study by De Vivo et al. (24) was conducted in a moderately severe iodine-deficient area, and the population in our study stems from an iodine-sufficient area, which is another likely explanation for the discrepancy between that study and ours.
At gestational weeks 4–8, the normal immune suppression of pregnancy is weakest, and thyroid autoantibody titers are higher than at other gestational weeks. A major strength of our study is its prospective design, which allowed us to collect detailed demographic, obstetric, and laboratory data. We assessed the iodine nutritional status of the pregnant women to confirm that they were iodine sufficient because both iodine deficiency and iodine excess can have adverse effects on thyroid function (2,28–30). In addition, our sample size is large, providing sufficient power for detection of the clinical significance of the differences. One limitation of the present study is that we did not exclude women with histories of previous miscarriage. Hence, this study population is relatively low risk. Another shortcoming of the study is that we did not provide interventions for the women with SCH or/and TAI, which would have allowed us to determine whether levothyroxine can abate the risk of miscarriage. The third limitation is that because of the low prevalence of the coexistence of SCH and TAI, there were only 34 participants who met the criteria for SCH+TAI 2. Therefore, the findings in Table 3 may be biased by the smaller number of participants. Although serum TSH levels in isolated TAI were higher than in ET (1.6 vs. 1.4 mIU/L), the difference was not statistically different. This may be due to the small group with isolated TAI. Furthermore, it is known that even a minor increase in TSH might be associated with an increased miscarriage rate (3).
In conclusion, the results of this large prospective cohort study indicate that low-risk women in iodine-sufficient regions who experience SCH, TAI, or both before eight weeks' gestation are more likely to suffer pregnancy loss. Further, miscarriages among women with these abnormalities were also found to occur at earlier gestational ages. To some extent, these findings support universal screening of women of reproductive age for thyroid function and TAI early during pregnancy or during the preconception period.
Acknowledgments
We gratefully acknowledge the invaluable contribution of the physicians from the gynecology and obstetrics clinics in the participating 13 hospitals and six prenatal clinics in Liaoning Province, and thank the residents who participated in this study. This work was supported by 973 Science and Technology Research Foundation, Ministry of Science and Technology in China (Grant 2011CB512112); the Chinese National Natural Science Foundation (Grant 81170730); Health and Medicine Research Foundation, Ministry of Health in China (Grant 201002002); and Research Foundation, Department of Science and Technology, Liaoning Province government, China (Grant 2012225020).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W.2011Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneuer FJ, Nassar N, Tasevski V, Morris JM, Roberts CL.2012Association and predictive accuracy of high TSH serum levels in first trimester and adverse pregnancy outcomes. J Clin Endocrinol Metab 97:3115–3122 [DOI] [PubMed] [Google Scholar]
- 3.Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ.2009Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 160:985–991 [DOI] [PubMed] [Google Scholar]
- 4.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green 2010Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 95:E44–48 [DOI] [PubMed] [Google Scholar]
- 5.Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH.2010Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 20:989–993 [DOI] [PubMed] [Google Scholar]
- 6.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG.2005Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 105:239–245 [DOI] [PubMed] [Google Scholar]
- 7.Wang WW, Teng WP, Shan ZY, Wang S, Li JX, Zhu L, Zhou J, Mao JY, Yu XH, Li J, Chen YY, Haibo Xue, Fan CL, Wang H, Zhang HM, Li CY.2011The prevalence of thyroid disorders during early pregnancy in China: the benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol 164:263–268 [DOI] [PubMed] [Google Scholar]
