Abstract
This work investigated the effect of flow perfusion bioreactor culture with and without transforming growth factor-β3 (TGF-β3) supplementation on the proliferation, extracellular matrix (ECM) production, and chondrogenic gene expression of chondrocytes both in monoculture and in co-culture with bone marrow-derived mesenchymal stem cells (MSCs). Both cell populations were cultured on electrospun poly(ɛ-caprolactone) scaffolds for 2 weeks in static or flow perfusion culture with and without TGF-β3. Overall, it was observed that without growth factors, flow perfusion culture resulted in increased cell proliferation and ECM with a more cartilage-like composition. While with TGF-β3 induction, flow perfusion constructs generally had lower chondrogenic gene expression than the corresponding static cultures, the growth factor still had an inductive effect on the cells with enhanced gene expression compared with the corresponding noninduced cultures. In addition, while flow perfusion cultures generally had reduced overall ECM content, the ECM distribution was more homogenous compared with the corresponding static cultures. These results are significant in that they indicate that while flow perfusion culture has some beneficial effects on the chondrogenic phenotype of articular chondrocytes, flow perfusion alone is not sufficient to maintain the chondrogenic phenotype of chondrocytes in either monoculture or co-culture, thus demonstrating the advantages of using exogenously added growth factors in flow perfusion culture. Furthermore, the results demonstrate the advantages of flow perfusion culture for the creation of large tissue engineered constructs and the potential of co-cultures of articular chondrocytes and MSCs to be used in flow perfusion culture.
Introduction
Articular cartilage lines the surfaces of bones in synovial joints and enables smooth gliding of the joints and protection of the underlying bones. Articular cartilage has a unique structure that imparts it with distinctive properties which are crucial to its function.1 This unique structure leads to a very low propensity for tissue healing, and current treatment options are not able to completely heal damage. Thus, injury to articular cartilage can cause long-term pain and disability, and there is a significant need for new treatment options.2 For this reason, tissue engineering seeks to develop new techniques and knowledge that can be used to enhance articular cartilage repair.
The culture of chondrocytes is not without its challenges. Specifically, articular chondrocytes can be difficult to isolate in sufficient numbers to be used in an effective treatment, and while they are easily expandable they dedifferentiate on expansion, lose their phenotype, and become more fibroblast-like cells.3 For these reasons, researchers have been investigating methods that would reduce the demand for chondrocytes and/or enhance the phenotype of the cells. One area of investigation that has shown great promise has been the use of co-cultures of chondrocytes with mesenchymal stem cells (MSCs). Such co-cultures have recently been investigated, and it has been observed that co-cultures can be used to reduce the total number of chondrocytes needed for the culture as the MSCs have been observed to enhance the phenotype of the chondrocytes when in co-culture.4 Furthermore, such co-cultures have been investigated in both normoxic and hypoxic conditions,5 with a range of chondrocyte passage numbers,6 and they have been found to be more sensitive to the chondrogenic stimulus transforming growth factor-β3 (TGF-β3) than chondrocyte cultures alone.7
Flow perfusion culture is one tissue engineering technique that has shown to be beneficial for the culture of a variety of cell types.8 Flow perfusion culture aims at perfusing the pores of three-dimensional scaffolds with culture medium in order to improve the mass transfer into the interior of the scaffolds and to apply shear stress to the cells in the scaffolds.9 This culture method has been shown to create tissue constructs with a more uniform distribution of cells and extracellular matrix (ECM) compared with static cultures, and the application of shear stress to the cells is known to have a variety of effects depending on the cell type. With chondrocyte cultures, the use of flow perfusion has been shown to increase the cell proliferation and cartilage-like ECM production.10–12 While TGF-β3 is known to improve the chondrogenic phenotype of both chondrocytes and co-cultures of chondrocytes and MSCs,7 its effect on these cell populations in flow perfusion culture has not been evaluated.
The present study aimed at investigating the effects of flow perfusion culture, growth factor stimulation, and MSC co-culture on the phenotype of bovine articular chondrocytes over a 2 week culture on electrospun poly(ɛ-caprolactone) (PCL) scaffolds. We hypothesized that the combined effects of flow perfusion culture and TGF-β3 induction would enhance the chondrogenic phenotype of both cell populations, and we aimed at mechanistically investigating the effects of each factor.
Materials and Methods
Experimental design
Bovine articular chondrocytes were seeded in monoculture or in co-culture with rabbit bone marrow-derived MSCs (1:1 ratio of chondrocytes:MSCs) on electrospun PCL scaffolds and cultured for 2 weeks in static or flow perfusion culture with and without 10 ng/mL of TGF-β3. After 2 weeks, constructs were analyzed via DNA, glycosaminoglycan (GAG), and hydroxyproline (HYP) quantification, chondrogenic gene expression analysis, and histology.
Cell isolation
Bovine articular chondrocytes were isolated from the femoral condyles of 7–10 day-old calves (Research 87) using previously described methods,13 rinsed with phosphate-buffered saline (PBS), pooled, and frozen for storage. Bone marrow-derived MSCs were harvested and isolated from the tibiae of 5-week-old New Zealand White rabbits (Charles River Laboratories) as previously described.5 Briefly, isolated bone marrow was plated on tissue culture flasks and rinsed after 48 h to remove nonadherent cells. Adherent cells were then cultured in general medium (Dulbecco's modified Eagle's medium [DMEM], 10% fetal bovine serum [FBS], penicillin, streptomycin, fungizone [PSF]) until confluent, pooled, and frozen. Similar to previous studies,4 two species of cells were used in co-culture in order to investigate the relative portion of chondrocyte gene expression.
