Abstract
Link N is a naturally occurring peptide that can stimulate proteoglycan synthesis in intervertebral disc (IVD) cells. IVD repair can also potentially be enhanced by mesenchymal stem cell (MSC) supplementation to maximize extracellular matrix (ECM) production. In a previous study, we have shown that Link N can inhibit osteogenesis and increase the chondrogenesis of MSCs in vitro. The aim of the present study was to determine the potential of MSCs and Link N alone or in combination with regard to tissue repair in the degenerate disc. Bovine IVDs with trypsin-induced degeneration were treated with MSCs, Link N, or a combination of MSCs and Link N. Trypsin-treated discs were also injected with phosphate-buffered saline to serve as a degeneration control. The ECM proteins and proteoglycans were extracted from the inner nucleus pulposus (NP) of the discs, and sulfated glycosaminoglycans (GAGs) were analyzed by the dimethyl methylene blue dye-binding assay. The expression of type II collagen was analyzed by western blot. To track the MSCs after injection, MSCs were labeled with PKH67 and observed under confocal microscopy after the 2 week culture period. The GAG content significantly increased compared with the degeneration control when degenerate discs were treated with MSCs, Link N, or a combination of both Link N and MSCs. Histological analysis revealed that the newly synthesized proteoglycan was able to diffuse throughout the ECM and restore tissue content even in areas remote from the cells. The quantity of extractable type II collagen was also increased when the degenerate discs were treated with MSCs and Link N, either alone or together. MSCs survived, integrated in the tissue, and were found distributed throughout the NP after the 2 week culture period. MSCs and Link N can restore GAG content in degenerate discs, when administered separately or together. Treatment with MSCs and Link N can also increase the expression of type II collagen. The results support the concept that biological repair of disc degeneration is feasible, and that the administration of either MSCs or Link N has therapeutic potential in early stages of the disease.
Introduction
The intervertebral discs (IVDs) link adjacent vertebrae within the spine. They are composed of the peripheral annulus fibrosus (AF) and the central nucleus pulposus (NP). The AF is a fibrosus tissue with concentric lamellae that are rich in collagen fibrils.1 The NP has a more amorphous consistency, with collagen fibrils that have a random orientation and a high content of aggrecan which give it a gelatinous appearance and provide for the ability to resist compressive loads. Aggrecan is a large proteoglycan with numerous glycosaminoglycan (GAG) chains attached to its core protein, which in the NP provides the osmotic properties needed to counter the effects of compression.
All the mechanisms that contribute to degenerative changes in the disc lead to biochemical alterations in the composition and structure of extracellular matrix (ECM) due to both depleted synthesis and increased degradation, with aggrecan being particularly susceptible to proteolytic damage and loss. Aging, poor nutrition, biomechanical,2–5 biochemical,6–10 and genetic influences11–14 are associated with increased IVD degeneration. During degeneration, loss of GAG content in the NP occurs, changing it from a gelatinous structure to a fibrotic texture as it becomes more collagenous, and fissures appear in both the NP and AF.15,16 This is commonly associated with low back pain, possibly due to the nerve ingrowth and loss of disc height, which are facilitated by proteoglycan depletion.17 Currently, there is no medical treatment for IVD degeneration, ultimately leaving surgical excision of the damaged tissue, insertion of a cage or prosthesis to restore the IVD space, and vertebral bone fusion as the only offered options. While this may provide relatively good clinical short-term results18 in pain relief, in many instances it also alters spine biomechanics, resulting in subsequent adjacent-level disc degeneration.
Biological repair of the degenerating IVD would be preferable to surgical excision, and the type of biological repair needed would change depending on the grade of degeneration. The strategies for biological repair might require supplementing the NP with cells, scaffolds, and/or growth factors to stimulate the production of ECM components, particularly aggrecan, to restore its function.
In this work, we used an organ culture model of early disc degeneration, involving proteoglycan depletion but no substantial collagen disruption, to study the effect of molecular and cell-based therapies, using Link N as an economic growth factor analog and mesenchymal stem cells (MSCs) as a cell supplement.
Materials and Methods
MSC culture
Human MSCs harvested from bone marrow were obtained from Lonza (Basel, Switzerland). According to the supplier, the cells were positive for CD105, CD166, CD29, and CD44 and negative for CD14, CD34, and CD45. In addition, the cells were confirmed to be able to differentiate into osteogenic, chondrogenic, and adipogenic lineages. All cells were expanded in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin and were used within four passages.19,20 All culture reagents were from Wisent, Inc. (St-Bruno, Canada).