- 8.Shan ZY, Chen YY, Teng WP, Yu XH, Li CY, Zhou WW, Gao B, Zhou JR, Ding B, Ma Y, Wu Y, Liu Q, Xu H, Liu W, Li J, Wang WW, Li YB, Fan CL, Wang H, Guo R, Zhang HM.2009A study for maternal thyroid hormone deficiency during the first half of pregnancy in China. Eur J Clin Invest 39:37–42 [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Teng WP, Li JX, Wang WW, Shan ZY.2012Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest 35:322–325 [DOI] [PubMed] [Google Scholar]
- 10.Dal Lago A, Vaquero E, Pasqualetti P, Lazzarin N, De Carolis C, Perricone R, and Morett C.2011Prediction of early pregnancy maternal thyroid impairment in women affected with unexplained recurrent miscarriage. Hum Reprod 26:1324–1330 [DOI] [PubMed] [Google Scholar]
- 11.Prummel MF, Wiersinga WM.2004Thyroid autoimmunity and miscarriage. Eur J Endocrinol 150:751–755 [DOI] [PubMed] [Google Scholar]
- 12.Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A.2011Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 342:d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, Papadimas I.2010Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol 162:643–652 [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Hu RM.2011Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf) 74:513–519 [DOI] [PubMed] [Google Scholar]
- 15.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S.2012Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:2543–2565 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization UNCsF, and International Council for the Control of Iodine Deficiency Disorders 2007 World Health Organization: Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide for Programme Managers. Third edition. WHO, Geneva [Google Scholar]
- 17.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR.2003Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13:3–126 [DOI] [PubMed] [Google Scholar]
- 18.Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, Velkeniers B.2003Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab 88:4149–4152 [DOI] [PubMed] [Google Scholar]
- 19.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H.2006Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 91:2587–2591 [DOI] [PubMed] [Google Scholar]
- 20.Negro R, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A.2011Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab 96:E920–924 [DOI] [PubMed] [Google Scholar]
- 21.Cooper DS, Biondi B.2012Subclinical thyroid disease. Lancet 379:1142–1154 [DOI] [PubMed] [Google Scholar]
- 22.Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, Locorotondo G, Caroli P, Pezzarossa A, Dazzi D. and Hassan H.2005Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod 20:1529–1533 [DOI] [PubMed] [Google Scholar]
- 23.Mehran L, Tohidi M, Sarvghadi F, Delshad H, Amouzegar A, Soldin OP, Azizi F.2013Management of thyroid peroxidase antibody euthyroid women in pregnancy: comparison of the American Thyroid Association and the Endocrine Society Guidelines. J Thyroid Res 2013:542692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vivo A, Mancuso A, Giacobbe A, Moleti M, Maggio Savasta L, De Dominici R, Priolo AM, Vermiglio F.2010Thyroid function in women found to have early pregnancy loss. Thyroid 20:633–637 [DOI] [PubMed] [Google Scholar]
- 25.Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D, Braverman LE.2008Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract 14:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twig G, Shina A, Amital H, Shoenfeld Y.2012Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun 38:J275–J281 [DOI] [PubMed] [Google Scholar]
- 27.Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, Poppe K.2013Thyroglobulin autoantibodies: is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid 23:1022–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng WP, Shan ZY, Teng XC, Guan HX, Li YS, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen YY, Zhao D, Shi XG, Hu FN, Mao JY, Gu XL, Yang R, Tong YJ, Wang WB, Gao TS, Li CY.2006Effect of iodine intake on thyroid diseases in China. N Engl J Med 354:2783–2793 [DOI] [PubMed] [Google Scholar]
- 29.Rebagliato M, Murcia M, Espada M, Alvarez-Pedrerol M, Bolumar F, Vioque J, Basterrechea M, Blarduni E, Ramon R, Guxens M, Foradada CM, Ballester F, Ibarluzea J, Sunyer J.2010Iodine intake and maternal thyroid function during pregnancy. Epidemiology 21:62–69 [DOI] [PubMed] [Google Scholar]
- 30.Sang ZN, Wei W, Zhao N, Zhang GQ, Chen W, Liu H, Shen J, Liu JY, Yan YQ, Zhang WQ.2012Thyroid dysfunction during late gestation is associated with excessive iodine intake in pregnant women. J Clin Endocrinol Metab 97:E1363–E1369 [DOI] [PubMed] [Google Scholar]