Abstract
Background: Over 140 million people in the United States have at least one chronic medical condition, but they receive fewer than 60% of guideline-recommended services for these conditions. Increasing patients' involvement in their own care may improve the receipt of guideline-recommended services. We evaluated patients' patterns of responses to notifications regarding guideline-recommended services delivered through a personalized health record (PHR). Materials and Methods: We enrolled 584 participants with high cardiovascular disease risk from 73 primary care practices into an active PHR in which they received patient-centered decision support—notifications delivered via a PHR regarding prevention gaps (i.e., unmet preventive healthcare or chronic disease monitoring). Participants with prevention gaps received up to three weekly messages regarding all services due within a 2-month time frame. These three-message cycles could repeat up to every 2 months for a new, or continuing, prevention gap. Results: Of the 584 participants, 501 (86%) received at least one reminder. Approximately 61% of these participants accessed the PHR or received the care that triggered the message after the first message and 73% after the first two messages. In subsequent three-message cycles, we observed no change in the number of messages required prior to participants accessing the PHR or receiving recommended care (chi-squared=12.4, p=0.3). Of the 2,656 prevention gaps these participants had over 1 year, 1,539 (58%) were closed. Conclusions: In this low-intensity intervention, participants accessed the PHR and received recommended care. Providing notification through the PHR allows patients to choose when they receive, and take action on, the message. Notifications can be provided to patients through a PHR without alert fatigue and may be an additional tool to help patients achieve better health.
Key words: : patient engagement, health information technology, chronic disease, patient activation
Introduction
There are currently over 140 million people in the United States with chronic conditions, and that number is expected to rise to over 170 million by 2030.1 Over 30% of the Medicare population have a chronic condition, and 13% and 8% have two or three chronic conditions, respectively.2 Chronic diseases that contribute to the development of coronary vascular disease, such as hypertension, hyperlipidemia, and diabetes, are common, affecting 26%, 13%, and 10% of people in the United States, respectively.3 Costs related to coronary vascular disease exceeded $440 billion in 2010 and are expected to triple by 2040.4 Delivering high-quality care to patients with these complex illnesses may decrease complications and reduce costs. Yet, care for patients with chronic illness is not optimal.5 Overall, patients receive fewer than 60% of services recommended by guidelines for chronic disease care, with fewer than 50% of these chronic disease services being delivered for diabetes and hyperlipidemia.5
Efforts to improve disease management by increasing rates of guideline-recommended care delivery have been modestly successful and focused on clinician-centered solutions and modifying systems of care.6,7 Clinician-centered solutions include point-of-care decision support (e.g., electronic health record [EHR] alerts during the clinical encounter) and registry feedback to clinicians.7–9 There is some evidence, however, that clinicians exhibit “alert fatigue” and stop responding to such decision support.10,11
Personal health records (PHRs) provide patients with a secure view into their clinician-maintained EHRs. PHRs allow patients to view laboratory test results and health reminders and may allow practice staff and patients to electronically communicate with each other. Studies to date have found that patients who register for PHRs have higher rates of compliance with disease management goals12 and that those who access PHRs have higher rates of guideline-based mammography and influenza vaccination.13 The appropriate timing and frequency of reminders through the PHR to activate patients to participate more in their chronic disease care are unknown.
The objective of this study was to evaluate Self-Management & Reminders with Technology: Appraisal of an Integrated PHR (SMART-PHR), which delivered patient-centered decision support through a patient-specific, active PHR to patients with complex illness and conditions that contribute to the development of cardiovascular disease.
We evaluated the patients' patterns of response to patient-centered decision support delivered through a PHR. Specifically, we examined whether and when patients logged-on to a PHR and/or receiving care in relation to notifications regarding prevention gaps (i.e., unmet preventive healthcare or chronic disease monitoring) and asked if there were differences over the course of the study as patients received more notifications.
Materials and Methods
Individuals were eligible to participate in SMART-PHR if they had documentation in the EHR (visit diagnosis, past medical history, or problem list entry) of (1) coronary artery disease, (2) congestive heart failure, (3) diabetes mellitus, or (4) hypertension or hyperlipidemia and were taking a medication that required routine laboratory monitoring (e.g., diuretic). In addition, individuals had to be able to provide informed consent and those without an existing PHR account had to be willing to sign up for the PHR.