MSC labeling and tracking
MSCs were labeled with PKH67 (Sigma-Aldrich, Oakville, Canada) following the instructions of the supplier. Briefly, 2×106 MSCs were washed with DMEM without FBS and collected as a loose pellet by centrifugation at 400 g for 5 min. The pellet was re-suspended in Diluent C and quickly mixed with the dye solution. The cell/dye suspension was then incubated for 5 min, after which the reaction was stopped by adding an equal volume of FBS. Viability of the cells was measured by staining with trypan blue. An aliquot was cultured in monolayer (Sarstedt, Saint-Léonard, Canada) for 2 days to track the labeling efficiency. The remaining cells were re-suspended in either phosphate-buffered saline (PBS; Wisent, Inc.), or PBS supplemented with 1 mg/mL Link N (CanPeptide, Montreal, Canada). The cell suspension was then injected into bovine discs pretreated with trypsin to induce degeneration.21
Disc isolation and culture
The largest first 3–4 caudal discs were isolated from the tails of 24- to 30-month-old steers, as previously described.21,22 Briefly, the tails were dissected free of skin, muscles, and ligaments, and pedicles for each segment were removed. The bone and the adjacent calcified part of the cartilaginous endplate were removed, so that the surface of the disc was soft and flexible without detectable calcified tissue. After the discs were rinsed in PBS supplemented with 1000 U/mL penicillin, 1000 μg/mL streptomycin, and 0.25 μg/mL fungizone (GIBCO, Burlington, Canada), they were preconditioned for 3 days in sterile 80 mL specimen containers (STARPLEX Scientific, Etobicoke, Canada) containing 50 mL culture medium (DMEM with 2 mM Glutamax and 25 mM Hepes, supplemented with 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL l-ascorbate). Degeneration was induced by a single injection of 100 μg trypsin (Sigma-Aldrich) dissolved in 75 μL PBS into the center of the disc using a 28G1/2 needle21 The needle was placed on top of the disc to measure the distance needed to reach the center and was then inserted to the same depth. Once in the center, the trypsin soulution was slowly injected and the needle was then gradually pulled out to avoid back flow. The discs were then cultured for another 4 days, before an injection of MSCs (105 cells), Link N (75 μg), or a combination of MSCs (105 cells) and Link N (75 μg) in a final volume of 75 μL PBS. The Link N concentration was based on the optimal dose for isolated bovine disc cells (1 μg/mL) assuming an average volume of the bovine discs to be 7.5 mL. The number of MSCs used was based on a study by Liebscher et al.,23 which measured the cell density of healthy human discs. About half the number of cells found in a healthy adult human disc were used in this study, in order to avoid a potential detrimental effect on cell survival due to nutrient deprivation as the bovine discs already have a high cell density. For all experimental conditions, seven discs were injected. Seven of the trypsin-treated discs were injected with PBS alone to serve as degeneration controls and to verify that the trypsin was active in degrading the proteoglycan content of the NP. Seven discs were cultured without any injection to serve as nondegeneration controls. The discs were then cultured for 14 days, and the media were changed every 3 days. The discs were randomized as indicated in Table 1.
Table 1.
Randomization of Discs
| Animal | Degeneration control | Nondegeneration control | Link N | MSCs | Link N+MSCs |
|---|---|---|---|---|---|
| 1 | x | x | x | x | |
| 2 | x | x | x | x | |
| 3 | x | x | x | x | |
| 4 | x | x | x | x | |
| 5 | x | x | x | x | |
| 6 | x | x | x | x | |
| 7 | x | x | x | x | |
| 8 | x | x | x | x | |
| 9 | x | x | x |
MSCs, mesenchymal stem cells.
Analysis of discs by microscopy and histology
At termination of culture, two sections were taken through the center of the discs using an in-house designed cutting tool consisting of two microtome blades.21 This gives two 750 μm thick slices about 3 cm wide (disc diameter) and 1 cm high (disc height). One slice was fixed in formalin-free fixative (Accustain; Sigma-Aldrich) for histology analysis. Fixed samples were embedded in paraffin wax, and 5-μm-thick sections were cut and stained with hematoxylin and Safranin O-fast green.24 The other slice was used fresh to study the distribution of MSCs in the disc tissue. The labeled stem cells populating the discs were visualized using an inverted confocal laser scanning microscope (CLSM; Zeiss LSM 510). Twenty consecutive 6 μm sections were imaged, and CLSM stacks were split into single images.
Extraction of ECM proteins and proteoglycans
The remaining NP tissue was collected, and the wet weight was recorded.21 The tissue was cut into small pieces and suspended in 14 volumes of extraction buffer (4 M guanidinium chloride, 50 mM sodium acetate, pH 5.8, 10 mM ethylenediaminetetraaceticacid, COMPLETE®; Roche, Laval, Canada) for protein and proteoglycan extraction. The tissue was extracted with continuous stirring at 4°C for 48 h, and the extracts were cleared by centrifugation at 12,000 g for 30 min at 4°C. The supernatants were collected and stored at −80°C for further analysis.
GAG analysis
Sulfated GAGs were quantified in tissue extracts by a modified dimethyl methylene blue (DMMB) dye-binding assay.25,26 Samples were diluted to fall within the middle of the linear range of the standard curve. An extraction buffer of an equal volume as the tissue extracts was added to the standard curve to compensate for possible interference.
Proteoglycan analysis by agarose gel electrophoresis
Proteoglycan composition was analyzed by agarose gel electrophoresis.27 Proteoglycans in 10 μL aliquots of disc extracts were precipitated with anhydrous ethanol and dissolved in distilled water. The samples were mixed with sample buffer (0.1 M Tris-HCl, 0.768 M glycine, 0.01% Bromophenol blue, 1.2% glycerol, 0.05% sodium dodecyl sulfate [SDS], pH 8.3) and boiled for 10 min. The proteoglycans were separated by electrophoresis in 1.2% agarose gels. The gel was stained with 0.02% (w/v) Toluidine blue in 3% acetic acid with 0.5% (w/v) Triton X 100, and destained with 3% acetic acid and then distilled water.