Scaffold preparation
Electrospun PCL fiber mats were fabricated as previously described,14 using an 18 wt% solution of PCL (Inherent viscosity range 1.0–1.3 dL/g; Durect Corporation with a number-average molecular weight [Mn] of 71,000±2300 Da and a polydispersity index [Mw/Mn] of 2.2±0.07, as determined by gel permeation chromatography [Phenogel Linear Column with 5-μm particles; Phenomenex, Differential Refractometer 410, n=3; Waters] and a calibration curve generated from polystyrene standards [Fluka]), created by dissolving the polymer in a 5:1 v/v ratio of chloroform to methanol. The polymer solution was extruded at a flow rate of 40 mL/h through a 16 G needle, charged with a voltage of 30 kV, and directed toward a grounded collecting plate, 33 cm from the needle tip. Fiber morphology was inspected using scanning electron microscopy.
Scaffolds with an average fiber diameter of 10.2±2.3 μm (n=30) were punched from electrospun mats using a 3 mm dermal biopsy punch. Scaffolds, ∼1.5 mm in thickness, were press-fit into polycarbonate scaffold holders, designed to support the scaffolds during perfusion culture,8 sterilized by exposure to ethylene oxide, prewetted in a graded ethanol series, rinsed with PBS, and incubated in general medium for 3 days.
Cell seeding and culture
Chondrocytes and MSCs were thawed and expanded for one and two passages, respectively, lifted from culture using 0.05% trypsin-EDTA, and suspended in chondrocyte growth medium (DMEM, 10% FBS, 1% nonessential amino acids, 50 μg/mL ascorbic acid, 46 μg/mL L-proline, 20 mM HEPES, and 1% PSF). All scaffolds were seeded with a total of 50,000 cells in 30 μL of culture medium. Scaffolds were seeded with chondrocytes or a 1:1 ratio of chondrocytes to MSCs. The cultures were incubated overnight in chondrocyte growth medium to enable cell attachment.15 After incubation, static cultures were removed from loading cassettes and placed in ultralow attachment 24-well plates for culture. Dynamic cultures were performed in a flow perfusion bioreactor as previously described8,13 with 10 scaffolds per bioreactor unit, and a flow rate of 10 μL/min through each 3 mm scaffold. Each bioreactor unit and static sample were given an equal volume of serum-free chondrogenic medium (high-glucose DMEM, 1% ITS+premix [BD Biosciences], 50 mg/mL ascorbic acid, 100 nM dexamethasone, and PSF). In addition, half of the cultures were supplemented with TGF-β3 (PeproTech) at a concentration of 10 ng/mL of culture medium. Half of the medium was replenished thrice a week with serum-free medium with or without TGF-β3 and cultured for 2 weeks.
Biochemical assays
After culture, samples from each group (n=12–16) were rinsed in PBS and stored at −20°C for 2 weeks until the biochemical assays were started. Samples were thawed and digested in proteinase K solution (1 mg/mL proteinase K, 0.01 mg/mL pepstatin A, and 0.185 mg/mL iodoacetamide in a 50 mM tris(hydroxymethyl aminomethane), 1 mM ethylenediaminetetraacetic acid buffer, pH 7.6) at 56°C for 16 h.15 After proteinase K digestion, samples underwent three freeze/thaw cycles followed by 20 min of sonication. Double-stranded DNA content of the constructs was quantified using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) following previously described methods.4 Cell lysate, assay buffer, and dye solution were combined in an opaque 96-well plate in duplicate, incubated for 10 min at room temperature, and fluorescence was measured using excitation and emission wavelengths of 485 and 528 nm, respectively (FL x800 Fluorescence Microplate Reader; BioTek Instruments). DNA concentrations were determined relative to a lambda DNA standard curve.
Sulfated GAG content was determined using the colorimetric dimethylmethylene blue assay as previously described.15 Cell lysate and color reagent were combined in a transparent 96-well plate in duplicate. After being incubated at room temperature for 7 min, absorbance at 520 nm was measured (PowerWave x340 Microplate Reader; BioTek Instruments). GAG concentrations were determined relative to a chondroitin sulfate standard curve.
HYP content, an indicator of total collagen content, was measured using a colorimetric assay.15 Cell lysate was combined with an equal volume of 12 M HCl and heated at 115°C for 4 h. Samples were then evaporated under nitrogen flow and reconstituted in ddH2O. Samples were divided into duplicate reactions, with chloramine-T and p-dimethylaminobenzaldehyde solutions. Absorbance was read at 570 nm. HYP concentrations were determined relative to a trans-4-hydroxy-L-proline standard curve.
Real-time reverse transcription polymerase chain reaction
Pelleted cell seeding stocks and cultured constructs (n=6–8 samples) were rinsed in PBS, placed in 600 μL lysis buffer (Qiagen), and vortexed before storing at −80°C. After thawing, cell lysate was centrifuged through a QIA shredder homogenization column and combined with an equal volume of 70% ethanol. Total RNA was isolated using an RNeasy mini kit (Qiagen), following the manufacturer's instructions for the isolation of RNA from animal cells. Reverse transcription was performed using Oligo(dT) primers (Promega) and SuperScript III reverse transcriptase (Invitrogen). Real-time PCR (7300 Real-Time PCR System; Applied Biosystems) was performed on cDNA samples using SYBR Green detection (PerfeCTa SYBR Green FastMix, ROX; Quanta Biosciences) with previously established primer sequences (Integrated DNA Technologies).4
All samples were first analyzed using primer sequences that were designed to amplify bovine targets with a high level of specificity compared with rabbit targets. Target gene expression was first normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same sample (ΔCt), then to the average baseline expression of that target gene measured in the chondrocyte cell stock used to seed the scaffolds (ΔΔCt). The 2−ΔΔCt method was used to convert normalized gene expression levels into fold differences,16 and statistical analysis was performed on these values. 2−ΔCt was used to calculate the ratios of collagen II/collagen I. The primer sequences used in this analysis were 4 Collagen type I: 5′-CGGGTCTTGCTGGTCATCAT-3′, 5′-TGCACCAGGCTGTCCAATG-3′; Collagen type II: 5′-AGTGGAAGAGCGGAGACTACTG-3′; 5′-GTTGGGAGCCAGGTTGTCAT-3′; Aggrecan: 5′-AGAGAGCCAAACAGCCGACA-3′; 5′-TAGTCCTGGGCATTGTTGTTGA-3′; GAPDH: 5′-GAGTCCACTGGGGTCTTCACT-3′; 5′-GCGTGGACAGTGGTCATAAGTC-3′.