Participants were recruited from 73 primary care practices affiliated with a single health system and located throughout Western Pennsylvania between June 2010 and September 2011. Physicians in these practices provide care to over 280,000 patients. Participants were recruited through several strategies. We used EHR alerts to inform physicians when they were seeing a patient who met SMART-PHR eligibility criteria. If the patient was interested in hearing more about the study, the physician accepted the alert. This generated a message to the study staff, who confirmed the patient's interest in the study. We also mailed invitations to all patients in our health system who had PHR accounts and had EHR-based past medical history or problem list diagnoses that met SMART-PHR eligibility criteria. From these invitations, participants could express interest in the study or opt out of receiving further information about the study. The health system followed these invitations with telephone calls to discuss the study with patients and forwarded the names of interested and eligible patients to the study staff. In five large practices, study staff were intermittently available on-site to discuss the study with interested patients and assist the practice staff with registering patients for the PHR. Finally, in a single practice, the practice's computerized patient intake form was used to inform patients about the study and identify patients who were interested in hearing more about the study.14,15
SMART-PHR was approved by the University of Pittsburgh and Weill Cornell Medical College's Institutional Review Boards.
Identification of Prevention Gaps
In all of the practices, physicians use a single EHR (EpicCare®; Epic Systems, Verona, WI) that includes a PHR and a health maintenance module. The health maintenance module is patient-specific, taking into account a patient's age and recommendations pertaining to each patient's medical conditions or needs. For example, the health maintenance module for a patient with diabetes would include annual dilated retinal examination, low-density lipoprotein and urine microalbumin testing, and influenza vaccination (in season), periodic hemoglobin A1C (A1C) testing, and completion of the pneumococcal vaccination. In addition, the health maintenance module can be modified by the provider by removing (or adding) services and by changing the intervals on services, as appropriate for the patient.
Health maintenance topics can be satisfied automatically or manually. If a report of a diagnostic test (e.g., A1C, colonoscopy), immunization, or other service is entered in the EHR, that health maintenance topic is automatically satisfied, and the completion date is displayed. If a test is one without discrete field documentation (e.g., diabetic foot exam) or the office learns of a service that has been provided for which there is no report to be entered into the record, the practice or provider can manually satisfy the health maintenance topic.
A prevention gap was defined as a patient's health maintenance topic that was unsatisfied or would need to be satisfied soon. In that way, prevention gaps were personalized—only the health maintenance module that was appropriate for the patient based on disease state and provider modification of health maintenance module (in the context of shared decision making with the patient) were considered prevention gaps.
Intervention
Participants received an enhanced, active version of the standard PHR, which was designed in conjunction with patients who regularly used the standard PHR.16
The intervention PHR contained all of the features of the standard PHR, including the ability to view health maintenance topics, in addition to active notifications regarding prevention gaps. Once a notification was triggered, the message received by the participant would include a list of all services due within the next 3 months. Notifications were delivered in cycles of up to three messages (described below). The initiation of each cycle was participant-specific and based solely on that participant's prevention gaps.
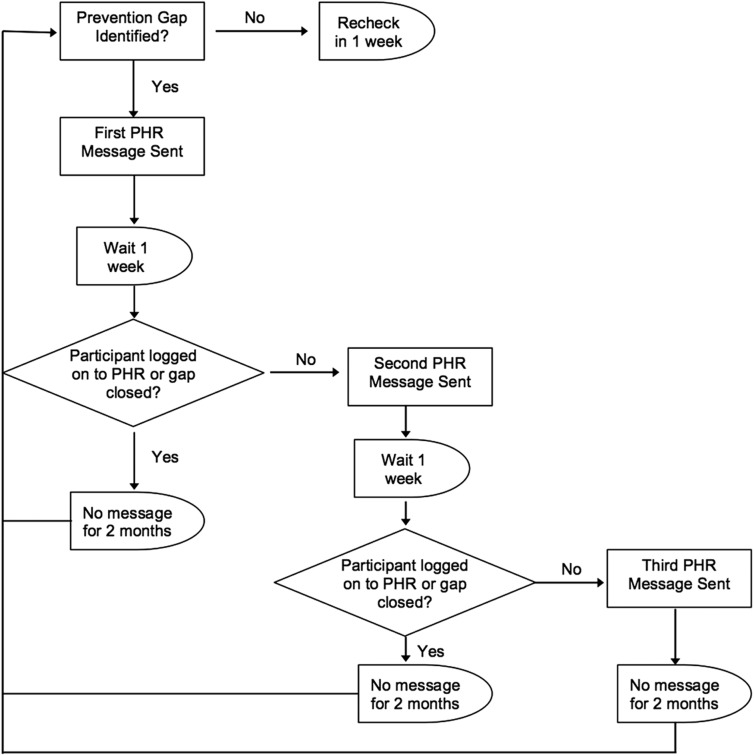
Every week the system checked to see if a participant had a prevention gap. Once the first prevention gap was identified, the participant entered messaging-cycle 1. The participant received an initial “tickler” message to his or her e-mail telling them that the subject had a secure message in the PHR (first PHR message). If he or she did not log-on to the PHR or close the prevention gap(s) in 1 week, the participant received a second “tickler” message and secure PHR message (second PHR message). If the participant still did not log-on to the PHR or close the prevention gap(s) in 1 week, he or she received a third and final “tickler” message and secure PHR message (third PHR message), as well as a postal letter regarding the prevention gap(s). Once the participant did log-on to the PHR, closed the prevention gap(s), or received the third message, the system did not recheck for prevention gaps for 2 months. If a gap was identified at that point, the next cycle started; if not, the system rechecked in 1 week (Fig. 1).