Aggrecan and type II collagen analysis by western blot
Proteins and proteoglycan in 10 μL aliquots of disc extracts were precipitated by the addition of nine volumes of anhydrous ethanol, washed twice in 95% ethanol, and finally lyophilized. Samples for analysis of type II collagen were dissolved in distilled water. Samples for analysis of aggrecan were dissolved in buffer (0.05 M Tris-HCl, with 0.03 M Sodium acetate, pH 7.4, COMPLETE) and digested by keratanase I and chondroitinase ABC (Amsbio, Lake Forest, CA). The samples from the same treatment group were pooled, mixed with SDS sample buffer, and boiled for 10 min. Then, the proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (4–12% Bio-Rad® gels) under reducing conditions. Separated proteins were transferred to nitrocellulose membranes that were blocked with 1% bovine serum albumin in PBS with 0.2% Tween 20 (blocking buffer). Then, they were incubated with the primary antibodies at a 1:2000 dilution in blocking buffer at 4°C overnight, followed by incubation with the secondary antibody conjugated with horseradish peroxidase (1:5000 dilution; Sigma-Aldrich) in blocking buffer. The primary antibody recognizing collagen type II was from Abcam (Toronto, Canada); the primary antibody recognizing the aggrecan G1 domain was prepared as previously described.28 The bound antibody was visualized by chemiluminescence (GE Healthcare Baie d'Urfe Canada) and analyzed using a Bio-Rad VersaDoc image analysis system (Bio-Rad, Mississauga, Canada).
Statistical analysis
Statistical analysis was performed by using analysis of variance followed by Fisher-protected least significant difference post hoc test by using Statview (SAS Institute, Inc., Cary, NC). Results are presented as the mean±standard deviation of seven independent experiments with discs from different bovine tails. Differences were considered statistically significant where p<0.05.
Results
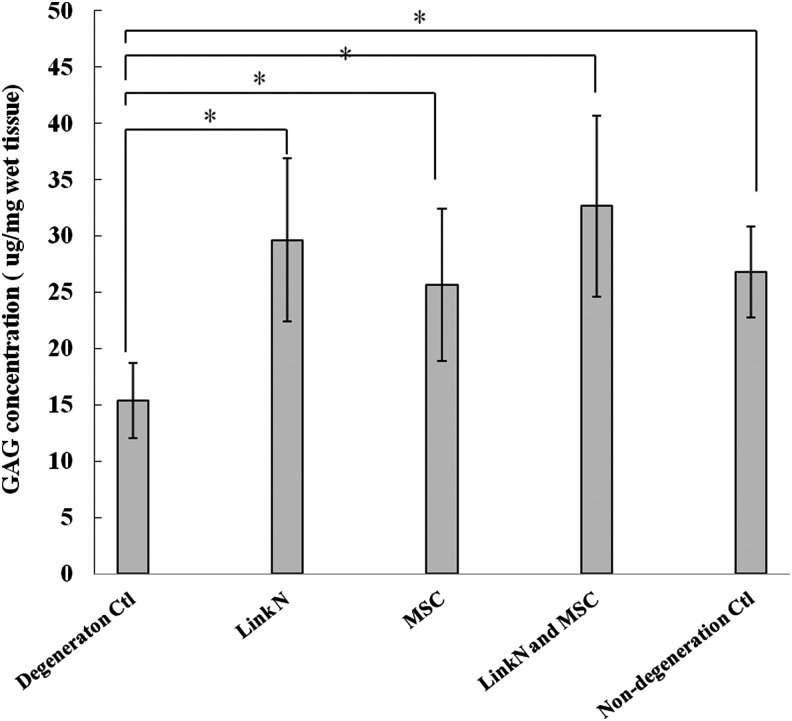
Link N is known to induce proteoglycan synthesis by isolated disc cells and in degenerate rabbit discs, and to enhance chondrogenesis of MSCs in vitro.20,29–31 It is however, not known whether Link N or MSCs alone can restore the proteoglycan content in larger discs with early degeneration. It is also not known whether a combination of Link N and MSCs would have an additive effect. To test this, bovine discs with proteolytically induced aggrecan depletion were treated with Link N or MSCs alone or with a combination of Link N and MSCs. The discs were cultured for a 2 week period after treatment, and the concentration of extractable proteoglycans was quantified by the DMMB assay (Fig. 1). Without intervention, the GAG content in degenerate discs dropped to about 50% of that in nondegenerate controls. In contrast, Link N and MSCs alone, or in combination, significantly increased the GAG content of the discs compared with the GAG content in degeneration control discs (p<0.05). The proteoglycan concentrations in treated discs were similar to that in nondegenerated discs. However, no statistical significance was observed among the treated groups (p>0.05). This indicates that in early degeneration, either Link N or MSCs alone has the potential to restore proteoglycan to its original level and no additional benefit is achieved by a combination therapy.
FIG. 1.
Proteoglycan concentration in the discs. Proteoglycan concentrations were determined in discs with induced degeneration, discs treated with Link N, mesenchymal stem cells (MSCs), both Link N and MSCs, and nondegeneration control discs. The results are represented as mean±standard deviation (SD) of seven discs from different bovine tails. (*p<0.05).