In addition, co-cultured samples were also analyzed using previously established primer sequences that were designed to amplify both rabbit and bovine targets with equal efficiencies. 2−ΔCt was used to calculate the ratios of bovine-specific/cross-species expression within individual samples.4 The primer sequences used in this analysis were 4 Collagen type I: 5′-CCCAGAATGGAGCAGTGGTTACT-3′, 5′-AGCAGACGCATGAAGGCAAG-3′; Collagen type II: 5′-GGCTTCCACTTCAGCTATGGAG-3, 5′-GTGTGTTTCGTGCAGCCATC-3′; Aggrecan: 5′-GAGCAGGAGTTTGTCAACAACAA-3′, 5′-CCTCCCCAGTGGCAAAGAAG-3′; GAPDH: 5′-CCATCTTCCAGGAGCGAGAT-3′, 5′-GGTTCACGCCCATCACAAAC-3′. The bovine-specific GAPDH signal of the co-cultures increased from 0.1 to ∼1.5 irrespective of the culture conditions (data not shown), indicating enrichment of the bovine chondrocytes.4
Histological analysis
Samples were fixed using 10% neutral-buffered formalin (Fisher Scientific), dehydrated in 70% ethanol, embedded in HistoPrep freezing medium (Fisher Scientific), and cut into 5 μm-thick sections using a cryostat (Leica CM 1850 UV; Leica Biosystems Nussloch GmbH). Sections were mounted onto glass slides, stained using Alcian Blue and Picrosirius Red, and counterstained with Fast Green to visualize the presence and distribution of GAGs and collagen and cells, respectively. Images were obtained using a light microscope with a digital camera attachment (Axio Imager.Z2 equipped with AxioCam MRc5; Carl Zeiss MicroImaging GmbH).
Statistical analysis
Results are reported as mean+standard deviation. Results of biochemical assays were evaluated using one-way analysis of variance and Tukey–Kramer multiple-comparison tests to determine significant differences (p<0.05). Results of reverse transcription polymerase chain reaction (RT-PCR) were evaluated using the Kruskal–Wallis test followed by the Mann–Whitney U test (p<0.05).
Results
Biochemical assays
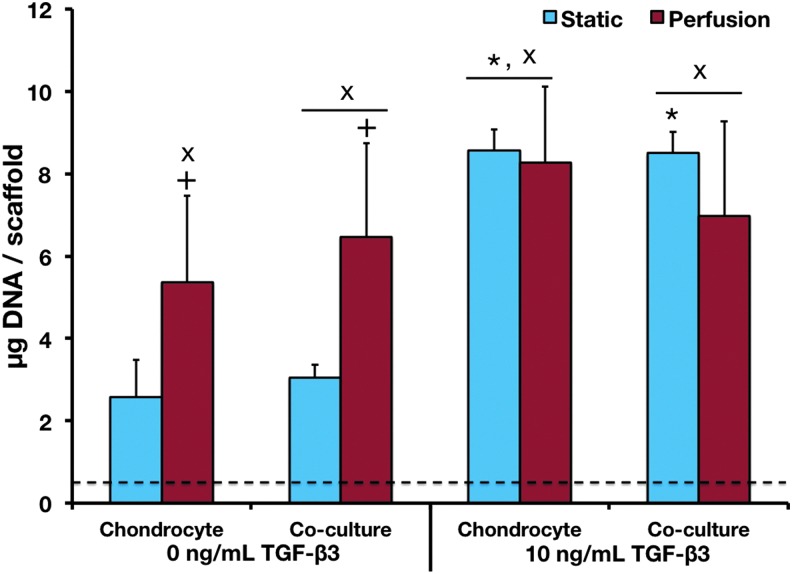
As shown in Figure 1, after 14 days of culture, all groups exhibited an increase in cell content from the day 0 levels except the noninduced, static chondrocyte constructs. However, no difference in DNA content was observed between the two cell populations in any of the culture conditions. Without TGF-β3 present, there was a noticeable effect of perfusion culture, resulting in an increase in DNA content in both cell populations. However, in inductive cultures, perfusion and static groups had similar cellularity. In perfusion, TGF-β3 increased DNA content in only the chondrocyte population, but in static culture there was a significant effect of TGF-β3 in both cell populations.
FIG. 1.