Fig. 1.
Self-Management & Reminders with Technology: Appraisal of an Integrated Personal Health Record (PHR) messaging cycle. Note that a participant's first cycle for any gap is messaging-cycle 1. A participant's first cycle for a specific gap is gap-cycle 1.
In addition to messaging-cycles (described above), we also defined an individual-level gap-centered definition of cycles. Gap-cycle 1 was defined as the first time a participant received a message for a particular prevention gap. Although each participant could only have one messaging-cycle 1 (i.e., the first time he or she received any message), he or she could have multiple gap-cycle 1 events (e.g., the first time a colon cancer screening gap was identified could be different than the first time a lipid screening gap was identified). When a participant closed a gap (not just accessed the PHR), he or she would not receive further messages about that gap. By defining gap-cycles differently than messaging-cycles, we are to understand how many cycles it took for a patient to close a particular gap (the gap is necessary to designate a cycle) as well as the trends in patients' responsiveness over multiple cycles of notifications.
Statistical Analysis
Participant characteristics and prevention gaps were summarized using frequencies and measures of central tendencies. For purposes of these analyses, we considered a prevention gap closed if the participant did not receive messages for that gap in subsequent cycles, meaning that the health maintenance topic was satisfied. We used chi-squared statistics to compare differences in PHR access and gap closures between cycles. All analyses were conducted using STATA version 11 software (StataCorp, College Station, TX).
Results
Five hundred eight-four individuals (30% of those referred to the study) participated in SMART-PHR. Of those, 501 (86%) received at least one message regarding a prevention gap between June 2010 and September 2012 and form the basis of our analyses. Those who received messages were more likely to be women and less likely to have been signed up for the PHR prior to the study (Table 1).
Table 1.
Self-Management & Reminders with Technology: Appraisal of an Integrated Personal Health Record Study Participant Characteristics
| ENROLLED IN ACTIVE PHR (N=584) | RECEIVED A MESSAGE (N=501)a | |
|---|---|---|
| Age (years) | 58.5 (10.6) | 58.7 (10.2) |
| Female | 319 (55) | 299 (60)b |
| Race | ||
| Black | 68 (12) | 58 (12) |
| White | 511 (88) | 439 (88) |
| Asian | 4 (1) | 4 (1) |
| Native American | 5 (1) | 3 (1) |
| Education | ||
| ≤High school | 4 (9) | 39 (9) |
| Some college | 5 (18) | 75 (18) |
| Completed college | 18 (40) | 162 (39) |
| Graduate school | 16 (33) | 136 (33) |
| Signed up for PHR prior to study | 393 (67) | 327 (65)c |
Data are number (%) except for age, which is mean (standard deviation).
All comparisons p>0.20, except as indicated, using t tests and chi-squared tests as appropriate.
p<0.001, indicating that women were more likely to receive a message by chi-squared test.
p=0.01, indicating that those who were signed up for the personal health record (PHR) prior to the study were less likely to receive a message by chi-squared test.
The prevention gaps prompting messages were diverse and ranged from liver function test (6% of messages) to mammogram (14% of messages). All of the prevention gap topics and associated numbers of gaps are shown in Table 2.