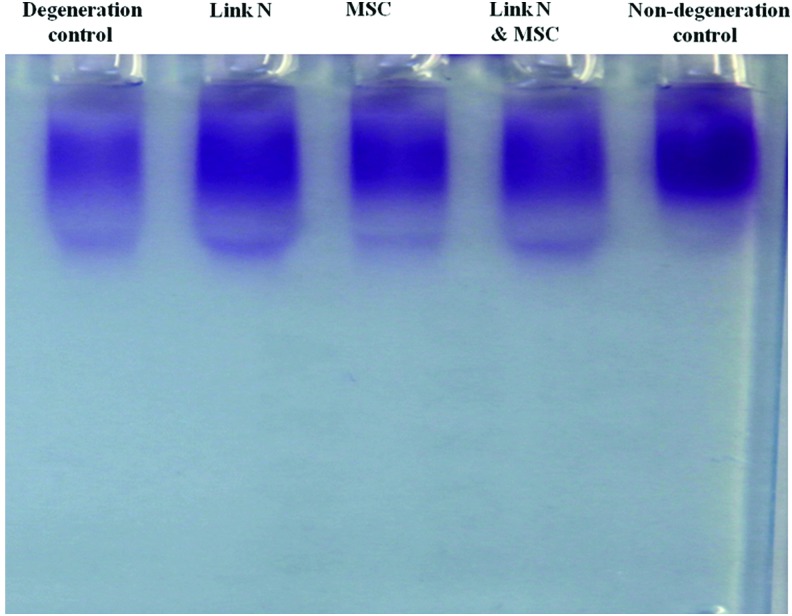
Having equal proteoglycan content does not necessarily imply that the structure is the same as that in the normal disc. To address this, extracted proteoglycans were analyzed by agarose gel electrophoresis. The size distribution and intensity of staining in the treated discs is equivalent to that of nondegenerated control discs, whereas the intensity of the staining is lower in degeneration control discs (Fig. 2). The data demonstrate that the newly synthesized proteoglycans produced in the treated discs are of the same size range as those of the nondegenerate discs.
FIG. 2.
Size distribution of proteoglycans in the discs. The proteoglycan isolated from seven discs with different treatments was pooled and analyzed by agarose gel electrophoresis. Proteoglycan was visualized by Toluidine blue staining. Color images available online at www.liebertpub.com/tea
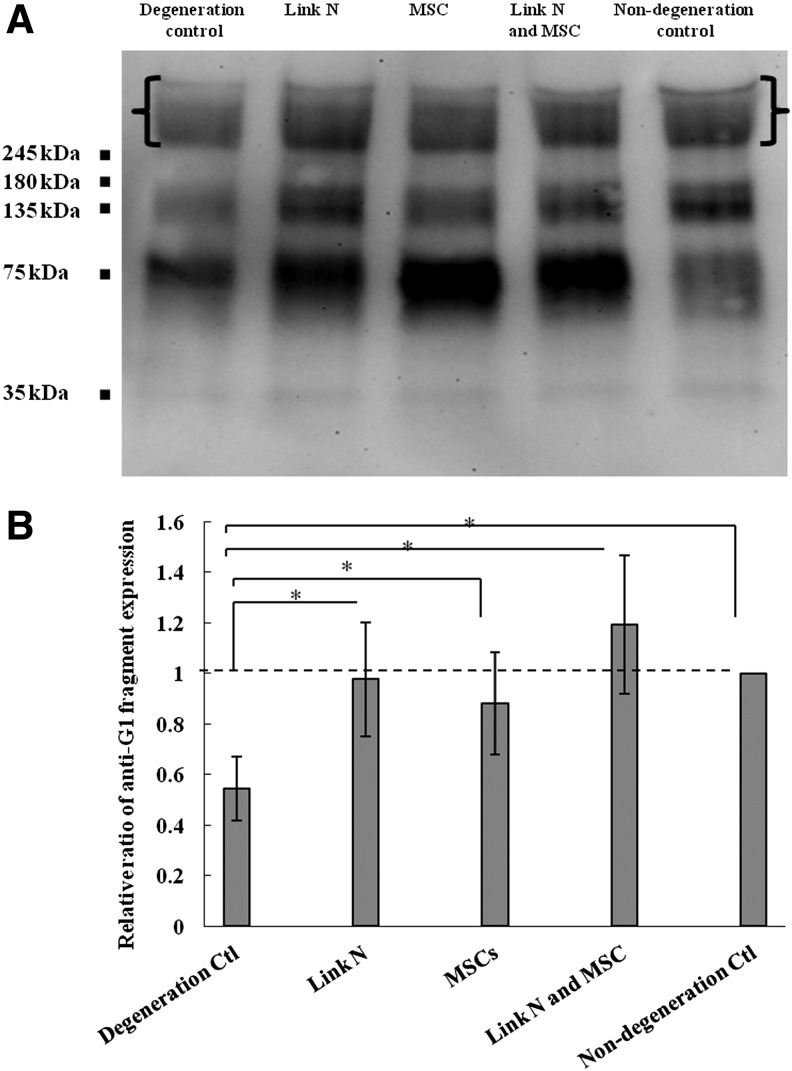
In addition, the presence and abundance of intact aggrecan core protein was evaluated by SDS-PAGE. Intact aggrecan core protein with a mass larger than 250 kDa was significantly lower in degeneration control discs compared with nondegenerated control discs (p<0.05), whereas the injection of Link N and/or MSCs significantly increased the quantity to a level comparable to the nondegenerate control discs (Fig. 3).