DNA content of samples after 14 days of culture. The dashed line indicates the day 0 DNA levels. ×Statistical difference between the day 14 and day 0 samples. +Statistical difference between the corresponding static and flow perfusion samples. *Statistical difference between cultures exposed to TGF-β3 and the corresponding cultures not exposed to TGF-β3. p<0.05 for all statistical comparisons. Error bars designate standard deviation for n=12–16. TGF-β3, transforming growth factor-β3. Color images available online at www.liebertpub.com/tea
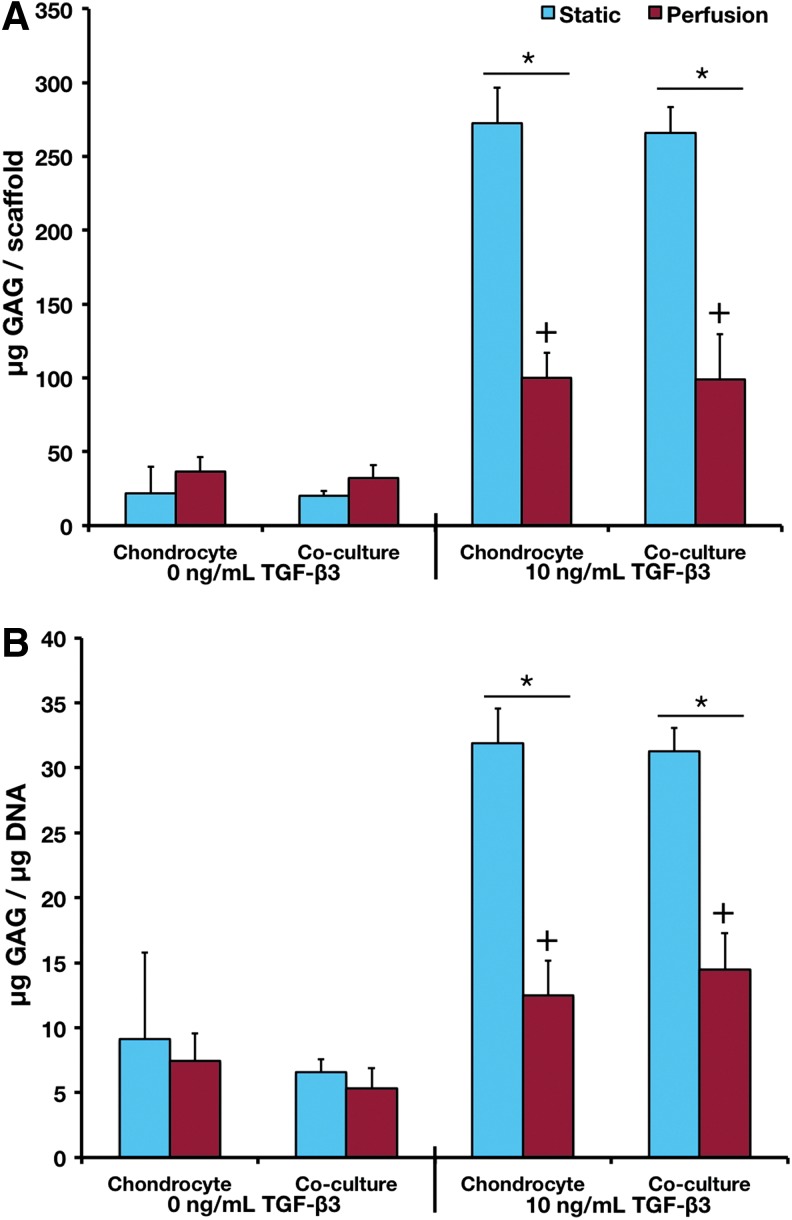
Total GAG content and GAG/DNA ratio followed similar trends (Fig. 2). All TGF-β3 cultures demonstrated greater GAG contents and GAG/DNA ratios than noninductive cultures. The difference in GAG production as a result of perfusion culture was most drastic in the cultures with TGF-β3, as there was no effect of perfusion culture on GAG production in the noninductive cultures. Conversely, the inductive perfusion cultures had significantly less GAG and lower GAG/DNA ratios than the corresponding static cultures.
FIG. 2.
(A) GAG content and (B) GAG/DNA ratio of samples after 14 days of culture. +Statistical difference between the corresponding static and flow perfusion samples. *Statistical difference between cultures exposed to TGF-β3 and the corresponding cultures not exposed to TGF-β3. p<0.05 for all statistical comparisons. Error bars designate standard deviation for n=12–16. GAG, glycosaminoglycan. Color images available online at www.liebertpub.com/tea
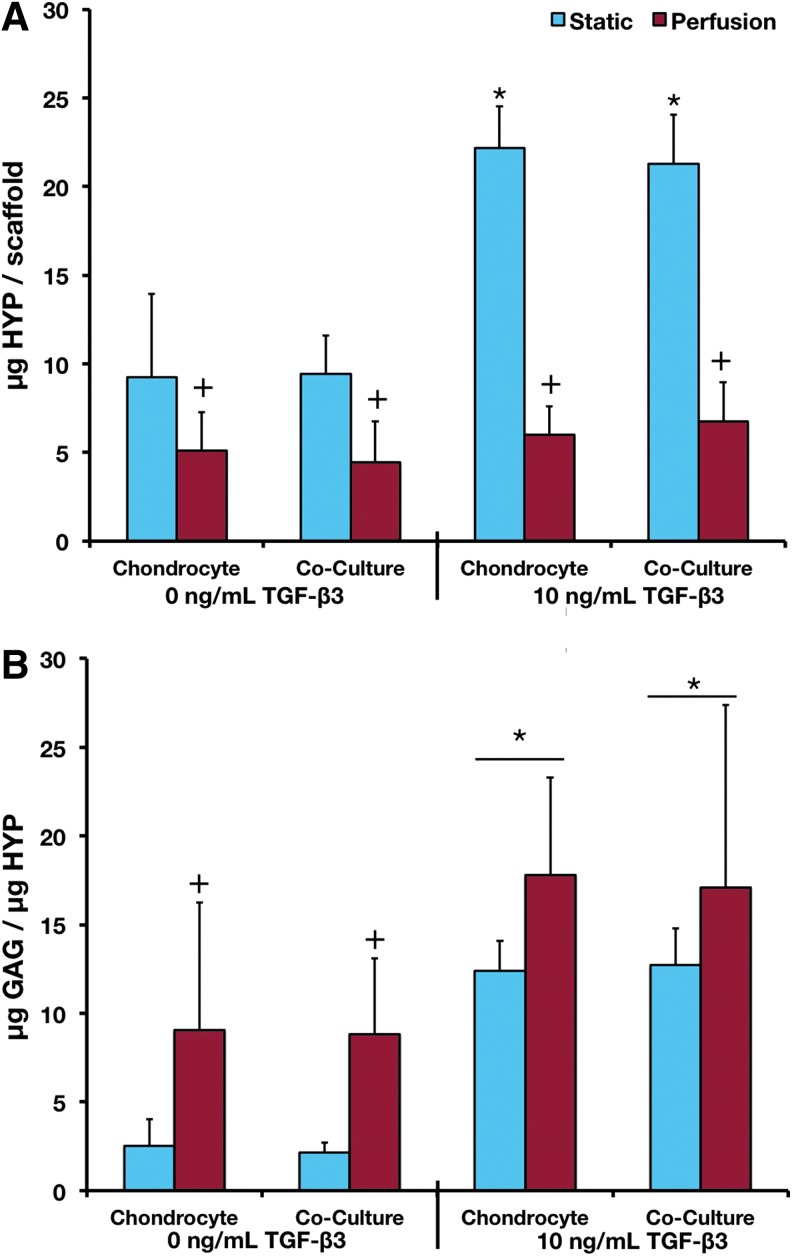
Total HYP contents and GAG/HYP ratios are shown in Figure 3. Total HYP content of the constructs was lower in all perfused constructs compared with static constructs. Exposure to TGF-β3 increased HYP content in static culture but not in perfusion. Evaluating the type of ECM present in the constructs, perfusion increased the GAG/HYP ratio only in noninduced cultures; whereas TGF-β3 exposure always increased the ratio.
FIG. 3.
(A) HYP content and (B) GAG/HYP ratio of samples after 14 days of culture. +Statistical difference between the corresponding static and flow perfusion samples. *Statistical difference between cultures exposed to TGF-β3 and the corresponding cultures not exposed to TGF-β3. p<0.05 for all statistical comparisons. Error bars designate standard deviation for n=12–16. HYP, hydroxyproline. Color images available online at www.liebertpub.com/tea
Real-time reverse transcription polymerase chain reaction
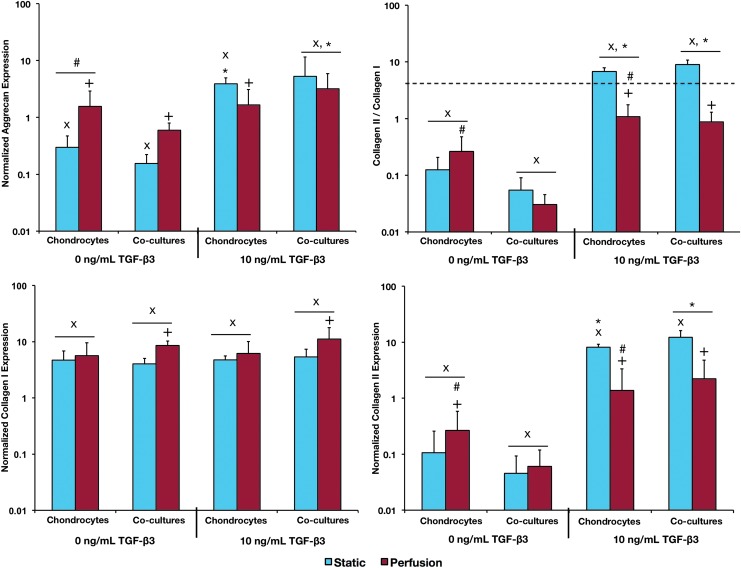