Table 2.
Disease Management and Prevention Gaps That Prompted Messaging and Were Subsequently Closed in the Self-Management & Reminders with Technology: Appraisal of an Integrated Personal Health Record Study (n=2,656 Total Gaps Open)
| GAP IN | GAPS OPEN | GAP CLOSEDa |
|---|---|---|
| Diabetic foot exam | 220 (8.3) | 127 (57.7) |
| Dilated retinal exam | 250 (9.4) | 144 (57.6) |
| Hemoglobin A1C | 346 (13.0) | 238 (68.8) |
| Urine albumin | 210 (7.9) | 135 (64.3) |
| Liver function test | 147 (5.5) | 109 (74.2) |
| Lipid testing | 263 (9.9) | 144 (54.8) |
| Creatinine testing | 264 (9.9) | 127 (48.1) |
| Pneumonia vaccines | 171 (6.4) | 84 (49.1) |
| Colon cancer screening | 157 (5.9) | 68 (43.3) |
| Pap smear | 253 (9.5) | 174 (68.8) |
| Mammogram | 375 (14.1) | 189 (50.4) |
Data are number (%).
Gap closure percentage is of the total gaps open for the individual gap.
Messaging-Cycles
The 501 participants who got notifications received a total of 2,833 first PHR messages, 1,119 second PHR messages, and 778 third PHR messages with an accompanying postal letter. Approximately 61% of participants accessed the PHR or closed the prevention gap that triggered the notification after the first message, and a total of 73% of participants accessed the PHR or closed the prevention gap after the first two messages. There was no effect of the messaging-cycle on the number of messages required prior to PHR access or gap closure (chi squared=12.4, p=0.3), meaning that participants continued to respond to messages in subsequent messaging-cycles. Figure 2 shows the number of messages sent per messaging-cycle for the first 8 cycles.
Fig. 2.
Number of first, second, and third messages sent for the first eight cycles of Self-Management & Reminders with Technology: Appraisal of an Integrated Personal Health Record.
Gap-Cycles
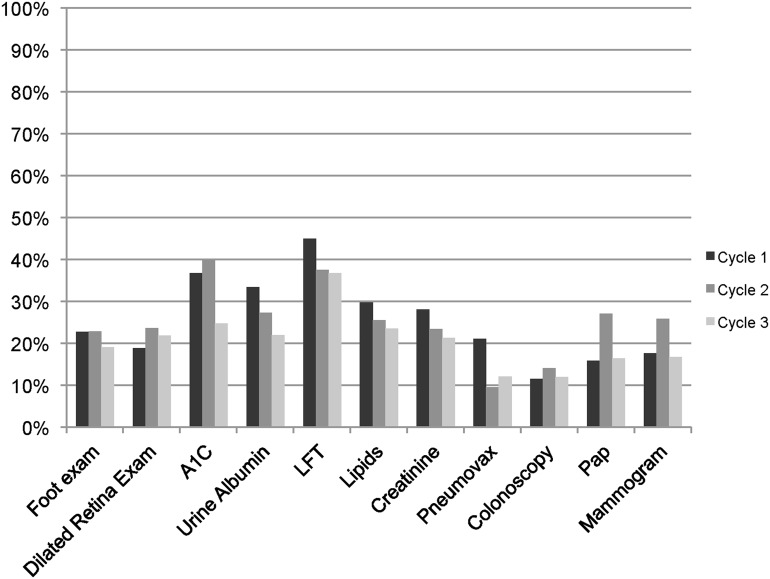
Over the course of the study, the participants had 2,656 prevention gaps. Of these, 1,539 (58%) prevention gaps were closed (as indicated by not receiving another cycle of notifications regarding the gap), ranging from 43% of colonoscopy gaps to 74% of liver function test (Table 2). Figure 3 shows the percentage of gaps closures that occurred each cycle. Gaps that may have resulted from missing documentation (e.g., influenza vaccination received outside the clinical setting) had a higher percentage of closures after gap-cycle 1 than did gaps that required lead-time to schedule (e.g., mammography) (p<0.001). Regardless of gap-cycle, over 50% of all gap closures occurred after the first PHR message.
Fig. 3.
Percentage of open gaps closed for each of the first three message cycles. Note that cycle 1 is the first time a patient receives a message for that gap or the gap reopens. A1C, hemoglobin A1C; LFT, liver function test.