FIG. 3.
Analysis of aggrecan core protein in the discs. (A) Western blot analysis of aggrecan core protein in degeneration control, Link N treated, MSCs treated, both Link N and MSCs treated, and no degeneration control discs. (B) Semi-quantitative analysis of intact aggrecan core protein with a molecular weight of about 250 kDa ([ ]). The results are represented as mean±SD of seven discs from different bovine tails. (*p<0.05).
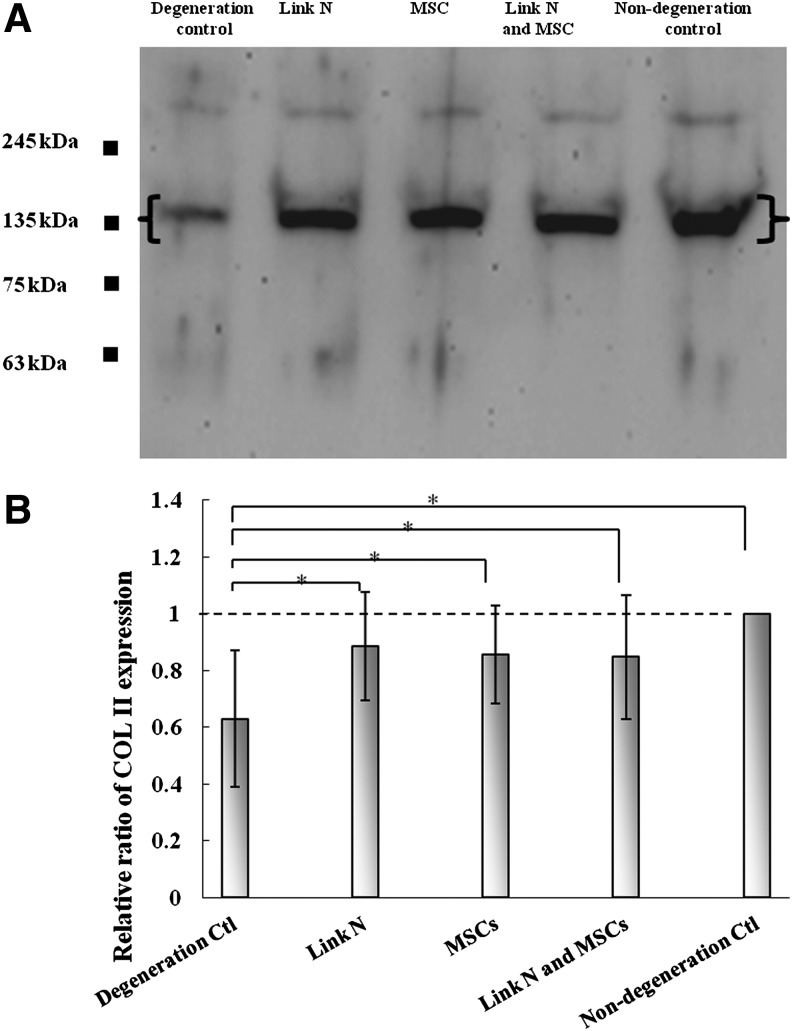
Disc repair also requires collagen production to generate a stable matrix. Therefore, the levels of recently produced extractable type II collagen were assessed (Fig. 4). The quantity of type II collagen extracted from the degeneration control discs was significantly lower than in nondegenerated control discs. When the discs were injected with MSCs and/or Link N, the quantities of type II collagen were increased to a similar level to that detected in nondegenerate control discs. Thus, both Link N and MSCs stimulate not only aggrecan production, but also that of type II collagen.
FIG. 4.
Analysis of newly synthesized type II collagen in the discs. (A) Western blot analysis of type II collagen in degeneration control, Link N treated, MSCs treated, both Link N and MSCs treated, and no degeneration control discs. (B) Semi-quantitative analysis of collagen alpha chains with a molecular weight of 135 kDa ([ ]). The results are represented as mean±SD of seven discs from different bovine tails. (*p<0.05).
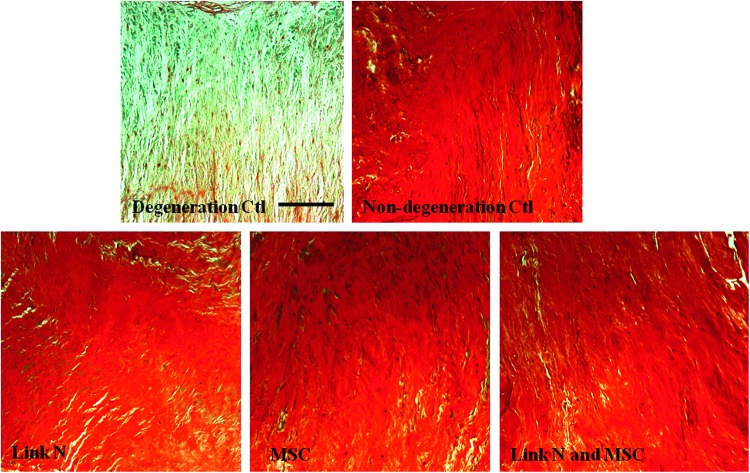
Histological analysis was used to evaluate proteoglycan distribution within the repair tissue. Safranin O and fast green staining of tissue sections confirmed a uniform loss of proteoglycans in the degeneration control discs, where little Safranin O (red) staining was found (Fig. 5). The results further confirmed that the proteoglycan content in degeneration control discs was depleted throughout the NP region. In the discs treated with MSCs or Link N alone or together, the intensity and distribution of the Safranin O staining showed an even distribution throughout the NP region, similar to that of nondegeneration control discs. Thus, the newly synthesized proteoglycan was able to diffuse throughout the ECM and restore tissue content even in areas remote from the cells.