As shown in Figure 4, aggrecan expression of noninduced static cultures decreased over the 2 week culture period with the chondrocytes in co-culture exhibiting lower aggrecan expression than the chondrocytes in monoculture. Conversely, noninduced perfusion culture led to maintenance of the initial level of aggrecan expression and higher expression compared with the static culture. TGF-β3 induction generally led to an increase in aggrecan expression compared with the initial levels and the levels in the noninduced cultures, with the exception of the chondrocyte perfusion group, whose aggrecan expression was no greater than the corresponding noninduced cultures or the initial aggrecan expression and was less than the induced static culture.
FIG. 4.
Gene expression of samples after 14 days of culture. Aggrecan, collagen I, and collagen II expression are normalized to the expression levels of chondrocyte seeding stock. The dashed line indicates the day 0 Collagen II/Collagen I ratio of chondrocytes in the seeding stocks. ×Statistical difference between the day 14 and day 0 samples. +Statistical difference between the corresponding static and flow perfusion samples. *Statistical difference between cultures exposed to TGF-β3 and the corresponding cultures not exposed to TGF-β3. #Statistical difference between the gene expression of the chondrocytes in monoculture and the corresponding chondrocytes in co-culture. p<0.05 for all statistical comparisons. Error bars designate standard deviation for n=6–8. Color images available online at www.liebertpub.com/tea
Collagen I expression increased over the duration of the culture in all groups with perfusion culture adding an additional increased effect in chondrocytes in both co-culture conditions. Interestingly, chondrocytes in co-culture demonstrated increased collagen I expression when exposed to perfusion than the corresponding co-culture in static condition. Collagen II expression decreased from the initial levels in all noninduced cultures; however, perfusion culture increased the expression in chondrocyte cultures compared with the corresponding static and co-culture groups. Induced cultures generally had greater collagen II expression than noninduced cultures, with the exception of the chondrocyte perfusion group. Induced cultures in static condition exhibited an increase in collagen II expression over the duration of the culture, and while the induced perfusion cultures maintained the initial level of collagen II expression, it was significantly lower than the corresponding static cultures. Furthermore, in static culture, the chondrocytes in induced co-culture exhibited higher collagen II expression than the corresponding chondrocytes in monoculture.