Discussion
In this low-intensity intervention, participants accessed the PHR and closed prevention gaps. After two messages, approximately 73% of participants accessed the PHR, a rate that did not vary based on the number of cycles in which a participant had received messages. This is encouraging. Had the proportion of participants accessing messages declined over time, such an intervention would be less useful. However, because it remained stable, the intervention timing and frequency appear to be acceptable to the participants.
By providing reminders through the PHR, patients are able to choose when they receive the message (log-on to the PHR) and are able to take action regarding the message immediately by scheduling an appointment or sending a request to the clinician's office to have a test or procedure ordered. This is in contrast to both telephone reminders and traditionally mailed reminders. Telephone reminders come at a time that a patient may not be prepared to receive the message and, if the message is automated, a time that the patient may not be able to take action on the message because the office is closed. Patients are able to choose when they open traditionally mailed reminders, but those are not immediately actionable. The patient must call the clinician's office during business hours to schedule an appointment or, if a reply card is included, return that card. The combination of both being prepared to receive the message and being able to take action within the same system at the same time may allow PHR-based reminders to be more successful than other reminders have been. This is particularly relevant given the findings of Wright et al.13 that individuals who access health maintenance modules in response to reminders were more likely to receive some preventive care.
Although we cannot comment on why patients chose to receive, or not receive the care to close their prevention gaps, we found that gaps that can be easily closed through documentation or blood testing required fewer notification cycles than those necessitating an office visit or other scheduled appointment, and we saw gap closure rates that were higher than in interventions using telephone, mailed, or passive reminders. McDowell et al.17 used telephone outreach and mailed letters to achieve a 24.1% and 35.7% gap closure rate in blood pressure screening, respectively (compared with a 21.1% gap closure rate with usual care). Mailing fecal occult blood testing cards and reminder letters has been associated with a 27–41% colon cancer screening gap closure.18,19 Telephone reminders are associated with 37% of patients closing colon cancer screening gaps.19 In a meta-analysis, Yabroff and Mandelblatt20 found that 11–40% of women closed mammography gaps following a mailed reminder. Telephone reminders were associated with 43–54% of mammography gaps closing.21,22 Lantz et al.22 found mailed and telephone reminders were associated with 22% of Pap smear gaps closing. In a study using a passive PHR, Krist et al.23 found those who accessed the PHR were up to date with 25% of all prevention at 16 months, compared with 14% of individuals invited to participate in a PHR and 13% of the control population.
In their active PHR study, Wright et al.13 found that, compared with a passive PHR, significantly more patients received mammograms (49% versus 30%) and influenza vaccinations (22% versus 14%). Compared with usual care, Green et al.24 found an increase in colorectal cancer screening among participants receiving active PHR-based reminders (51% versus 26%). These results are similar to our findings.
This study has several limitations. Although all medical personnel were trained to use the EHR health maintenance module, we do not know how medical personnel use this module and if they are completely documenting gap closures from outside sources (e.g., Pap smears done by an outside gynecologist). In addition, this work was conducted in a single health system with a single EHR. However, the practices and populations in this health system are diverse, with over 100 practices and locations in both urban and rural areas.
Finally, in these analyses, we are able to describe access patterns, but not compare gap closures to a control group. Although a control group is necessary to evaluate the effect of PHR notifications on gap closures compared with usual care, the primary aim of these analyses was to look at whether and when patients receiving notifications about gaps would either view the message or close the gap, and whether this pattern of response would change as patients experienced more messaging-cycles. A control group would not aid in these analyses.
Optimizing care for patients with chronic medical conditions is challenging. Most chronic medical conditions require periodic to daily monitoring that is best accomplished by a patient–provider team. Active notifications through a PHR can be used to provide patient-centered decision support, without incurring alert fatigue. These tools may help achieve better care and health for patients. Future work should evaluate the effect of such tools on chronic disease outcomes, may vary time between notifications or take scheduled procedures into account, and should continue to evaluate the threshold at which patients begin to ignore notifications or exhibit alert fatigue.
Acknowledgments
This work is funded by a grant from the Agency for Healthcare Research and Quality (R18-HS018167) and as such is subject to federal open access policies.
Disclosure Statement
No competing financial interests exist.