FIG. 5.
Proteoglycan distribution in the nucleus pulposus region of the discs. Discs with trypsin-induced degeneration were cultured for 14 days after an injection with Link N, MSC, or Link N plus MSCs. These were compared with degeneration control and nondegeneration control discs. The discs were evaluated by histology using Safranin O staining (scale bar, 100 μm).
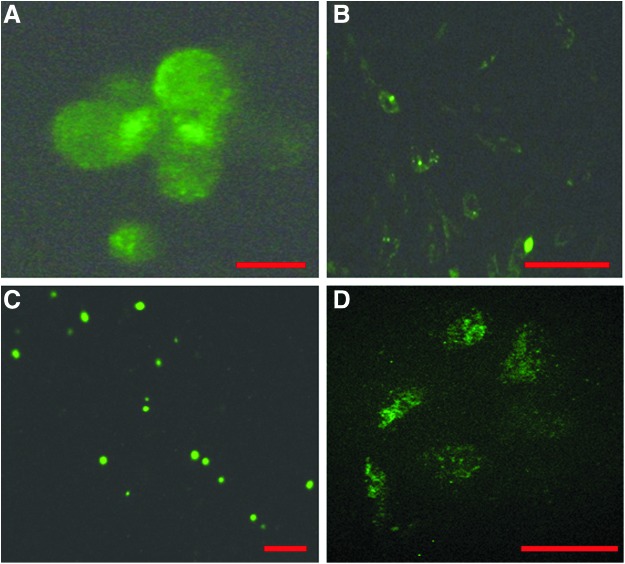
For MSC-induced repair processes to be sustained, the injected stem cells need to remain viable and distributed throughout the repair tissue. To address this, MSCs were labeled with PKH67 (Fig. 6A, B) and cultured for 2 days in a monolayer to evaluate labeling efficiency and dye sustainability. MSC viability was higher than 90% when the cells were labeled and suspended in PBS or Link N/PBS solution before injecting into the trypsin-treated discs. To evaluate whether the injected MSCs survived and integrated in the ECM of the discs, cells were traced by confocal microscopy. Labeled MSCs were found distributed throughout the NP region after the 2 week organ culture period (Fig. 6C, D), indicating the feasibility of a sustainable repair process.
FIG. 6.
Labeling and tracking of the MSCs. (A) MSC cell membranes were labeled using the PKH67 kit (green fluorescence), and the labeling efficiency was evaluated using fluorescence microscopy (Scale bar, 10 μm). (B) Labeled MSCs were cultured in expansion medium for 2 days, and maintained labeling was verified using fluorescence microscopy (Scale bar, 50 μm). (C) The presence of labeled MSCs was determined in the nucleus pulposus region after 14 days in organ culture (scale bar, 50 μm). (D) Magnification of (C) (scale bar, 10 μm).
Discussion
In the current study, we used an organ culture model of early disc degeneration to study the potential of molecular and cell-based therapies to restore IVD proteoglycan content. We used Link N as a molecular agent and MSCs as a cell supplement. The degenerate discs were treated with either therapy separately or in combination, and the results revealed that Link N or MSCs alone have the ability to restore tissue proteoglycan and that no additional effect was observed by a combination of the two.
Previous work has demonstrated the potential of Link N to stimulate disc repair.30–34 Although Link N is cleaved by AF cells, the resulting N-terminal eight amino acid peptide appears to be proteolytically stable and retains biological activity (unpublished data). Studies utilizing isolated IVD cells in vitro showed that Link N could induce collagen and proteoglycan message levels and result in increased incorporation of radioactive 35SO4 into newly synthesized proteoglycans.33,34 In addition, Link N injection into intact human IVDs ex vivo33 resulted in increased incorporation of radioactive 35SO4 in newly synthesized proteoglycans, and Link N led to partial restoration of disc height when injected into rabbit discs in a stab model of disc degeneration.30 However, the current study is the first which demonstrates that Link N can actually restore proteoglycan content, which is an essential prerequisite for any biological IVD repair technique designed to restore the functional properties of the degenerate disc.
The model used in the current study mimics early-stage degeneration in a young adult, where the tissue has sufficient numbers of cells that can respond to Link N stimulation. In contrast, diminishing cell numbers, cell senescence, and possibly an inflammatory environment, on the other hand, often characterize human disc degeneration. Previous work from our group has shown that Link N is equally potent in an inflammatory environment33 However, it still needs to be established at what stage of degeneration, cell number and activity drops below a level where a bioactive substance alone is insufficient to induce repair. At this stage, it might be necessary to also supply additional cells that are capable of synthesizing disc ECM.