The collagen II/collagen I ratio decreased over time in all noninduced cultures with the monoculture chondrocytes in perfusion having a higher collagen II/collagen I ratio than the co-culture chondrocytes in perfusion. TGF-β3 induction had a positive effect on the collagen II/collagen I ratio in all cultures with the effect being most noticeable in the static cultures. In static culture, induction medium led to an increase in the collagen II/collagen I ratio over time; however, in perfusion culture, the ratio was not only lower than the static culture but also decreased over time. In addition, in static conditions, co-culturing chondrocytes led to an additional increase in collagen II/collagen I ratio in comparison to monocultures.
Histology
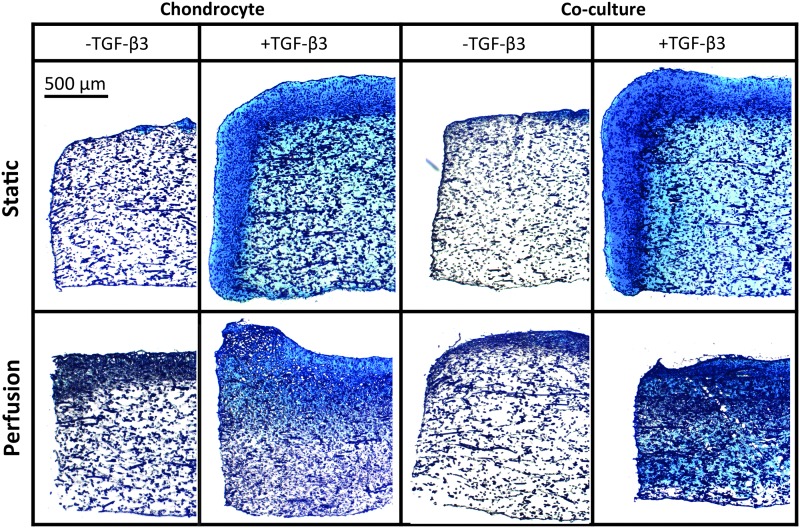
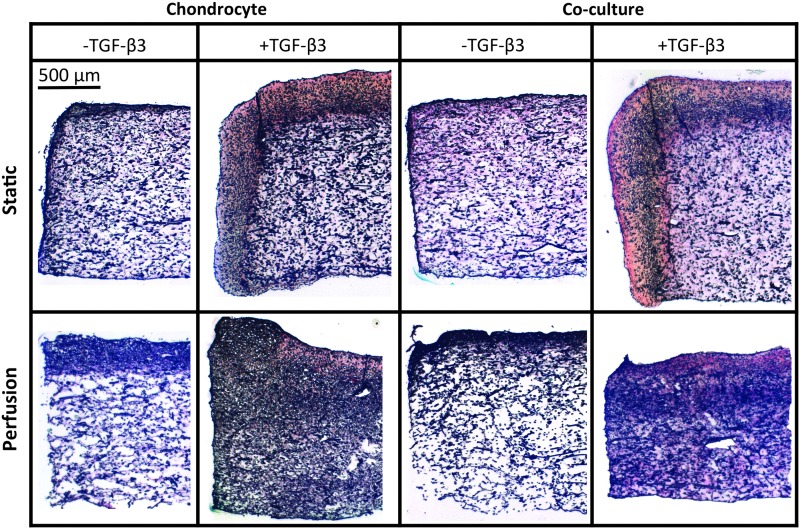
Histological evaluation corroborated the results of the biochemical assays. Representative images of sections stained for GAGs and collagen are shown in Figures 5 and 6, respectively. Denser Alcian Blue and Picrosirius Red staining was observed in constructs exposed to TGF-β3. Furthermore, no difference in staining was observed between chondrocyte and co-cultured constructs. Static cultured constructs had a dense peripheral region of ECM deposition, compared with flow perfusion cultured constructs, which had a more homogeneous distribution of ECM.
FIG. 5.
Alcian blue staining for GAGs in 5 μm-thick sections of constructs cultured for 14 days. Scale bar represents 500 μm in all images.
FIG. 6.
Picrosirius red staining for collagen and fast green staining for cells in 5 μm-thick sections of constructs cultured for 14 days. Scale bar represents 500 μm in all images.
Discussion
Previous studies investigating flow perfusion culture of articular chondrocytes have demonstrated that flow perfusion is capable of enhancing chondrocyte proliferation and matrix production, which could aid in the production of large tissue engineered constructs.10–12 However, studies investigating the gene expression of chondrocytes in perfusion have generally observed a decrease in chondrogenic gene expression compared with static cultures.13,17 In our previous work, we investigated the use of flow perfusion culture in combination with hypoxia and found that while there were beneficial effects of both flow perfusion and hypoxic culture, neither condition was capable of maintaining the chondrogenic phenotype of the cells in the absence of exogenously added growth factors.13 Instead, we observed high levels of cellular proliferation, and with that rapid dedifferentiation of the cells, as the ratio of collagen II to collagen I gene expression decreased dramatically over even a very short period of culture (6 days).13 Thus, while flow perfusion enhanced the chondrocyte proliferation, ECM production, and spatial distribution of cells and ECM, flow perfusion conditions were not sufficient to maintain the initial levels of chondrogenic gene expression. When cells were exposed to hypoxia under flow perfusion, the level of chondrogenic gene expression was maintained at a higher level, but while hypoxia has been shown to support chondrogenesis,5,18 it was not able to fully mitigate the dedifferentiation that occurred in both perfusion and static culture. Therefore, there was a need for a more potent chondrogenic signal. Previous studies in flow perfusion have traditionally used medium with 10% serum content and no additional exogenous growth factors.10–12,17,19–21 While these conditions are beneficial for cell proliferation, they may not be ideal for the maintenance of the chondrogenic phenotype. Accordingly, the present study aimed at mechanistically evaluating the effects of flow perfusion culture, with minimal serum, with and without the chondrogenic stimulus TGF-β3, on the chondrogenic phenotype of chondrocytes in both monoculture and co-culture. It was hypothesized that combined exposure to the chondrogenic growth factor and perfusion culture could support the chondrogenic phenotype of the cells in both monoculture and co-culture. To evaluate this hypothesis, bovine articular chondrocytes were cultured for 14 days on PCL fiber mesh scaffolds in static and flow perfusion conditions with and without TGF-β3 and in monoculture or a 1:1 co-culture ratio with rabbit MSCs.