R.H., G.S.F., S.C., and M.S.R. designed the SMART-PHR study, monitored data collection, designed the statistical analysis plan, and drafted and revised the article. S.M.S., X.D., and S.C. cleaned and analyzed the data. M.W. and C.Z. implemented the data collection drafted and revised the article. M.S.R. initiated the collaborative project. R.H. is the guarantor of the article.
References
- 1.Anderson G. The Number of People with Chronic Conditions Is Rapidly Increasing. Chronic Care: Making the Case for Ongoing Care. 2010. Available at www.rwjf.org/pr/product.jsp?id=56828 (last accessed July20, 2012)
- 2.Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes 2009;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson G, Herbert R, Zeffiro T, Johnson N. Chronic conditions: Making the case for ongoing care. Princeton, NJ: Partnership for Solutions, Robert Wood Johnson Foundation, 2004 [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011;123:933–944 [DOI] [PubMed] [Google Scholar]
- 5.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med 2003;348:2635–2645 [DOI] [PubMed] [Google Scholar]
- 6.Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Eff Clin Pract 1998;1:2–4 [PubMed] [Google Scholar]
- 7.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA 2005;293:1223–1238 [DOI] [PubMed] [Google Scholar]
- 9.Fischer GS, Ling B, Simak D, Kapoor WN. Increasing utilization of preventative services in an internal medicine clinic [abstract]. J Gen Intern Med 2004;19(Suppl):106 [Google Scholar]
- 10.Russ AL, Zillich AJ, McManus MS, Doebbeling BN, Saleem JJ. Prescribers' interactions with medication alerts at the point of prescribing: A multi-method, in situ investigation of the human-computer interaction. Int J Med Inform 2012;81:232–243 [DOI] [PubMed] [Google Scholar]
- 11.Embi PJ, Leonard AC. Evaluating alert fatigue over time to EHR-based clinical trial alerts: Findings from a randomized controlled study. J Am Med Inform Assoc 2012;19:e145–e148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenforde M, Nowacki A, Jain A, Hickner J. The association between personal health record use and diabetes quality measures. J Gen Intern Med 2012;27:420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright A, Poon EG, Wald J, et al. Randomized controlled trial of health maintenance reminders provided directly to patients through an electronic PHR. J Gen Intern Med 2012;27:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess R, Matthews K, McNeil M, Chang CH, Kapoor W, Bryce C. Health services research in the privacy age. J Gen Intern Med 2005;20:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess R, Santucci A, McTigue K, Fischer G, Kapoor W. Patient difficulty using tablet computers to screen in primary care. J Gen Intern Med 2008;23:476–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer GS, Hess R, Landeen B, et al. Electronic reminders to patients within an interactive patient health record. Telemed J E Health 2013;19:497–500 [DOI] [PubMed] [Google Scholar]
- 17.McDowell I, Newell C, Rosser W. A randomized trial of computerized reminders for blood pressure screening in primary care. Med Care 1989;27:297–305 [DOI] [PubMed] [Google Scholar]
- 18.Goldberg D, Schiff GD, McNutt R, Furumoto-Dawson A, Hammerman M, Hoffman A. Mailings timed to patients' appointments: A controlled trial of fecal occult blood test cards. Am J Prev Med 2004;26:431–435 [DOI] [PubMed] [Google Scholar]
- 19.Myers RE, Ross EA, Wolf TA, Balshem A, Jepson C, Millner L. Behavioral interventions to increase adherence in colorectal cancer screening. Med Care 1991;29:1039–1050 [DOI] [PubMed] [Google Scholar]
- 20.Yabroff KR, Mandelblatt JS. Interventions targeted toward patients to increase mammography use. Cancer Epidemiol Biomarkers Prev 1999;8:749–757 [PubMed] [Google Scholar]
- 21.Mohler PJ. Enhancing compliance with screening mammography recommendations: A clinical trial in a primary care office. Fam Med 1995;27:117–121 [PubMed] [Google Scholar]
- 22.Lantz PM, Stencil D, Lippert MT, Beversdorf S, Jaros L, Remington PL. Breast and cervical cancer screening in a low-income managed care sample: The efficacy of physician letters and phone calls. Am J Public Health 1995;85:834–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krist AH, Woolf SH, Rothemich SF, et al. Interactive preventive health record to enhance delivery of recommended care: A randomized trial. Ann Fam Med 2012;10:312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green BB, Wang CY, Anderson ML, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: A randomized trial. Ann Intern Med 2013;158:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]