There is no benign site where autologous IVD cells can be harvested and used as a cell source for IVD repair, leaving MSCs as an attractive option. The potential use of MSCs for IVD repair has been described in small animals.35–37 Favorable results in rabbits demonstrate increased disc height, as well as ECM deposition and hydration. However, other studies in the rabbit report osteophyte formation, especially when MSCs were administered without a scaffold or without sealing of the AF.38 Since Link N is known to promote chondrogenesis and to reduce osteogenesis of human MSCs in vitro, it may be considered an ideal candidate for a combination therapy.20 In addition to animal studies, a small-scale human clinical trial has reported improved pain and disability score.39 No increase in disc height was found in the clinical trials, but an increase in hydration measured by magnetic resonance imaging (MRI) could be detected. Our results indicate that MSC supplementation could be a viable option in early degeneration. However, since endplate calcification is associated with degeneration,40 it remains to be seen whether the resulting compromised nutritional pathway in degenerate discs would support the metabolic activity of additional cells.41,42
While the use of MSCs for IVD repair is promising, it remains to be established at what degree of degeneration they would be a therapeutic option. It is also not clear whether the cells should be administered alone or in a hydrogel, and whether bioactive components such as Link N should be included. The current model does not result in the generation of fissures but only molecular depletion, whereas natural disc degeneration often involves the creation of fissures. To repair such lesions, it may require injecting Link N and stem cells in a polymerizable scaffold that will fill the lesions and enable uniform distribution of the therapeutic agents.
The source of stem cells is another critical point. While either autologous adipose or bone marrow stem cells are a readily available source, their differentiation potential is reported to decrease with age and in disorders such as arthritis.43 Another possibility is the use of induced pluripotent stem cells, but safety issues with these cells still need to be evaluated. Further work is required in all of these areas if stem cell therapy is to become a viable option.
Reliable detection methods of early degeneration will be key to selecting patients who are suitable for the different treatment options, and the development of more sensitive and quantitative MRI methods will help in patient selection.44–46 One part of the controversy is whether one should treat only the painful degenerate disc rather than disc degeneration per se. It is not unreasonable to suggest that one should treat degenerate (nonpainful) discs in a prophylactic manner in an attempt to repair or at least retard degeneration and possibly so, prevent future development of pain and delay the need for surgery.
Conclusion
In conclusion, the results support the concept that biological repair is a feasible process for treating the degenerate disc. They also imply that during the early stages of disc degeneration in the young adult, the administration of either Link N or MSCs has therapeutic potential.
Acknowledgments
This research is supported by CIHR (MOP111084) and AOSpine (SRN_2011_04_10498) grants. The authors also thank Janet Moir, Rahul Gawri, and Insaf Hadjab for their technical help during the experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mwale F.Collagen and other proteins of the nucleus pulposus, annulus fibrosus, and cartilage endplates. In: Shapiro I.M., and Risbud M.V., eds. The Intervertebral Discs, Wien: Springer; 2014, vol. 5, 2013, p. 79 [Google Scholar]
- 2.Ishihara H., McNally D.S., Urban J.P., and Hall A.C.Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol (1985) 80,839, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Handa T., Ishihara H., Ohshima H., Osada R., Tsuji H., and Obata K.Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 22,1085, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Hutton W.C., Elmer W.A., Boden S.D., Hyon S., Toribatake Y., Tomita K., and Hair G.A.The effect of hydrostatic pressure on intervertebral disc metabolism. Spine (Phila Pa 1976) 24,1507, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Hsieh A.H., and Lotz J.C.Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine (Phila Pa 1976) 28,1781, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kang J.D., Stefanovic-Racic M., McIntyre L.A., Georgescu H.I., and Evans C.H.Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 22,1065, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Goupille P., Jayson M.I., Valat J.P., and Freemont A.J.Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine (Phila Pa 1976) 23,1612, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Oegema T.R., Jr, Johnson S.L., Aguiar D.J., and Ogilvie J.W.Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine 25,2742, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Roberts S., Caterson B., Menage J., Evans E.H., Jaffray D.C., and Eisenstein S.M. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976) 25,3005, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Akhatib B., Onnerfjord P., Gawri R., Ouellet J., Jarzem P., Heinegard D., Mort J., Roughley P., and Haglund L.Chondroadherin fragmentation mediated by the protease HTRA1 distinguishes human intervertebral disc degeneration from normal aging. J Biol Chem 288,19280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunen S., Paassilta P., Lohiniva J., Perala M., Pihlajamaa T., Karppinen J., Tervonen O., Kroger H., Lahde S., Vanharanta H., Ryhanen L., Goring H.H., Ott J., Prockop D.J., and Ala-Kokko L.An allele of COL9A2 associated with intervertebral disc disease. Science 285,409, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi Y., Osada R., Kanamori M., Ishihara H., Ohmori K., Matsui H., and Kimura T.Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine 24,2456, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Ala-Kokko L.Genetic risk factors for lumbar disc disease. Ann Med 34,42, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Roughley P., Martens D., Rantakokko J., Alini M., Mwale F., and Antoniou J.The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater 11,1; discussion 7 2006 [PubMed] [Google Scholar]