Cell proliferation was evaluated by the total DNA content of the constructs after 14 days of culture. Similar to previous studies, flow perfusion culture increased proliferation in noninduced cultures of chondrocytes.13 However, when exposed to TGF-β3, no difference in proliferation was observed between static and perfusion cultures, as the growth factor led to high levels of cell proliferation. These results correspond well with previous findings that when exposed to shear stress, chondrocytes proliferate as a result of TGF-β1 up-regulation and the subsequent autocrine signaling.22 In the present study with TGF-β3 supplementation, enhanced proliferation due to perfusion was not observed, which may occur according to the described mechanism as the effect of intrinsic growth factor up-regulation would be masked by supplemented TGF-β3.
Chondrogenic ECM deposition was evaluated by total GAG content, GAG/DNA, and GAG/HYP of the constructs after 14 days. The utility of co-cultures rests in the assurance that co-cultures of MSCs and chondrocytes can achieve levels of normalized GAG and total GAG on par with chondrocytes alone.4,7 This is a striking fact considering that in as little as 14 days of culture in the present study, one cell population starting with half the chondrogenic potential of another can deposit an equivalent level of chondrogenic ECM. An interesting question is raised as to whether this striking effect of co-cultures can persist in the presence of other inductive environments, namely perfusion with or without TGF-β3 supplementation, which was shown in the present study to be the case. In each case, the co-cultures achieved chondrogenic ECM depostion on par with chondrocytes alone.
The present experiment expanded earlier analysis to isolate the effects of perfusion and TGF-β3 supplementation. With added TGF-β3, perfusion cultures consistently produced lower multiples in total GAG and normalized GAG but equivalent GAG/HYP compared with static culture. It appears that perfusion culture decreased the levels of both GAG and HYP in the constructs so that greater overall ECM production in static did not translate to an enhanced chondrogenic make-up, which may be a result of ECM production or poor retention of ECM in the perfusion constructs.23 Comparing TGF-β3 supplemented to nonsupplemented cultures in perfusion for the first time, TGF-β3 induced the chondrogenesis of articular chondrocytes and co-cultures by creating an additive effect to perfusion alone. This proved true in terms of GAG/DNA, total GAG, and GAG/HYP.
Our gene expression analysis indicated that perfusion alone had a positive chondrogenic effect on the chondrocytes in culture. This effect was demonstrated by the level of aggrecan expression of the chondrocytes in both monoculture and co-culture without TGF-β3 supplementation. Here, the aggrecan expression was maintained in perfusion culture from the day 0 levels, as compared with the static cultures that exhibited a decreased level of aggrecan expression over the 14 day culture. This result corroborates the increase in GAG/HYP described earlier. With TGF-β3 supplementation, there was a further increase in aggrecan expression. However, similar to previous studies, the addition of growth factor to co-cultures led to a more dramatic chondrogenic effect compared with the chondrocytes in monoculture.7 This effect was noticable, as the growth factor supplementation led to no increase in aggrecan expression in the chondrocyte perfusion cultures. Interestingly, with TGF-β3 there was an increase in co-culture aggrecan expression to levels where no difference between static and perfusion culture was observed. This result may further indicate that the difference in GAG content of the constructs in static and perfusion culture may be the result of the washing out of GAGs from the scaffolds, rather than a difference in ECM production.23
While perfusion alone was able to maintain the aggrecan expression, the ratio of collagen II/I expression was not maintained from the initial level in perfusion culture. In fact, without growth factor supplementation, there was no effect of flow perfusion culture on the expression ratio. Without TGF-β3, monocultured chondrocytes in perfusion exhibited a higher collagen II/I ratio than chondrocytes co-cultured in perfusion, but again with growth factor induction there was a more drastic increase in collagen II/I expression in chondrocytes in co-culture than in monoculture. Interestingly, collagen I expression was higher for all co-cultured chondrocytes in perfusion compared with static culture. Perhaps the flow perfusion culture lowered the density of cell-to-cell contacts, causing an increase in the more fibrocartilage-associated collagen.24 The highest level of collagen II/I expression was observed in the static chondrocytes in co-culture with TGF-β3 supplementation. However, with TGF-β3 present, perfusion culture exhibited a lower level of expression than static and day 0. While the collagen II/I ratio still decreased over time in perfusion, the growth factor was still effective in enhancing the chondrogenic phenotype of the cells in perfusion culture compared with chondrocytes not supplemented with growth factors.
In static culture with TGF-β3, there were significant ECM deposits outside the scaffold, compared with the perfusion cultures, which had minimal ECM deposits outside the scaffold. This could be the result of poor diffusion of nutrients into the interior of the scaffold, a known effect of static cultures in constructs of this size.25 Similarly, the TGF-β3 diffusion would be greatly diminished to the interior of the construct as diffusion of large proteins through dense ECM may be inhibited,26 leading to the creation of a local growth factor gradient. Such an effect would potentially create a positive feedback to the creation of an even thicker exterior. On the other hand, in perfusion, a more even distribution of ECM can be seen in the perfusion cultures, possibly due to the more even distribution of TGF-β3 when it is supplemented to the scaffolds through convection. These results demonstrate the need for the use of flow perfusion or convection in cultures supplemented with growth factors to form large tissue engineered constructs.
Conclusions
The effect of TGF-β3 and perfusion, which were hypothesized to increase chondrogenesis in these cultures, was confirmed in this work. Perfusion culture alone, although exhibiting advantages to the distribution of ECM production in our PCL constructs, was not capable of maintaining chondrogenesis. While perfusion alone promoted higher aggrecan expression than the corresponding static cultures, perfusion alone was not capable of maintaining the collagen II/I expression. However, when combined with TGF-β3 supplementation, the perfusion groups achieved some of the chondrogenic benefit while retaining the previously established advantages of perfusion cultures, namely a more homogenous distribution of ECM compared with static cultures. Overall, the results provide a rationale for creating tissue engineering chondrogenic constructs on a larger scale by using perfusion culture.
Acknowledgment
This work was supported by the National Institutes of Health grant R01 AR057083.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cohen N.P., Foster R.J., and Mow V.C.Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther 28,203, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Temenoff J.S., and Mikos A.G.Review: tissue engineering for regeneration of articular cartilage. Biomaterials 21,431, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Benya P.D., and Shaffer J.D.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30,215, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Meretoja V.V., Dahlin R.L., Kasper F.K., and Mikos A.G.Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials 33,6362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meretoja V.V., Dahlin R.L., Wright S., Kasper F.K., and Mikos A.G.The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 34,4266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meretoja V.V., Dahlin R.L., Wright S., Kasper F.K., and Mikos A.G.Articular chondrocyte redifferentiation in 3D co-cultures with mesenchymal stem cells. Tissue Eng Part C Methods 2014[Epub ahead of print]; DOI: 10.1089/ten.tec.2013.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlin R.L., Ni M., Meretoja V.V., Kasper F.K., and Mikos A.G.TGF-beta3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials 35,123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlin R.L., Meretoja V.V., Ni M., Kasper F.K., and Mikos A.G.Design of a high-throughput flow perfusion bioreactor system for tissue engineering. Tissue Eng Part C Methods 18,817, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bancroft G.N., Sikavitsas V.I., and Mikos A.G.Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng 9,549, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Gemmiti C.V., and Guldberg R.E.Fluid flow increases type II collagen deposition and tensile mechanical properties in bioreactor-grown tissue-engineered cartilage. Tissue Eng 12,469, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Davisson T., Sah R.L., and Ratcliffe A.Perfusion increases cell content and matrix synthesis in chondrocyte three-dimensional cultures. Tissue Eng 8,807, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Pazzano D., Mercier K.A., Moran J.M., Fong S.S., DiBiasio D.D., Rulfs J.X., et al. Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnol Prog 16,893, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Dahlin R.L., Meretoja V.V., Ni M., Kasper F.K., and Mikos A.G.Hypoxia and flow perfusion modulate proliferation and gene expression of articular chondrocytes on porous scaffolds. AIChE J 59,3158, 2012 [Google Scholar]
- 14.Pham Q.P., Sharma U., and Mikos A.G.Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 7,2796, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Liao J.H., Guo X.A., Grande-Allen K.J., Kasper F.K., and Mikos A.G.Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials 31,8911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmittgen T.D., and Livak K.J.Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3,1101, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mizuno S., Allemann F., and Glowacki J.Effects of medium perfusion on matrix production by bovine chondrocytes in three-dimensional collagen sponges. J Biomed Mater Res 56,368, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Foldager C.B., Nielsen A.B., Munir S., Ulrich-Vinther M., Soballe K., Bunger C., et al. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop 82,234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raimondi M.T., Boschetti F., Falcone L., Migliavacca F., Remuzzi A., and Dubini G.The effect of media perfusion on three-dimensional cultures of human chondrocytes: integration of experimental and computational approaches. Biorheology 41,401, 2004 [PubMed] [Google Scholar]
- 20.Raimondi M.T., Candiani G., Cabras M., Cioffi M., Lagana K., Moretti M., et al. Engineered cartilage constructs subject to very low regimens of interstitial perfusion. Biorheology 45,471, 2008 [PubMed] [Google Scholar]
- 21.Raimondi M.T., Moretti M., Cioffi M., Giordano C., Boschetti F., Lagana K., et al. The effect of hydrodynamic shear on 3D engineered chondrocyte systems subject to direct perfusion. Biorheology 43,215, 2006 [PubMed] [Google Scholar]
- 22.Malaviya P., and Nerem R.M.Fluid-induced shear stress stimulates chondrocyte proliferation partially mediated via TGF-beta1. Tissue Eng 8,581, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Shahin K., and Doran P.M.Strategies for enhancing the accumulation and retention of extracellular matrix in tissue-engineered cartilage cultured in bioreactors. PLoS One 6,e23119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlin R.L., Gershovich J.G., Kasper F.K., and Mikos A.G.Flow perfusion co-culture of human mesenchymal stem cells and endothelial cells on biodegradable polymer scaffolds. Ann Biomed Eng 2013[Epub ahead of print]; DOI: 10.1007/s10439-013-0862-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freyer J.P., and Sutherland R.M.Proliferative and clonogenic heterogeneity of cells from EMT6/Ro multicellular spheroids induced by the glucose and oxygen supply. Cancer Res 46,3513, 1986 [PubMed] [Google Scholar]
- 26.Chen F.M., Zhang M., and Wu Z.F.Toward delivery of multiple growth factors in tissue engineering. Biomaterials 31,6279, 2010 [DOI] [PubMed] [Google Scholar]