- 15.Thompson J.P., Pearce R.H., Schechter M.T., Adams M.E., Tsang I.K., and Bishop P.B.Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 15,411, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Mwale F., Roughley P., and Antoniou J.Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater 8,58, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Wuertz K., and Haglund L.Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J 3,175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbaszade I., Liu R.Q., Yang F., Rosenfeld S.A., Ross O.H., Link J.R., Ellis D.M., Tortorella M.D., Pratta M.A., Hollis J.M., Wynn R., Duke J.L., George H.J., Hillman M.C., Jr, Murphy K., Wiswall B.H., Copeland R.A., Decicco C.P., Bruckner R., Nagase H., Itoh Y., Newton R.C., Magolda R.L., Trzaskos J.M., and Burn T.C.Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol.Chem 274,23443, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Almaawi A., Wang H.T., Ciobanu O., Rowas S.A., Rampersad S., Antoniou J., and Mwale F.Effect of acetaminophen and nonsteroidal anti-inflammatory drugs on gene expression of mesenchymal stem cells. Tissue Eng Part A 19,1039, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Antoniou J., Wang H.T., Alaseem A.M., Haglund L., Roughley P.J., and Mwale F.The effect of link N on differentiation of human bone marrow-derived mesenchymal stem cells. Arthritis Res Ther 14,R267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jim B., Steffen T., Moir J., Roughley P., and Haglund L.Development of an intact intervertebral disc organ culture system in which degeneration can be induced as a prelude to studying repair potential. Eur Spine J 20,1244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demers C.N., Antoniou J., and Mwale F.Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine (Phila Pa 1976) 29,2793, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Liebscher T., Haefeli M., Wuertz K., Nerlich A.G., and Boos N.Age-related variation in cell density of human lumbar intervertebral disc. Spine (Phila Pa 1976) 36,153, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg L.Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am 53,69, 1971 [PubMed] [Google Scholar]
- 25.Barbosa I., Garcia S., Barbier-Chassefiere V., Caruelle J.P., Martelly I., and Papy-Garcia D.Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology 13,647, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Mort J.S., and Roughley P.J.Measurement of glycosaminoglycan release from cartilage explants. Methods Mol Med 135,201, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Bjornsson S.Size-dependent separation of proteoglycans by electrophoresis in gels of pure agarose. Anal Biochem 210,292, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Sztrolovics R., White R.J., Roughley P.J., and Mort J.S.The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J 362,465, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mwale F., Demers C.N., Petit A., Roughley P., Poole A.R., Steffen T., Aebi M., and Antoniou J.A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem 88,1202, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Mwale F., Masuda K., Pichika R., Epure L.M., Yoshikawa T., Hemmad A., Roughley P.J., and Antoniou J.The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther 13,R120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., Weitzmann M.N., Sangadala S., Hutton W.C., and Yoon S.T.Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. J Biol Chem 288,28243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z., Hutton W.C., and Yoon S.T.ISSLS prize winner: effect of link protein peptide on human intervertebral disc cells. Spine (Phila Pa 1976) 38,1501, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Gawri R., Antoniou J., Ouellet J., Awwad W., Steffen T., Roughley P., Haglund L., and Mwale F.Best paper NASS 2013: link-N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur Cells Mater 26,107, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Petit A., Yao G., Rowas S.A., Gawri R., Epure L., Antoniou J., and Mwale F.Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells. Tissue Eng Part A 17,899, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Yang H., Wu J., Liu J., Ebraheim M., Castillo S., Liu X., Tang T., and Ebraheim N.A.Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J 10,802, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Hiyama A., Mochida J., Iwashina T., Omi H., Watanabe T., Serigano K., Tamura F., and Sakai D.Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res 26,589, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Sakai D., Mochida J., Yamamoto Y., Nomura T., Okuma M., Nishimura K., Nakai T., Ando K., and Hotta T.Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 24,3531, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Vadala G., Sowa G., Hubert M., Gilbertson L.G., Denaro V., and Kang J.D.Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 6,348, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Orozco L., Soler R., Morera C., Alberca M., Sanchez A., and Garcia-Sancho J.Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92,822, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Hristova G.I., Jarzem P., Ouellet J.A., Roughley P.J., Epure L.M., Antoniou J., and Mwale F.Calcification in human intervertebral disc degeneration and scoliosis. J Orthop Res 29,1888, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Nachemson A., Lewin T., Maroudas A., and Freeman M.A.In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand 41,589, 1970 [DOI] [PubMed] [Google Scholar]
- 42.Urban J.P., and Roberts S.Degeneration of the intervertebral disc. Arthritis Res Ther 5,120, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oreffo R.O., Bord S., and Triffitt J.T.Skeletal progenitor cells and ageing human populations. Clin Sci (Lond) 94,549, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Antoniou J., Epure L.M., Michalek A.J., Grant M.P., Iatridis J.C., and Mwale F.Analysis of quantitative magnetic resonance imaging and biomechanical parameters on human discs with different grades of degeneration. J Magn Reson Imaging 38,1402, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majumdar S., Link T.M., Steinbach L.S., Hu S., and Kurhanewicz J.Diagnostic tools and imaging methods in intervertebral disk degeneration. Orthop Clin North Am 42,501, viii, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Borthakur A., Maurer P.M., Fenty M., Wang C., Berger R., Yoder J., Balderston R.A., and Elliott D.M.T1rho magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine (Phila Pa 1976) 36,2190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]